Abstract
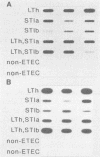
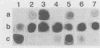
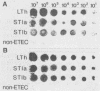
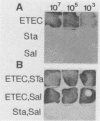
A 1,268-bp polynucleotide probe for heat-labile and heat-stable enterotoxins (LTh, STIa, STIb) was conjugated with horseradish peroxidase (HRP). The HRP-conjugated trivalent probe was applied to the detection of enterotoxigenic Escherichia coli (ETEC) by colony and stool hybridizations. The binding of the probe to its targets was assayed by the addition of HRP substrates hydrogen peroxide and luminol in the presence of an enhancer, and the chemiluminescence was recorded by exposure to X-ray film. Slot blot hybridization demonstrated that the HRP-conjugated trivalent probe specifically hybridized with the DNA isolated from ETEC strains. The trivalent probe also specifically identified bacterial colonies of ETEC that produced LTh, STIa, STIb, LTh-STIa, or LTh-STIb. Treatment of targets with sodium dodecyl sulfate and proteinase K remarkably reduced nonspecific hybridization to DNAs of non-ETEC strains. Furthermore, this probe was able to detect stool specimens seeded with 10(2) original ETEC cells per 5 mg of feces. These results suggest that the HRP-conjugated trivalent probe is a candidate for use in the clinical laboratory to detect ETEC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danbara H., Komase K., Arita H., Abe H., Yoshikawa M. Molecular analysis of enterotoxin plasmids of enterotoxigenic Escherichia coli of 14 different O serotypes. Infect Immun. 1988 Jun;56(6):1513–1517. doi: 10.1128/iai.56.6.1513-1517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirii Y., Danbara H., Komase K., Arita H., Yoshikawa M. Detection of enterotoxigenic Escherichia coli by colony hybridization with biotinylated enterotoxin probes. J Clin Microbiol. 1987 Oct;25(10):1962–1965. doi: 10.1128/jcm.25.10.1962-1965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Hardy J. W., Hug M. I., Echeverria P., Falkow S. Isolation and nucleotide sequence determination of a gene encoding a heat-stable enterotoxin of Escherichia coli. Infect Immun. 1983 Mar;39(3):1167–1174. doi: 10.1128/iai.39.3.1167-1174.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz M., Kurz C. A colorimetric method for DNA hybridization. Nucleic Acids Res. 1984 Apr 25;12(8):3435–3444. doi: 10.1093/nar/12.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., McCarthy B. J. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Tamura T., Yokota T. Primary structure of heat-labile enterotoxin produced by Escherichia coli pathogenic for humans. J Biol Chem. 1984 Apr 25;259(8):5037–5044. [PubMed] [Google Scholar]