Abstract
Black lipid membranes (BLMs) are widely used for recording the activity of incorporated ion channel proteins. However, BLMs are inherently unstable structures that typically rupture within a few hours after formation. Here, stabilized BLMs were formed using the polymerizable lipid bis-dienoyl phosphatidylcholine (bis-DenPC) on glass pipettes of ∼10 μm (I.D.). After polymerization, these BLMs maintained steady conductance values for several weeks, as compared to a few hours for unpolymerized membranes. The activity of an ion channel, α-hemolysin, incorporated into bis-DenPC BLMs prior to polymerization, was maintained for 1 week after BLM formation and polymerization. These lifetimes are a substantial improvement over those achievable with conventional BLM technologies. Polymerized BLMs containing functional ion channels may represent an enabling technology for development of robust biosensors and drug screening devices.
Suspended phospholipid bilayers, also known as black lipid membranes (BLMs), are an important tool for investigating the activity of reconstituted ion channels.(1) Ligands can be added to the buffer on either side of a BLM, allowing binding to both sides of a transmembrane ion channel to be probed. However, long-term monitoring of ion channel activity using BLMs composed of naturally occurring lipids is limited by the inherent instability of the bilayer. The lipids are self-associated via a network of relatively weak intermolecular forces; as a consequence, membrane rupture usually occurs in <24 h.(2) Herein, we demonstrate that lipid polymerization is an effective strategy to significantly extend BLM lifetimes.
A number of approaches have been utilized to improve the physiochemical stability of BLMs, including miniaturization of the aperture across which the BLM is suspended,1b,3 sandwiching the BLM between agarose layers,(4) and doping the membrane with stabilizing agents (e.g., cholesterol).(5) The enhanced stability obtained using these approaches is attributed to introduction of additional noncovalent intermolecular interactions or minimization of stresses that disrupt intermolecular interactions. For example, suspending a BLM across a glass nanopore minimizes the membrane surface area susceptible to rupture.(2) While these types of BLMs are more stable than a conventional construct (e.g., a bilayer suspended across an ∼100 μm aperture in a Teflon sheet), their long-term robustness is inherently limited by the noncovalent nature of their assembly. Stabilization by lipid polymerization is a strategy that addresses this limitation.(6)
Reactive lipid monomers have been used to prepare both linear and cross-linked poly(lipid) networks.(7) A wide variety of polymerizable lipids have been synthesized, differing in the type of the reactive moiety, its location in the lipid, and the number per lipid.(7) Much of the initial work in this field was performed using bis-diacetylene lipids which tend to produce relatively rigid polymers with numerous defects.(8) Polymerization of BLMs prepared from mixtures of nonpolymerizable and monodiacetylene lipids resulted in a moderate increase in membrane lifetime (from 13 to 31 min), and single channel recordings of gramicidin A in these BLMs were measured.(9) An earlier study showed that the activity of α-hemolysin (α-HL) was maintained after partial linear polymerization of a BLM composed of a monodiacetylene lipid; however stability data were not reported.(10)
Poly(dienes) are rubber-like materials;(7) thus dienoyl lipids appear to be a better choice for creating a stable poly(BLM) that maintains the activity of incorporated ion channels. Among these are bis-dienoyl phosphatidylcholine (bis-DenPC; Scheme 1) and bis-sorbyl phosphatidylcholine (bis-SorbPC); both readily form poly(lipid) vesicles and planar supported bilayers with markedly increased stability to surfactants, solvents, drying, and long-term storage.(8) Furthermore, poly(bis-SorbPC) planar membranes have been utilized for reconstitution of functional bovine rhodopsin.(11)
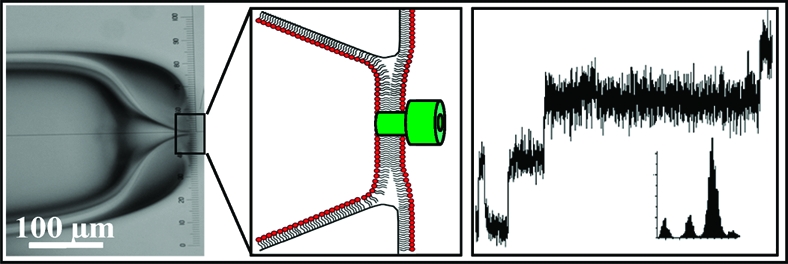
Scheme 1. Structures of (A) Diphytanoyl Phosphatidylcholine (DPhPC) and (B) Bis-dienoyl Phosphatidylcholine (bis-DenPC) and (C) Schematic of an Ion Channel Functionalized BLM Suspended across the Opening of a Micropipet.
The aperture of the pipet ranges from 5 to 20 μm.
In an effort to significantly improve the long-term stability of single ion channel recordings, we fabricated and characterized BLMs composed of poly(bis-DenPC). BLMs were formed across apertures prepared using borosilicate glass pipettes (Scheme 1 and Supporting Information (SI)). Aperture diameters ranging from 5 to 20 μm (I.D.) were formed by first breaking the pipet and then using a microforge to anneal the tip. The inner and outer surfaces of pipettes were covalently modified with 3-cyanopropyldimethylchlorosilane to provide a moderately hydrophobic surface that supports formation of a lipid monolayer.(2) BLMs were formed using a method similar to that of Montal and Mueller.(12) Briefly, solutions of lipid (DPhPC or bis-DenPC) were dried and resuspended in n-decane (∼10 mg/mL). This solution (3 μL) was applied to the pipet tip and allowed to dry, prior to backfilling the pipet with recording buffer (1 M KCl, 5 mM HEPES, pH 7.5). The pipet was mounted onto a holder and the lipid solution was reapplied, followed by immersion into a bath containing recording buffer. Successful BLM formation was verified by an increase in the resistance monitored using a patch clamp amplifier.
In the absence of a BLM, pipet resistances ranged from 10 to 100 kΩ and were dependent upon the aperture diameter. Upon BLM formation with DPhPC or bis-DenPC, the resistance values were 3.6 (±0.4) (n = 5) and 4.2 (±0.6) (n = 5) GΩ/cm2, respectively (also see SI). The statistical equivalence of these values indicates that the dienoyl moieties in bis-DenPC do not measurably increase bilayer permeability. Subsequent UV irradiation (see SI) of bis-DenPC to yield cross-linked BLMs resulted in a 5−10% increase in conductance, whereas no increase was observed upon irradiation of DPhPC BLMs (see SI). This increase likely arises from defects in lipid packing generated upon cross-linking and/or changes in the material properties of the bilayer, e.g., the curvature (bending) elastic modulus.
The stability of BLMs was investigated by monitoring the conductance of BLMs prepared using DPhPC, bis-DenPC, and poly(bis-DenPC). Pipettes with BLMs were stored in recording buffer and measured at defined intervals. The mean BLM lifetime was 240 ± 60 min (n = 4) for bis-DenPC compared to 260 ± 30 min (n = 4) for DPhPC. In contrast, conductances indicative of an intact BLM were maintained for ca. 3 weeks (22 ± 4 days, n = 4) for poly(bis-DenPC). We also explored the effect of passing BLMs repeatedly across the air-buffer interface. Unpolymerized bis-DenPC and DPhPC BLMs degraded after 3 ± 1 and 2 ± 1 passes (n = 12 for each type of BLM), whereas no BLM degradation was observed for poly(bis-DenPC) BLMs after 30 passes (n = 5). Conductance data are listed in the SI.
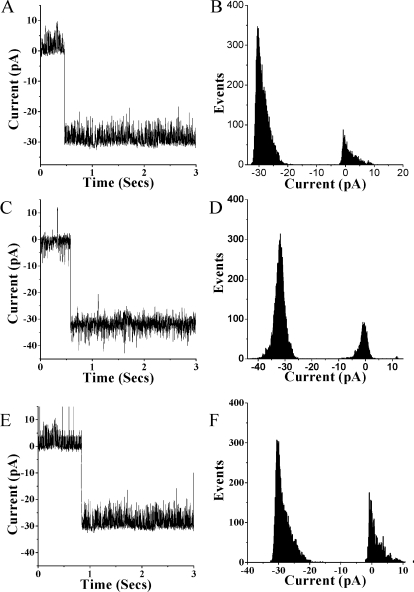
BLMs were functionalized with α-HL (Scheme 1), a protein that forms a heptameric pore complex upon insertion into lipid membranes.(13) The resulting ca. 1 nS pore(5) has a molecular weight cutoff of ca. 3400 Da(14) and is well characterized in BLMs. Following BLM formation, the pipet potential was adjusted to −40 mV and α-HL (3 μL of 0.5 mg/mL) was added to the bath (500 μL of recording buffer) in contact with the exterior surface of the BLM. Upon insertion of the first α-HL pore, the buffer was exchanged to minimize the probability of multiple channel insertions. Insertion of an α-HL pore is observed as a stepwise, negative decrease in the measured current (Figure 1). For multiple channel experiments (see SI), longer incubation times were allowed. Single channel recordings were performed with α-HL inserted into DPhPC, bis-DenPC, and poly(bis-DenPC) BLMs (Figure 1); in the latter case, lipid polymerization was performed after α-HL insertion. In all three types of BLMs, openings of ca. −30 pA were obtained, which correlates well with published data.(5) Using poly(bis-DenPC) BLMs, single channel recordings were obtained daily, with minimal effects on α-HL activity, for an average of 1 week (n = 4 pipettes, with lifetimes of 4, 6, 7, and 10 days). An example of this unprecedented long-term stability is shown in Figure 2. In all cases, after α-HL activity was no longer observed, the BLM remained intact. Thus for poly(bis-DenPC) BLMs, loss of α-HL activity (not membrane rupture) is the cause of failure.
Figure 1.
Single ion channel recordings of α-HL in DPhPC (A), bis-DenPC (C), and poly(bis-DenPC) (E) BLMs. All-points histograms of α-HL channel recordings in DPhPC (B), bis-DenPC (D), and poly(bis-DenPC) (F) BLMs.
Figure 2.
All-points histograms of single α-HL recordings in a poly(bis-DenPC) BLM on day 1 (A) and day 7 (B). Between recordings, the pipet was stored in recording buffer at 4 °C.
In conclusion, we have prepared polymeric BLMs that exhibit greatly enhanced long-term stability compared to unpolymerized BLMs. The functional lifetime of ion channels incorporated in poly(bis-DenPC) BLMs is measured in days, whereas it is at most a few hours for unpolymerized BLMs. These polymerized BLMs may represent an enabling technology for development of robust biosensors and drug screening devices.
Acknowledgments
This work was supported by grants from the NSF (CHE-0548167 and CHE-0518702) and the NIH (GM074522 and EB007047).
Supporting Information Available
Additional figures, tables, and experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Anrather D.; Smetazko M.; Saba M.; Alguel Y.; Schalkhammer T. J. Nanosci. Nanotechnol. 2004, 4, 1–22. [DOI] [PubMed] [Google Scholar]; b Reimhult E.; Kumar K. Trends Biotechnol. 2008, 26, 82–89. [DOI] [PubMed] [Google Scholar]
- White R. J.; Ervin E. N.; Yang T.; Chen X.; Daniel S.; Cremer P. S.; White H. S. J. Am. Chem. Soc. 2007, 129, 11766–11775. [DOI] [PubMed] [Google Scholar]
- Schmitt E. K.; Vrouenraets M.; Steinem C. Biophys. J. 2006, 91, 2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer J. A.; White V. E.; Dougherty D. A.; Nadeau J. L. Biosens. Bioelectron. 2007, 22, 2577–2584. [DOI] [PubMed] [Google Scholar]
- Raffy S.; Teissie J. Biophys. J. 1999, 76, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert R.; Laschewsky A.; Ringsdorf H. J. Am. Chem. Soc. 1985, 107, 4134–4141. [Google Scholar]
- Mueller A.; O’Brien D. F. Chem. Rev. 2002, 102, 727–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. E.; Rozanski L. J.; Spratt T.; Liu S. C.; O’Brien D. F.; Saavedra S. S. Langmuir 2003, 19, 1752–1765. [Google Scholar]
- Daly S. M.; Heffernan L. A.; Barger W. R.; Shenoy D. K. Langmuir 2006, 22, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Shenoy D. K.; Barger W. R.; Singh A.; Panchal R. G.; Misakian M.; Stanford V. M.; Kasianowicz J. J. Nano Lett. 2005, 5, 1181–1185. [DOI] [PubMed] [Google Scholar]
- Subramaniam V.; Alves I. D.; Salgado G. F. J.; Lau P. W.; Wysocki R. J.; Salamon Z.; Tollin G.; Hruby V. J.; Brown M. F.; Saavedra S. S. J. Am. Chem. Soc. 2005, 127, 5320–5321. [DOI] [PubMed] [Google Scholar]
- Montal M.; Mueller P. Proc. Natl. Acad. Sci. U.S.A. 1972, 69, 3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux J. E.; Braha O.; Hobaugh M. R.; Song L.; Cheley S.; Shustak C.; Bayley H. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 12828–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezrukov S. M.; Vodyanoy I.; Brutyan R. A.; Kasianowicz J. J. Macromolecules 1996, 29, 8517–8522. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.