Abstract
In Europe and the United States, there is an increasing prevalence of the use of autologous blood products to facilitate healing in a variety of applications. Recently, we have learned more about specific growth factors, which play a crucial role in the healing process. With that knowledge there is abundant enthusiasm in the application of concentrated platelets, which release a supra-maximal quantity of these growth factors to stimulate recovery in non-healing injuries. For 20 years, the application of autologous PRP has been safely used and documented in many fields including; orthopedics, sports medicine, dentistry, ENT, neurosurgery, ophthalmology, urology, wound healing, cosmetic, cardiothoracic, and maxillofacial surgery. This article introduces the reader to PRP therapy and reviews the current literature on this emerging treatment modality. In summary, PRP provides a promising alternative to surgery by promoting safe and natural healing. However, there are few controlled trials, and mostly anecdotal or case reports. Additionally the sample sizes are frequently small, limiting the generalization of the findings. Recently, there is emerging literature on the beneficial effects of PRP for chronic non-healing tendon injuries including lateral epicondylitis and plantar fasciitis and cartilage degeneration (Mishra and Pavelko, The American Journal of Sports Medicine 10(10):1–5, 2006; Barrett and Erredge, Podiatry Today 17:37–42, 2004). However, as clinical use increases, more controlled studies are needed to further understand this treatment.
Keywords: Platelet rich plasma, Injection, Growth factors, Tendon injury, Autologous blood, Musculoskeletal injuries, Chondropenia, Knee osteoarthritis
Introduction
In Europe, and more recently in the United States, an increased trend has emerged in the use of autologous blood products in an effort to facilitate healing in a variety of applications. In recent years, scientific research and technology has provided a new perspective on understanding the wound healing process. Initially platelets were thought to act exclusively with clotting. However, we have learned that platelets also release many bioactive proteins responsible for attracting macrophages, mesenchymal stem cells, and osteoblasts which not only promotes removal of necrotic tissue, but also enhances tissue regeneration and healing.
Based on this principle platelets are introduced to stimulate a supra-physiologic release of growth factors in an attempt to jump start healing in chronic injuries. The current literature reveals a paucity of randomized clinical trials. The existing literature is filled with mostly anecdotal reports or case series, which typically have small sample sizes and few control groups [1, 2]. A large multi-center trial is currently underway providing a more objective understanding of Platelet Rich Plasma (PRP) use in chronic epicondylitis.
According to the World Health Organization (WHO), musculoskeletal injuries are the most common cause of severe long-term pain and physical disability, and affect hundreds of millions of people around the world [3]. In fact, the years 2000–2010 have been termed “the decade of bone and joint” as a global initiative to promote further research on prevention, diagnosis, and treatment [3, 4]. Soft tissue injuries including tendon and ligament trauma represent 45% of all musculoskeletal injuries in the USA [4, 5]. The continued popularity of sporting activities has brought with it an epidemic of musculoskeletal disorders focusing attention on tendons. Additionally, modern imaging techniques including magnetic resonance imaging and musculoskeletal ultrasound have provided clinicians with further knowledge of these injuries.
Blood components
Blood contains plasma, red blood cells (RBC), white blood cells (WBC), and platelets. Plasma is the liquid component of blood, made mostly of water and acts as a transporter for cells. Plasma also contains fibrinogen, a protein that acts like a net and catches platelets at a wound site to form a clot. RBC helps pick up oxygen from the lungs and delivers it to other body cells, while removing carbon dioxide. WBC fights infection, kills germs, and carries off dead blood cells. Platelets are responsible for hemostasis, construction of new connective tissue, and revascularization. Typically a blood specimen contains 93% RBC, 6% Platelets, and 1% WBC [6]. The rationale for PRP benefit lies in reversing the blood ratio by decreasing RBC to 5%, which are less useful in the healing process, and increasing platelets to 94% to stimulate recovery [6].
Platelets
Platelets are small discoid blood cells made in bone marrow with a lifespan of 7–10 days. Inside the platelets are many intracellular structures containing glycogen, lysosomes, and two types of granules. The alpha granules contain the clotting and growth factors that are eventually released in the healing process. Normally at the resting state, platelets require a trigger to activate and become a participant in wound healing and hemostasis [7]. Upon activation by thrombin, the platelets morph into different shapes and develop branches, called pseudo-pods that spread over injured tissue. This process is termed aggregation. Eventually the granules contained within platelets release the growth factors, which stimulate the inflammatory cascade and healing [7].
PRP
Platelet Rich Plasma is defined as a volume of the plasma fraction of autologous blood having a platelet concentration above baseline [8, 9]. Normal platelet concentration is 200,000 platelets/ul. Studies have shown that clinical efficacy can be expected with a minimum increase of 4× this baseline (1million platelets/ul) [6]. Slight variability exists in the ability to concentrate platelets, largely depending on the manufacturer’s equipment. However, it has not been studied if too great an increased platelet concentration would have paradoxical effects.
The use of autologous PRP was first used in 1987 by Ferrari et al. [10] following an open heart surgery, to avoid excessive transfusion of homologous blood products. Since that time, the application of autologous PRP has been safely used and documented in many fields including; orthopedics, sports medicine, dentistry, ENT, neurosurgery, ophthalmology, urology, and wound healing; as well as cosmetic, cardiothoracic, and maxillofacial surgery. Studies suggest that PRP can affect inflammation, post-operative blood loss, infection, narcotic requirements, osteogenesis, wound, and soft tissue healing.
In addition to local hemostasis at sites of vascular injury, platelets contain an abundance of growth factors and cytokines that are pivotal in soft tissue healing and bone mineralization [4]. An increased awareness of platelets and their role in the healing process has lead to the concept of therapeutic applications.
Tendons
PRP is increasingly used in treatment of chronic non-healing tendon injuries including the elbow, patella, and the achilles among others. As a result of mechanical factors, tendons are vulnerable to injury and stubborn to heal. Tendons are made of specialized cells including tenocytes, water, and fibrous collagen proteins. Millions of these collagen proteins weave together to form a durable strand of flexible tissue to make up a tendon. They naturally anchor to the bone and form a resilient mineralized connection. Tendons also bear the responsibility of transferring a great deal of force, and as a result are susceptible to injury when they are overwhelmed. With repetitive overuse, collagen fibers in the tendon may form micro tears, leading to what is called tendonitis; or more appropriately tendinosis or tendinopathy. The injured tendons heal by scarring which adversely affects function and increases risk of re-injury. Furthermore, tendons heal at a slow rate compared with other connective tissues, secondary to poor vascularization [11–13]. Histologic samples from chronic cases indicate that there is not an inflammatory response, but rather a limitation of the normal tendon repair system with a fibroblastic and a vascular response called, angiofibroblastic degeneration [1, 14, 15]. Given the inherent nature of the tendon, new treatment options including dry needling, prolotherapy, and extracorporeal shockwave therapy are aimed at embracing inflammation rather than suppressing it.
Traditional therapies to treat these conditions do not alter the tendon’s inherent poor healing properties and involve long-term palliative care [16, 17]. A recent meta-analysis of 23 randomized controlled studies on physical therapy treatment for epicondylitis, concluded that there is insufficient supportive evidence of improved outcomes [1, 18]. Corticosteroids are commonly injected, however studies suggest adverse side effects including atrophy and permanent adverse structural changes in the tendon [14]. Medications including NSAIDs, while commonly used for tendinopathies, carry significant long-term risks including bleeding ulcers and kidney damage. Thus, organically based strategies to promote healing while facilitating the release of one’s own natural growth factors is attracting interest.
Growth factors
It is widely accepted that growth factors play a central role in the healing process and tissue regeneration [4, 19]. This conclusion has lead to significant research efforts examining varying growth factors and their role in repair of tissues [4, 20]. However, there are conflicting reports in the literature regarding potential benefits. Although some authors have reported improved bone formation and tissue healing with PRP, others have had less success [4, 21, 22]. These varying results are likely attributed to the need for additional standardized PRP protocols, preparations, and techniques. There are a variety of commercially FDA approved kits available with variable platelet concentrations, clot activators, and leukocyte counts which could theoretically affect the data.
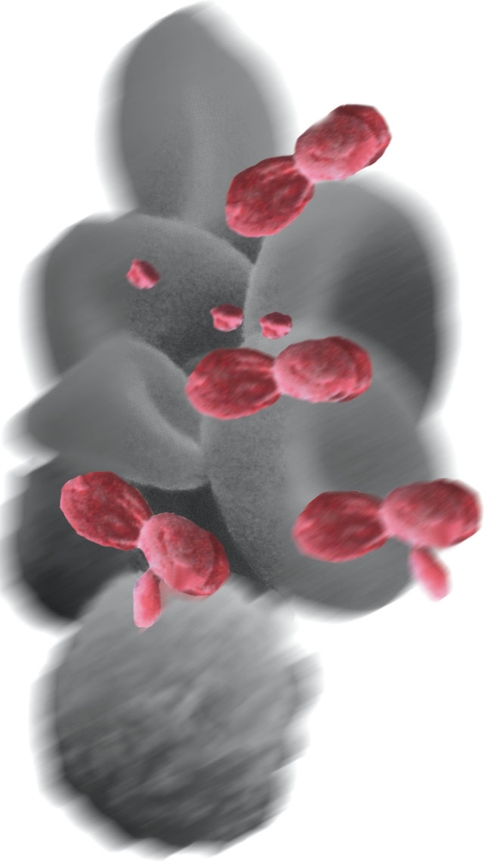
Alpha granules are storage units within platelets, which contain pre-packaged growth factors in an inactive form (Fig. 1). The main growth factors contained in these granules are transforming growth factor beta (TGFbeta), vascular endothelial growth factor (VEGF) platelet-derived growth factor (PDGF), and epithelial growth factor (EGF) (Table 1). The granules also contain vitronectin, a cell adhesion molecule which helps with osseointegration and osseoconduction.
Fig. 1.
Inactive platelets
Table 1.
Growth factor chart [Printed with permission from: Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004 November;114(6):1502–8]
| Platelet-derived growth factor (PDGF) | Stimulates cell replication |
| Promotes angiogenesis | |
| Promotes epithelialization | |
| Promotes granulation tissue formation | |
| Transforming growth factor (TGF) | Promotes formation of extracellular matrix |
| Regulates bone cell metabolism | |
| Vascular endothelial growth factor (VEGF)r | Promotes angiogenesis |
| Epidermal growth factor (EGF) | Promotes cell differentiation and stimulates re-epithelialisation, angiogenesis and collagenase activity |
| Fibroblast growth factor (FGF) | Promotes proliferation of endothelial cells and fibroblasts |
| Stimulates angiogenesis |
TGFbeta is active during inflammation, and influences the regulation of cellular migration and proliferation; stimulate cell replication, and fibronectin binding interactions [23] (Fig. 2). VEGF is produced at its highest levels only after the inflammatory phase, and is a potent stimulator of angiogenesis. Anitua et al. showed that in vitro VEGF and Hepatocyte Growth Factor (HGF) considerably increased following exposure to the pool of released growth factors; suggesting they accelerate tendon cell proliferation and stimulate type I collagen synthesis [11]. PDGF is produced following tendon damage and helps stimulate the production of other growth factors and has roles in tissue remodeling. PDGF promotes mesenchymal stem cell replication, osteoid production, endothelial cell replication, and collagen synthesis. It is likely the first growth factor present in a wound and starts connective tissue healing by promoting collagen and protein synthesis [7]. However, a recent animal study by Ranly et al. suggests that PDGF may actually inhibit bone growth [24].
Fig. 2.
Active platelets
In vitro and in vivo studies have shown that bFGF is both a powerful stimulator of angiogenesis and a regulator of cellular migration and proliferation [23]. IGF-I is highly expressed during the early inflammatory phase in a number of animal tendon healing models, and likely assists in the proliferation and migration of fibroblasts and to increase collagen production [23]. However, a laboratory analysis of human PRP samples demonstrated increased concentrations of PDGF, TGFbeta, VEGF, and EGF, while not showing an increase in IGF-1 [25]. EGF effects are limited to basal cells of skin and mucous membrane while inducing cell migration and replication.
PRP preparation
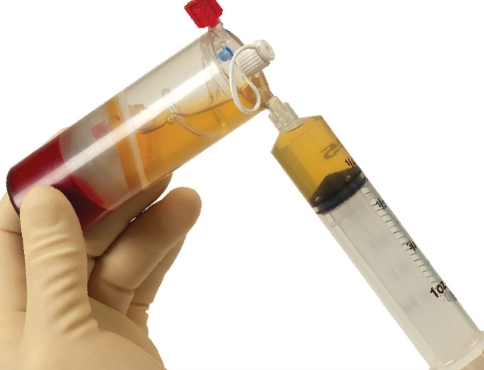
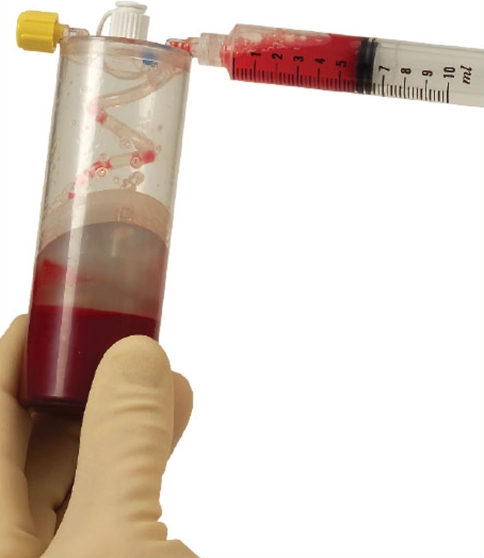
Various blood separation devices have differing preparation steps essentially accomplishing similar goals. The Biomet Biologics GPS III system is described here for simplicity. About 30–60 ml of venous blood is drawn with aseptic technique from the anticubital vein. An 18 or 19 g butterfly needle is advised, in efforts of avoiding irritation and trauma to the platelets which are in a resting state. The blood is then placed in an FDA approved device and centrifuged for 15 min at 3,200 rpm (Fig. 3). Afterward, the blood is separated into platelet poor plasma (PPP), RBC, and PRP. Next the PPP is extracted through a special port and discarded from the device (Fig. 4). While the PRP is in a vacuumed space, the device is shaken for 30 s to re-suspend the platelets. Afterwards the PRP is withdrawn (Fig. 5). Depending on the initial blood draw, there is approximately 3 or 6 cc of PRP available.
Fig. 3.
GPS III system and centrifuge
Fig. 4.
GPS III system, withdrawing of platelet poor plasma to be discarded
Fig. 5.
GPS III withdrawing of platelet rich plasma for injection/graft
Injection procedure
The area of injury is marked while taking into account the clinical exam, and data from imaging studies such as MRI and radiographs. It is recommended to use dynamic musculoskeletal ultrasound with a transducer of 6–13 Hz in an effort to more accurately localize the PRP injection. Under sterile conditions, the patient receives a PRP injection with or without approximately 1 cc of 1% lidocaine and 1 cc of 0.25 Marcaine directly into the area of injury. Calcium chloride and thrombin may be added to provide a gel matrix for the PRP to adhere to, potentially maximizing the benefit in the case of a joint space. We recommend using a peppering technique spreading in a clock-like manner to achieve a more expansive zone of delivery. The patient is observed in a supine position for 15–20 min afterwards, and is then discharged home. Patients typically experience minimal to moderate discomfort following the injection which may last for up to 1 week. They are instructed to ice the injected area if needed for pain control in addition to elevation of the limb and modification of activity as tolerated. We recommend acetaminophen as the optimal analgesic, or Vicodin for break through pain, and dissuade the use of NSAID’s in the early post-injection period (Fig. 6).
Fig. 6.
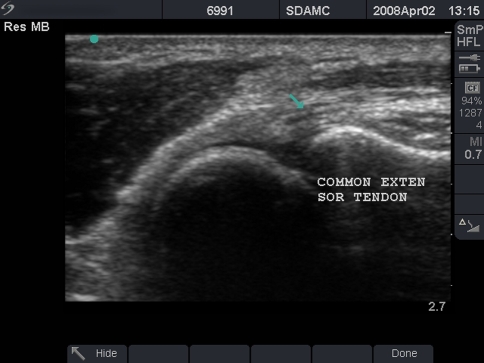
Musculoskeletal ultrasound, common extensor tendinosis
Safety
Any concerns of immunogenic reactions or disease transfer are eliminated because PRP is prepared from autologous blood. No studies have documented that PRP promotes hyperplasia, carcinogenesis, or tumor growth. Growth factors act on cell membranes rather than on the cell nucleus and activate normal gene expression [7]. Growth Factors are not mutagenic and naturally act through gene regulation and normal wound healing feed-back control mechanisms [6]. Relative contraindications include the presence of a tumor, metastatic disease, active infections, or platelet count < 10 5/ul Hgb < 10 g/dl. Pregnancy or active breastfeeding are contraindications. Patients with an allergy to Bupivicaine (Marcaine) should not receive a local anesthetic with these substances.
The patients should be informed of the possibility of temporary worsening symptoms after the injection. This is likely due to the stimulation of the body’s natural response to inflammatory mediators. Although adverse effects are uncommon, as with any injection there is a possibility of infection, no relief of symptoms, and neurovascular injury. Scar tissue formation and calcification at the injection site are also remote risks.
An allergic reaction or local toxicity to Bupivacaine HCL or Lidocaine, although uncommon could trigger an adverse reaction. Additionally, when used in surgical applications for grafting or with intra-articular injections, PRP may be combined with calcium chloride and bovine thrombin to form a gel matrix. This bovine thrombin which is used to activate PRP, in the past has been associated with life threatening coagulopathies as a result of antibodies to clotting factors V, XI, and thrombin [7, 26]. However, since 1997 production has eliminated contamination of bovine thrombin with bovine factor Va. Prior to 1997, Va levels were 50 mg/ml and now are <0.2 mg/ml with no further reports of complications [6].
Literature review
There is extensive documentation of both animal and human studies, with widespread applications, demonstrating the safety and efficacy of PRP for 20 years. However, most studies are pilot studies with small sample sizes. Recently, there is emerging literature on the beneficial effects of PRP for chronic non-healing tendon injuries including lateral epicondylitis and plantar fasciitis [1, 2]. Other orthopedic applications include diabetic wound management, treatment of non-unions, and use in acute tendon injuries. There is also a range of publications in other fields including ENT, cardiology, and plastic surgery. The following is a review of some of the more recent studies on PRP.
Elbow
In a recent study in the American Journal of Sports Medicine, Mishra et al. evaluated 140 patients with chronic epicondylar elbow pain. Of those patients, 20 met the study criteria and were surgical candidates who had failed conservative treatments. In total, 15 were treated with one PRP injection and five were controls with local anesthetic. The treatment group noted 60% improvement at 8 weeks, 81% at 6 months, and 93% at final follow-up at 12–38 months. Of note, there were no adverse effects or complications. Additionally, there was a 94% return to sporting activities and a 99% return to daily activities [1]. The major limitation of this study was the 60% attrition rate in the control group as 3/5 of the patients withdrew from the study or sought outside treatment at 8 weeks. This small retrospective series is considered a pilot study and a randomized clinical trial is needed to substantiate these findings.
In 2003 Edwards and Calandruccio, demonstrated that 22 of 28 patients (79%) with refractory chronic epicondylitis were completely pain free following autologous blood injection therapy [15]. There was no reported worsening or recurrence of pain and no other adverse events. Pain after autologous blood administration was variable, but most patients reported it to be similar to prior steroid injections they received before the study. One patient failed to improve satisfactorily and eventually underwent surgery [15]. This study is limited by its small sample size and lack of control group.
Foot and ankle
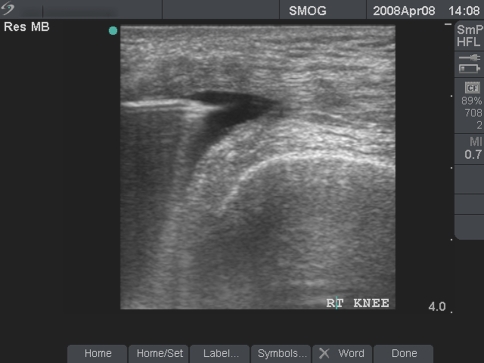
Barett et al. enrolled nine patients in a pilot study to evaluate PRP injections with plantar fasciitis. Patients met the criteria if they were willing to avoid conservative treatments including bracing, NSAIDS, and avoidance of a cortisone injection for 90 days prior. All patients demonstrated hypoechoic and thickened plantar fascia on ultrasound. While anesthetizing each patient with a block of the posterior tibial and sural nerve, 3 cc of autologous PRP was injected under ultrasound guidance (Fig. 7). Post-injection thickness and increased signal intensity of the fascial bands were seen on ultrasound. Six of nine patients achieved complete symptomatic relief after 2 months. One of the three unsuccessful patients eventually found complete relief following an additional PRP injection. At one year 77.9% patients had complete resolution of symptoms [2]. Again, this was a non-controlled pilot study with a small sample size.
Fig. 7.
Ultrasound guided suprapatella bursa injection/graft
Knee
After injecting rat patellar tendons with PRP, Kajikawa et al. showed increased quantity of circulation-derived cells in the early phase of tendon repair after injury versus controls. Unfortunately, these helpful cells normally disappear with time; therefore prolonging their presence is beneficial. Furthermore, they showed increased type I & III collagen and macrophages [27].
Taylor, et al. demonstrated safety and efficacy while injecting autologous blood into New Zealand white rabbits at the patellar tendon. After reviewing the histology at 6 and 12 weeks, there was no adverse change in histology or tendon stiffness. However, the tendons injected with blood were significantly stronger [28].
Berghoff et al. retrospectively reviewed a large series of patients in an effort to access autologous blood product effects in patients undergoing total knee arthroplasty (TKA). The study included 66 control patients and 71 patients treated with autologous blood products at the wound site. The intervention group demonstrated higher hemoglobin levels and fewer transfusions as well as shorter hospitalization and greater knee range of motion at 6 weeks. Additionally, no infections occurred and significantly fewer narcotics were required [29]. Although limited by the retrospective nature of the study, the results are compelling.
Gardner et al. performed a similar retrospective study in a series of patients undergoing TKA. The patients were treated with an intra-operative platelet gel; resulting in lower blood loss, improved early range of motion, and fewer narcotic requirements [30].
In a controlled study by Everts et al., of 160 patients undergoing Total Knee Replacements (TKA) 85 received Platelet gel and fibrin sealants; which resulted in decreased blood transfusion requirements, lower post-surgical wound disturbances, shorter hospital stay, and fewer infections [31].
Wounds
Non-healing cutaneous wounds represent a challenging problem and are commonly related to peripheral vascular disease, infection, trauma, neurologic and immunologic conditions, as well as neoplastic and metabolic disorders. These chronic ulcerative wounds represent significant impact both psychologically and socioeconomically. An analysis of the surfaces of chronic pressure wounds (decubitus ulcers) revealed a decreased growth factor concentration compared with an acute wound [32]. In a study by Crovetti et al., 24 patients with chronic cutaneous ulcers were treated with a series of PRP Gel treatments. Only three patients received autologous blood PRP due to medical issues, while the others received donor blood product. Nine patients demonstrated complete wound healing. Of those nine, one wound reopened at 4 months. There were two reports of wound infection, both with positive Staph Aureus which were successfully treated with oral antibiotics. There were no adverse effects encountered and all patients noted decreased pain [32].
Another wound study by McAleer et al., involved 24 patients with 33 chronic non-healing lower extremity wounds. Patients failed conservative treatment for >6 months with a lack of reduction of surface area. Surgical wound debridement was initially performed to convert chronic ulcers to acute wounds, in an effort to promote wound metabolism and chemotaxis. The wounds were injected with PRP every 2 weeks. Successful wound closure and epitheliazation was obtained in 20 wounds. The mean time for closure was 11.15 weeks. Five wounds displayed no improvement [33]. These findings were particularly significant because all patients had failed previously available treatment methods.
Bone
Diabetes impairs fracture healing with reduced early proliferation of cells, delayed osteogenesis, and diminished biomechanical properties of the fracture callus [34, 35]. In an animal study by Gandhi et al., male Wister rats received closed mid-diaphyseal fractures after 14 days of the onset of diabetes. PRP did not alter blood glucose levels or HbA1c. The study demonstrated that diabetic rats had decreased growth factors compared to non-diabetic group [34].
Not all studies on autologous growth factors have shown favorable results with promoting bone formation and healing. In a recent study by Ranly et al., PRP was shown to decrease osteoinductivity of demineralized bone matrix in immunocompromised mice. PRP from six healthy men was implanted as gelatin capsules in the calves of inbred nude mice. After 56 days the mice were killed and the studied calf muscles suggest that PDGF may actually reduce osteoinductivity [24]. The main criticism of this study is related to the PRP treatment protocol. Conventional PRP processing kits yield a 6-fold increase in platelet concentration. However, in the Ranly study the PRP concentration was only four times above baseline. Additionally, the timing of the assays looking at osteoinduction may have been too late to accurately access early bone formation.
Spine
Generally, maintaining arthrodesis in a posterolateral lumbar fusion can be challenging and may necessitate revision [36]. Subsequently multiple strategies have evolved to decrease non-union rates including screw instrumentation, interbody fusion, bone morphogenic protein, and limiting risk factors such as smoking, NSAID, and corticosteroid use [37]. There is mixed literature and controversy surrounding the efficacy of platelet gel to supplement autologous bone graft during instrumented posterolateral spinal fusion [37–39]. The potential efficacy of PRP to facilitate osteoinduction in spine fusion remains uncertain at present time.
A study by Carreon et al. investigated 76 patients with posterior lateral lumbar fusion with autologous iliac crest bone graft mixed with PRP compared to a control group. Using 500 ml of whole blood, 30 ml of platelet concentrate was obtained. Non-union was diagnosed by either a revision intra-operatively or via plain radiographs or CT scan. The study concluded that the PRP group had a 25% non-union rate versus 17% in the control group at a minimal 2-year follow-up [37]. Of note, platelet concentrations were not measured before or after preparation, as this is not routinely performed clinically.
A study of single-level intertransverse fusions by Weiner and Walker demonstrated a 62% fusion rate in iliac graft augmented with PRP versus 91% fusion rate in bone graft alone [40].
Lowery et al. retrospectively reviewed 19 spinal fusion patients with PRP after 13 months. There was no pseudoarthrosis seen on exploration or plain radiographs in 100% of cases [41].
Hee et al. examined 23 patients who underwent instrumented transforaminal lumbar interbody fusions with PRP versus control with a 2-year follow-up. Interestingly they found accelerated bony healing in the PRP group; however it did not result in increased fusion rates versus control [36]. Platelet concentrations were measured after preparation and were increased 489% from baseline [36].
Jenis et al. explored anterior interbody lumbar fusions in 22 patients with autograph using iliac crest bone graft versus 15 patients with allograft combined with PRP. CT scans at 6 months and plain radiographs at 12 and 24 months demonstrated an 85% fusion rate for autograft versus 89% with PRP and allograft [38]. This could potentially eradicate the morbidity from iliac crest harvesting, and provide a more cost effective alternative to costly bone induction techniques.
A study from Chen et al. demonstrated that PRP might potentially play a role in prevention of disc degeneration. They demonstrated that PRP can act as a growth factor cocktail to induce proliferation and differentiation and promote tissue-engineered nucleus formation regeneration via the Smad pathway [42]. This offers a conservative management option to patients with degenerative disc disease, besides traditional management options including cortosteroid injection and ultimately surgery.
Summary
In summary, for over 20 years PRP has been used safely in a variety of conditions with promising implications. Unfortunately, most studies to date are anecdotal or involve small sample sizes. Undoubtedly we are seeing increased clinical use of PRP, however more clinical trials are certainly needed. Little is documented in the literature regarding the expected timeframe of tendon healing post-PRP injection. Also, there are no studies to date that review the need of post-PRP injection rehabilitation, nor are there any protocols. However, it is assumed that Physical/Occupational therapy and restoring the kinetic chain will help facilitate recovery post injection.
The authors are currently expanding PRP injection applications from tendon injuries to other persistent ailments including greater trochanteric bursitis and knee osteoarthritis with favorable results. The authors also have had success in injecting professional soccer athletes with acute MCL knee injuries in an effort to accelerate their return to play (Fig. 8). Further understanding of this promising treatment is required to determine which particular diagnoses are amenable to PRP therapy. The authors will report results on this topic in the near future.
Fig. 8.
Ultrasound guided knee MCL injection/graft
The use of autologous growth factors in the form of PRP may be just the beginning of a new medical frontier known as “orthobiologics.” First generation injectables such as visco-supplementation have been successful in the treatment of pain for patients with osteoarthritis of the knee. These injections represent a non-biologic effort to influence the biochemical environment of the joint.
A second generation of injectables is now available with PRP. This technology provides delivery of a highly concentrated potent cocktail of growth factors to stimulate healing. TGF-b, contained in PRP has been linked to chondrogenesis in cartilage repair [43]. New reports presented at the 2007 International Cartilage Repair Society Meeting in Warsaw indicate PRP enhancement of chondrocyte cell proliferation and positive clinical effects on degenerative knee cartilage [44, 45]. Anitua and Sanchez recently demonstrated increased hyluronic acid concentration balancing angiogenesis in ten osteoarthritic knee patients [46]. Wu et al. documented PRP promotion of chondrogenesis as an injectable scaffold while seeded with chondrocytes in rabbit ears. Hard knobbles were found and seen on MRI, as well as histologic analysis and staining which confirmed cartilage growth [47].
Future generations of biologic injectables may target specific cells, rather than providing an assortment of non-specific healing properties. Currently clinical trials of intra-articular use of growth factor BMP 7 (OPI) are underway. Soft tissue applications of BMP7 (OPI) are also in its early stages. Bone marrow aspirate stem cell injections are seeing increased clinical use as well. Ultimately, stem cell therapy represents the greatest biologic healing potential.
References
- 1.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;10(10):1–5. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 2.Barrett S, Erredge S. Growth factors for chronic plantar fascitis. Podiatry Today. 2004;17:37–42. [Google Scholar]
- 3.Woolf AD, Pfleyer B. Burdon of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Anitua M, Sánchez E, Nurden A, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24(5):227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Praemer AF. Musculoskeletal conditions in the United States. 2. Rosemont: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 6.Marx R, Garg A. Dental and craniofacial applications of platelet-rich plasma. Carol Stream: Quintessence Publishing Co, Inc.; 2005. [Google Scholar]
- 7.Everts P, Knape J, Weirich G, Schonberger J, Hoffman J, Overdevest E, et al. Platelet-rich plasma and platelet gel: a review. JECT. 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrzak W, Eppley B. Scientific foundations platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–54. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 9.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari M, Zia S, Valbonesi M. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47–50. [PubMed] [Google Scholar]
- 11.Antitua E, Andia I, Sanchez M, Azofra J, Del Mar Zalduendo M, Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF productions by human tendon cells in culture. J Orthop Res. 2005;23:281–6. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick SA, Hazlelman BL, Riley GP. The vasulature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252–60. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayem G. Tenology: a new frontier. Joint, Bone, Spine. Rev Rhum. 2001;68:19–25. doi: 10.1016/s1297-319x(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 14.Jobe F, Ciccotti M. Lateral and medial epicondylitis of the elbow. J Am Acad Orthop Surg. 1994;2:1–8. doi: 10.5435/00124635-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. Am J Hand Surg. 2003;28(2):272–8. doi: 10.1053/jhsu.2003.50041. [DOI] [PubMed] [Google Scholar]
- 16.Antiua E, Sanchez M, Nurden A, Zalduendo M, Fuente M, Prive G, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res Pt A. 2006;77(2):285–93. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- 17.Kader D, Sakena A, Movin T, Magulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–49. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smidt N, Assendelft W, Arola H, et al. Effectiveness of physiotherapy for lateral epicondylitis: a systemic review. Ann Med. 2003;35:51–62. doi: 10.1080/07853890310004138. [DOI] [PubMed] [Google Scholar]
- 19.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 20.Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43:65–92. doi: 10.1016/S0169-409X(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 21.Froum SJ, Wallace S, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002;22:45–53. [PubMed] [Google Scholar]
- 22.Raghoebar GM, Schortinghuis J, Liem R, Ruben J, Wal J, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for the augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349–56. doi: 10.1111/j.1600-0501.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 23.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–94. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ranly D, Lohmann C, Andreacchio D, Boyan B, Schwartz Z. Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J Bone Joint Surg. 2007;89:139–46. doi: 10.2106/JBJS.F.00388. [DOI] [PubMed] [Google Scholar]
- 25.Eppley B, Woodell J, Higgins J. Platelet Quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–7. doi: 10.1097/01.PRS.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 26.Zehnder JL, Leung LLK. Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombim. Blood. 1990;76:2011–6. [PubMed] [Google Scholar]
- 27.Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–45. [DOI] [PubMed]
- 28.Taylor M, Norman T, Clovis N, Blaha D. The response of rabbit patellar tendons after autologous blood injection. Med Sci Sports Exerc. 2002;34(1):70–3. doi: 10.1097/00005768-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Berghoff W, Pietrzak W, Rhodes R. Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics. 2006;29(7):590–8. doi: 10.3928/01477447-20060701-11. [DOI] [PubMed] [Google Scholar]
- 30.Gardner MJ, Demetrakopoulos D, Klepchick P, Mooar P. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty: an analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2006;31:309–13. doi: 10.1007/s00264-006-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everts P, Devilee R, Mahoney C, Eeftinck-Schattenenkerk M, Knape J, Zundert A. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:593–9. doi: 10.1111/j.1399-6576.2006.001005.x. [DOI] [PubMed] [Google Scholar]
- 32.Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–51. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.McAleer JP, Kaplan E, Persich G. Efficacy of concentrated autologous platelet-derived growth factors in chronic lower-extremity wounds. J Am Podiatr Med Assoc. 2006;96(6):482–8. doi: 10.7547/0960482. [DOI] [PubMed] [Google Scholar]
- 34.Ghandi A, Dumas C, O’Connor J, Parsons J, Lin S. The effects of local platelet rich plasma delivery on diabetic bone fracture healing. Bone. 2006;38:540–6. doi: 10.1016/j.bone.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210–6. doi: 10.1016/S0736-0266(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 36.Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12(12):400–7. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carreon LY, Glassman SD, Anekstein Y, Puno RM. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30(9):E243–6. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 38.Jenis LG, Banco RJ, Kwon B. A prospective study of Autologous Growth Factors (AGF) in lumbar interbody fusion. Spine J. 2006;6(1):14–20. doi: 10.1016/j.spinee.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Castro FP., Jr Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17(5):380–4. doi: 10.1097/01.bsd.0000110342.54707.19. [DOI] [PubMed] [Google Scholar]
- 40.Weiner BK, Walker M. Efficacy of autologous growth factors in autologous intertransverse fusions. Spine. 2003;28:1968–70. doi: 10.1097/01.BRS.0000083141.02027.48. [DOI] [PubMed] [Google Scholar]
- 41.Lowery GL, Kulkarni S, Pennisi AE. Use of autologous growth factors in lumbar spine fusion. Bone. 1999;25:47S–50S. doi: 10.1016/S8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209(3):744–54. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 43.Hunziker EB, Driesang IM, Morris EA. Clinical orthopaedics and related research. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res. 2001;391(Suppl):S171–81. doi: 10.1097/00003086-200110001-00017. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K, Sasho T, Arai M, Kitahara S, Ogino S, Wada Y, et al. Effects of autologous platelet-rich plasma on the metabolism of human articular chondrocytes. Chiba and Ichihara, Japan. Electronic poster presentation P181. International Cartilage Repair Society Meeting, Warsaw Poland, October 2007.
- 45.Kon E, Filardo G, Presti ML, Delcogliano M, Iacono F, Montaperto C, et al. Utilization of platelet-derived growth factors for the treatment of cartilage degenerative pathology. Bologna, Italy. Electronic poster presentation 29.3. International Cartilage Repair Society Meeting, Warsaw Poland, October 2007.
- 46.Anitua E, Sánchez M, Nurden AT, Zalduendo MM, Fuente M, Azofra J, et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology. 2007;46(12):1769–72. doi: 10.1093/rheumatology/kem234. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65(10):1951–7. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]