Abstract
We evaluated long-term administration of estrogen after cardiac arrest and cardiopulmonary resuscitation (CA/CPR) on neurohistopathological and behavioral outcome. We also examined the effect of estrogen receptor (ER) stimulation using ER-α agonist propyl pyrazole triol (PPT) and ER-β agonist diarylpropionitrile (DPN) on neuronal survival after CA/CPR to determine whether possible neuroprotective effects of estrogen are ER-mediated. Male C57Bl/6 mice underwent 10 mins of CA/CPR and 3-day survival. In protocol 1, intravenous injection of vehicle (NaCl 0.9%) and 0.5 or 2.5 µg 17β-estradiol (E2 loading dose) was performed followed by subcutaneous implants containing vehicle (oil) or E2 (12.6 µg), according to a treatment group. In experimental protocol 2, mice were injected (intravenously) with the ER-α agonist PPT or ER-β agonist DPN followed by Alzet pump implants (subcutaneously) containing PPT (200 µg) or DPN (800 µg). Long-term E2 administration reduced neuronal injury in the striatum after administration of either loading dose (41% ± 19%, 35% ± 26% of injured neurons), as compared with vehicle (68% ± 7%, P< 0.01), with no effect in the hippocampal CA1 field. In protocol 2, treatment with ER-β agonist DPN reduced neuronal injury in the striatum (51% ± 13% injured neurons) as compared with ER-α agonist PPT (68% ± 10%) and vehicle (69% ± 11%; P < 0.01). Estrogen receptor-β agonist DPN reduced neuronal injury in the hippocampal CA1 field (29% ± 22% injured neurons) as compared with ER-α agonist PPT treatment (62% ± 33%; P < 0.05). Injury was not different in hippocampal CA1 between vehicle and ER-α agonist-treated animals. We conclude that long-term E2 administration after CA/CPR is neuro-protective and that this effect is most likely mediated via ER-β.
Keywords: cardiac arrest, cardiopulmonary resuscitation estradiol, erebral ischemia, estrogen receptor subtypes, neuroprotection
Introduction
Sudden cardiac arrest is and has been a leading cause of mortality and poor neurologic outcome for decades. Each year, approximately 500,000 people are victims of cardiac arrest in the United States alone (Eisenberg and Mengert, 2001) and despite improvements in cardiopulmonary resuscitation (CPR) techniques, survival and outcome remains dismal (Polentini et al, 2006). One possible reason for this is that most CPR strategies have focused on the restoration of cardiac function and circulation (chest compression, defibrillators, ventilation modes, artificial perfusion, and pharmacological approaches), whereas very few techniques and interventions are available for protection and restoration of neuronal survival and functional outcome. Thus far, only the induction of mild hypothermia has been shown in human clinical trials to improve neurologic outcome and 6-month survival from cardiac arrest (CA)/CPR (Bernard et al, 2002). Epidemiologic studies and outcome data from clinical trials of acute myocardial infarction (Cobb et al, 1999; Kim et al, 2001) have identified sex differences in incidence and risk factors for sudden death. Clinical studies have found gender differences in the incidence of cardiac arrest, survival, and outcome (Herlitz et al, 2001; Wigginton et al, 2002). When compared with men, women experience less sudden cardiac death, are more likely to be successfully resuscitated, and reach the hospital alive more frequently than men in a prehospital setting (Wigginton et al, 2002). However, sex-specific long-term survival and functional recovery have not been intensively studied.
In several in vitro and in vivo models of brain injury, the principal mammalian estrogen, 17β-estradiol (E2) is potentially neuroprotective (McCullough and Hurn, 2003) and we have shown earlier that a single low dose of E2 (intravenous) provides neuroprotection after CA/CPR in mice (Noppens et al, 2005). In contrast, some reports show a deleterious effect of estrogen after global cerebral ischemia (Harukuni et al, 2001). However, little is known about optimal dosing, time point, and the duration of treatment with E2. Estrogen has pleiotropic effects in the brain and acts through multiple pathways (Hurn and Brass, 2003). The classical pathway of estrogen signaling involves cytoplasmic receptors that translocate to the nucleus and act as ligand-activated transcription factors for target genes. A more recently introduced pathway involves receptor-mediated nontranscriptional, nongenomic signaling. Two subtypes of estrogen receptors (ER) have been identified: ER-α and ER-β, but little is known about which ER subtype is involved in neuroprotection and some studies are conflicting (Carswell et al, 2004b; Dubal et al, 2001; Sampei et al, 2000).
The effect of long-term administration of E2 after CA/CPR, reflecting the human clinical situation of complete global brain ischemia and circulatory stasis, has not been studied earlier. It is also unknown which ER subtype (ER-α or ER-β) is primarily involved in any possible neuroprotective effects of E2 after global cerebral ischemia. We examined in an animal model of transient global ischemia if chronic post-CA/CPR treatment with E2 is neuroprotective and if a different loading dose might alter outcome. We also administered ER-α (propyl pyrazole triol, PPT) and ER-β (diarylpropionitrile, DPN) agonists in a similar treatment regimen to determine whether direct ER stimulation mediates possible neuroprotective effects of estrogen and which ER subtype is involved.
Materials and methods
Subjects
Male C57Bl/6 mice (20–27 g, Charles River, Hollister, CA, USA) were treated according to National Institutes of Health guidelines for the care and use of animals in research, and experimental protocols were approved by the Institutional Animal Care and Use Committee. All animals were housed in a temperature-controlled room (22°C) with a 12-12 h dark/light cycle and had free access to food (RMH 1000, 5P07, Purina Mills, Animal Specialties, Hubbard, OR, USA) and water.
Animal Groups
Experimental protocol 1: neuroprotective potency of chronic E2 treatment after cardiac arrest and cardiopulmonary resuscitation and effect of loading dose
The aim of this protocol was to determine whether chronic E2 treatment after CA/CPR provides neuroprotection. Animals (n = 60) were randomized into one of the three treatment groups. Group 1 (vehicle group, n = 20) received a single bolus of NaCl 0.9% for 1.5 mins after the return of spontaneous circulation (ROSC), followed by implantation (subcutaneous) of a silastic capsule containing oil (20 mins of ROSC; 35 µL). Treatment groups received either a single intravenous injection (loading dose) of 0.5 µg (group 2, n = 20) or 2.5 µg (group 3, n = 20) estradiol (water soluble, cyclodextrin-encapsulated 17β-estradiol, E4389; Sigma-Aldrich, St Louis, MO, USA) at 1.5 mins after ROSC, followed by implantation (subcutaneous) of a silastic implant containing 12.6 µg E2 (diluted in oil, E2758; Sigma). We chose our estrogen doses on the basis of previous experiments (acute dose; Noppens et al, 2005) and preliminary results using different estrogen doses for the subcutaneous implants (data not shown).
Experimental protocol 2: involvement of estrogen receptor subtype in neuroprotection
To determine whether possible neuroprotection is mediated through ER-α and or ER-β, we randomized 47 additional mice to receive the ER-α agonist PPT (n = 16; Tocris Cookson Inc.), the ER-β agonist DPN (n = 15; Tocris Cookson Inc.), or vehicle (n = 16; 50% dimethyl sulfoxide in 0.9% NaCl), respectively. Animals received a single loading dose (50 µl, intravenously) of PPT (2 µg), DPN (8 µg), or vehicle, respectively. Osmotic mini-pumps (ALZET Osmotic Pumps, Cupertino, CA, USA, Model 1003D, 100 µL reservoir volume, release rate of 1 µL/h) were implanted 20 mins after ROSC for continuous drug (subcutaneous) administration. Pumps contained PPT (50 µg/day), DPN (200 µg/day), or vehicle according to treatment group. Doses for ER agonists were from an earlier published work using these agents (Carswell et al., 2004a, b).
For protocols 1 and 2 the investigator was blinded to treatment groups.
Cardiac Arrest Model
The experimental procedures were performed as described earlier (Kofler et al, 2004; Noppens et al, 2005). Animals were anesthetized (induction with 3% halothane and maintenance with 1%–1.5% in 30% oxygen/70% air) and temperature probes were placed into the left temporalis muscle and the rectum. Rectal temperature was controlled at 37°C during surgery. A PE-10 catheter was inserted into the right jugular vein for drug administration. Electrocardiogram was monitored throughout the experimental procedure. Mice were endotracheally intubated and ventilated. Body and pericranial temperatures were controlled using circulating water coils. Cardiac arrest was induced by the injection (intravenous) of 50 µL cold 0.5 mol/L KCl and confirmed by an isoelectric electrocardiogram. Anesthesia was ceased, mechanical ventilation was discontinued, and the endotracheal tube was disconnected from the ventilator. Cardiopulmonary resuscitation was initiated 10 mins after the induction of CA by slow injection of 0.5mL epinephrine (8 µg), chest compressions (approximately 300 per min), and ventilation with 100% oxygen. Immediately after ROSC was confirmed by electrocardiogram activity with palpable femoral artery pulse, CPR was ceased. If CA persisted, additional doses of 0.1mL epinephrine (1.6 µg) were administrated in 30 secs intervals. Resuscitation efforts were stopped if ROSC could not be achieved within 2.5 mins of initiating CPR. Body temperature was controlled using a warming mat and warming lamp during the recovery period. After 30mins of ROSC, the catheter and temperature probes were removed, incisions were closed, and the animals were weaned from the ventilator. After sufficient spontaneous breathing was confirmed, the endotracheal tube was removed and mice were returned to their home cage on a circulating warm water blanket. Mice that did not survive 3 days (72 h) after ROSC were excluded from further analysis.
Behavioral and Neurologic Deficit Evaluation
Neurologic and behavioral testing was performed in a quiet room between 1400 and 1600 hours by one investigator who was blinded to the experimental group assignments. The evaluation procedure was performed daily for 3 days. Weight gain, food/water intake, consciousness, motor function, overall activity, ability to interact with the environment, and the presence of seizures were scored according to a neurologic assessment score adapted for mice and this experimental setting (0–100 scale: 0 = normal performance, 100 = equivalent to brain death, most severe deficit) (Noppens et al, 2005). Animals that died prematurely (before 72 h) during the observation period were excluded from further data analysis.
Serum Estrogen Levels/Neurohistopathological Evaluation
At 3 days after CA/CPR, mice were deeply anesthetized with halothane, the chest was opened, and the heart was punctured for blood sample withdrawal and the brain perfused-fixed (10% phosphate-buffered formalin, as described earlier; Kofler et al, 2004). Serum estradiol levels were determined using radioimmunoassay as described earlier (Hurn et al, 1995). Tissue analysis was by standard hematoxylin and eosin histology. For analysis, viable and nonviable neurons were counted by an investigator blinded to treatment groups using light microscopy ( × 100) in the CA1 region of the hippocampus (bregma −1.5 mm), and the rostral and caudal caudoputamen (CP) (bregma −0.5 and −1mm, respectively). The entire length of the CA1 sector was counted, and six microscopic fields were evaluated following a distinctive pattern in both levels of the CP. Nonviable neurons were identified by the pink hypereosinophilic cytoplasm and a dark pyknotic nucleus, and the percentage of neurons that were nonviable was calculated for each region of interest.
Statistical Analysis
All data are expressed as mean ± s.d. Comparison of treatment groups for physiologic variables, cardiac arrest-related parameters, serum E2 levels, and neurohistopathology was performed using one-way analysis of variance followed by Tukey’s post hoc test. χ2 Test was used for survival analysis. Differences were considered statistically significant with P < 0.05.
Results
Cardiac Arrest and Cardiopulmonary Resuscitation-Related Parameters and Mortality
Immediate asystolic arrest after bolus injection of KCl was observed in all mice. All animals were successfully resuscitated within the predefined CPR time window of 2.5 mins. Body weight, CPR duration, and epinephrine dose were not different between groups in experimental protocols 1 and 2 (Table 1). Animal survival was improved in estrogen-treated animals (0.5 µg E2 + 12.6 µg: 80%; 2.5 µg E2 + 12.6 µg: 85%), as compared with vehicle-treated group (65%, P < 0.05). However, survival was not different between selective ER agonist-treated groups (PPT: 75% and DPN: 73%) and vehicle-treated groups (69%). No differences were seen between surviving animals and nonsurvivors regarding CA/CPR-related parameters (e.g., CPR time and epinephrine dose).
Table 1.
CA/CPR-related parameters
| Estrogen treatment | Estrogen receptor agonists | |||||
|---|---|---|---|---|---|---|
| Vehicle | 0.5 µg E2+12.6 µg | 2.5 µg E2+12.6 µg | Vehicle | PPT | DPN | |
| Body weight (g) | 25.1 ± 1.2 | 25.4 ± 1.6 | 24.5 ± 1.4 | 23.1 ± 1.3 | 22.9 ± 1.7 | 22.8 ± 1.5 |
| CPR duration (secs) | 35 ± 7 | 37 ± 11 | 39 ± 16 | 34 ± 11 | 42 ± 12 | 37.6 ± 12 |
| Epinephrine (µg) | 7 ± 1 | 8 ± 2 | 8 ± 2 | 6 ± 1 | 7 ± 2 | 7 ± 2 |
| Epinephrine (µg/g BW) | 0.3 ± 0.01 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Survival (%) | 65 (13/20) | 80 (16/20)* | 85 (17/20)* | 69 (11/16) | 75 (12/16) | 73 (11/15) |
BW, body weight; CA/CPR, cardiac arrest and cardiopulmonary resuscitation; DPN, estrogen receptor-β agonist diarylpropionitrile; E2, 17β-estradiol; PPT, estrogen receptor-α agonist propyl pyrazole triol.
P<0.05; mean ± s.d.
Pericranial and Rectal Temperatures
Pericranial and rectal temperatures were similar in all groups throughout the acute experiment (Table 2). During 10 mins of cardiac arrest, body temperature was decreased to a nadir of 27°C±0.5°C and recovered to baseline levels within 30 mins after ROSC. Pericranial temperature was increased to 39°C ± 0.2°C during cardiac standstill. As temperature in the temporalis muscle was not maintained after ROSC, cool body blood caused moderate hypothermic pericranial temperature during the early reperfusion period (nadir of 32.7°C ± 0.6°C) and recovered to baseline values within 30 mins after CA/CPR.
Table 2.
Body and pericranial temperatures during CA/CPR
| Baseline | CA 5 mins | CA 10 mins | ROSC 15 mins | ROSC 30 mins | ||
|---|---|---|---|---|---|---|
| Body temperature (°C) | ||||||
| Estrogen treatment | Vehicle | 37.9 ± 0.3 | 30.2 ± 0.5* | 27.4 ± 1.0* | 33.8 ± 1.2* | 37.9 ± 0.2 |
| 0.5 µg E2+12.6 µg | 37.8 ± 0.5 | 30.5 ± 0.5* | 27.2 ± 0.5* | 33.8 ± 1.5* | 37.9 ± 0.6 | |
| 2.5 µg E2+12.6 µg | 37.8 ± 0.5 | 30.2 ± 0.5* | 27.2 ± 0.3* | 34.4 ± 1* | 38 ± 0.2 | |
| Estrogen receptor agonists | Vehicle | 37.6 ± 0.2 | 30.8 ± 0.7* | 26.7 ± 0.5* | 34.5 ± 0.8* | 37.9 ± 0.3 |
| PPT | 37.7 ± 0.4 | 31 ± 0.6* | 26.9 ± 0.6* | 34.9 ± 1.3* | 38.1 ± 0.4 | |
| DPN | 37.5 ± 0.3 | 31.1 ± 0.3* | 26.7 ± 0.4* | 34.2 ± 1* | 37.9 ± 0.4 | |
| Pericranial temperature (°C) | ||||||
| Estrogen treatment | Vehicle | 35.1 ± 0.1 | 39 ± 0.1* | 39 ± 0.1* | 33.3 ± 0.6* | 35.5 ± 0.4 |
| 0.5 µg E2+12.6 µg | 34.6 ± 0.5 | 38.9 ± 0.1* | 38.9 ± 0.1* | 33.2 ± 0.9* | 35.4 ± 0.5 | |
| 2.5 µg E2+12.6 µg | 35.1 ± 0.6 | 39 ± 0.1* | 39 ± 0.1* | 33.7 ± 0.5* | 35.5 ± 0.4 | |
| Estrogen receptor agonists | Vehicle | 34.7 ± 0.2 | 39 ± 0.1* | 38.9 ± 0.1* | 33.1 ± 0.7* | 34.8 ± 0.3 |
| PPT | 34.5 ± 0.3 | 39 ± 0.1* | 39 ± 0.1* | 33.5 ± 0.4* | 34.8 ± 0.4 | |
| DPN | 34.7 ± 0.2 | 39 ± 0.1* | 39 ± 0.1* | 33.4 ± 0.7* | 34.7 ± 0.4 |
CA/CPR, cardiac arrest and cardiopulmonary resuscitation; DPN, estrogen receptor-β agonist diarylpropionitrile; E2, 17β-estradiol; PPT, estrogen receptor-α agonist propyl pyrazole triol; ROSC, return of spontaneous circulation.
P<0.05 from baseline; mean ± s.d.
Neurologic Recovery and Weight Loss
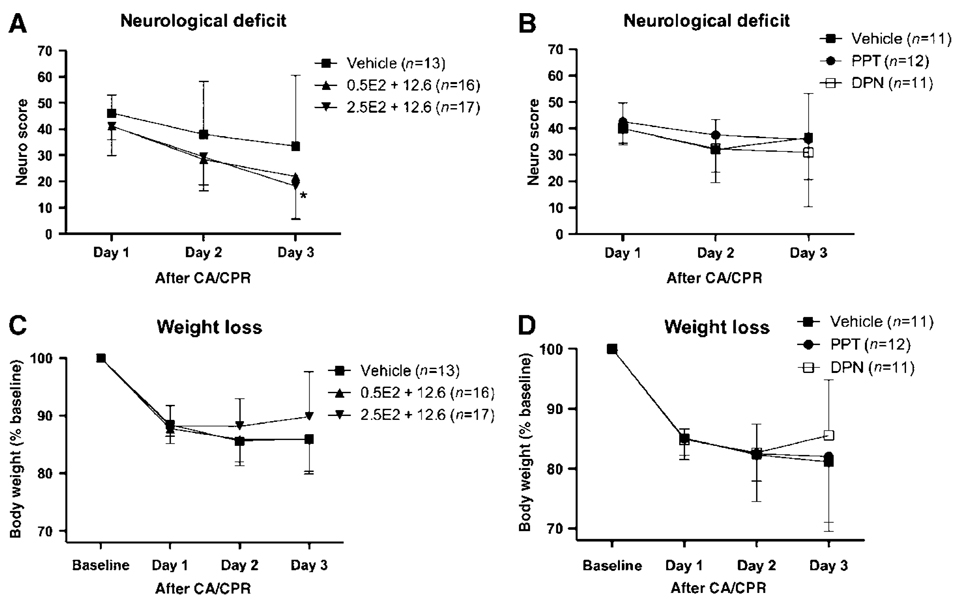
Neurologic deficit was most pronounced on the first day after CA/CPR in all groups and improved slowly throughout the 3-day observation period without full recovery (Figures 1A and 1B). However, in vehicle-treated animals in the estrogen treatment protocol (protocol 1), functional recovery was slower, as compared with animals receiving E2 regimens, and significance was only reached with the higher loading dose (2.5 µg E2 + 12.6µg) on day 3 after CA/CPR (P<0.05) (versus 2.5µg E2 + 12.6 µg, P<0.05). Treatment with ER agonists in experimental protocol 2 had no effect on functional recovery, as compared with vehicle treatment (Figure 1B). Body weight decreased by 14% 24h after CA/CPR, did not recover over the 3-day observation period, and did not differ between treatment groups in protocol 1 or 2 (Figures 1C and 1D).
Figure 1.
Neurologic deficit and weight loss recovery over the 3-day observation period of treatment protocol 1 (A) and protocol 2 (B). DPN, estrogen receptor-β agonist diarylpropionitrile; E2, 17β-estradiol; PPT, estrogen receptor-α agonist propyl pyrazole triol. *P<0.05; mean ± s.d.
Serum Estrogen Level
Estradiol serum levels were within physiologic range in vehicle-treated male C57Bl/6 mice (28.3 ± 7.3 pg/mL). As intended, estradiol-treated groups showed increased serum levels 3 days after CA/CPR (0.5 µg E2 + 12.6 µg: 164.8 ± 39.55 pg/mL, P < 0.001 and 2.5 µg E2 + 12.6 µg: 153 ± 45.9 pg/mL, P < 0.001). Serum levels were independent of initial E2 loading dose.
Neurohistopathology
All mice, regardless of treatment regimen, sustained histologic injury.
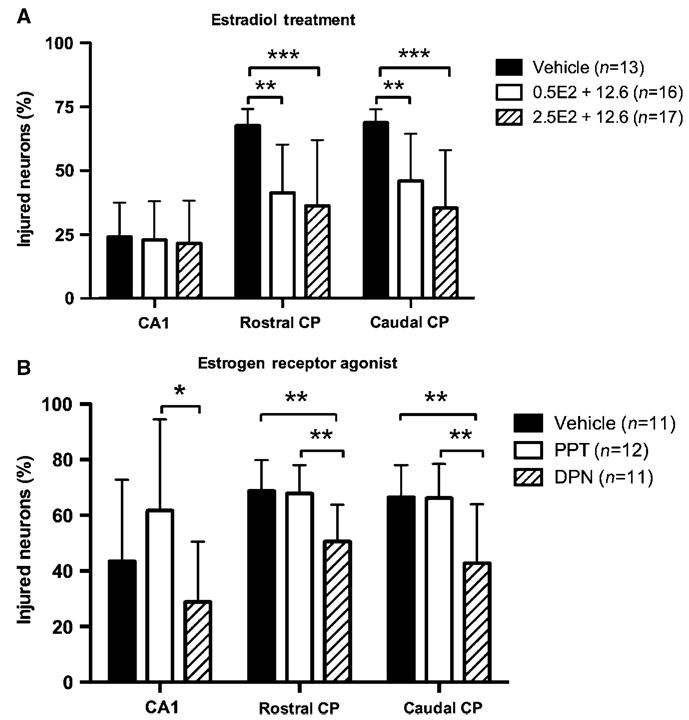
Protocol 1: Effects of chronic estradiol treatment
Animals in this group showed mild levels of neuronal injury in the hippocampal CA1 region. No differences were found between groups in this area (Figure 2A, P = 0.43). Neuronal injury was greatest in the rostral and caudal CP. Both estradiol-treated groups showed reduced brain injury in the rostral CP (0.5 µg E2 + 12.6 µg: 41.3% ± 19% injured neurons; 2.5 µg E2 + 12.6 µg: 35.3%±25.6%), as compared with vehicle-treated mice (67.7% ± 6.5%, P < 0.01 and P < 0.001, respectively). However, the initial E2 intravenous dose did not alter injury between estradiol-treated groups (P = 0.53). A similar pattern of neuronal injury was seen in the caudal CP: neuronal survival was greater in both E2-treated groups (0.5 µg E2 + 12.6 µg: 46.1% ± 18.4% injured neurons; 2.5 µg E2 + 12.6 µg: 35.4% ± 22.64%), as compared with vehicle mice (68.8% ± 5.2%, P < 0.01 and P < 0.001, respectively). Different E2 loading dose had no effect on neuronal injury.
Figure 2.
Neurohistopathology of estradiol treatment protocol (A) and estrogen receptor agonist (B). CA1, hippocampal CA1 region; CP, caudoputamen; DPN, estrogen receptor-β agonist diarylpropionitrile; E2, 17β-estradiol; PPT, estrogen receptor-α agonist propyl pyrazole triol. *P<0.05, **P<0.01, ***P<0.001; mean ± s.d.
Protocol 2: Selective ER agonists
Neuronal injury was not different in the hippocampal CA1 region between vehicle and ER agonist-treated groups. However, ER-β agonist DPN-treated mice showed less neuronal injury (28.9% ± 21.7% injured neurons) than ER-α agonist PPT-treated animals (61.8% ± 32.8%, P < 0.05). In the rostral CP, treatment with DPN reduced neuronal injury (50.7% ± 13.1% injured neurons), as compared with vehicle-treated animals (68.8% ± 11%, P < 0.01) and the PPT group (67.9% ± 10.1%). Brain injury was similar in the vehicle and PPT groups. Increased neuronal survival was found in the caudal CP in DPN mice (42.8% ± 21.2% injured neurons), as compared with vehicle (66.5% ± 11.6%, P < 0.01) and PPT (66.18% ± 12.4%). Vehicle and PPT mice had similar neuronal injury in the caudal CP.
Discussion
This study shows several important and novel findings. We show for the first time that long-term administration of 17β-estradiol in a model of transient ischemia after CA/CPR is neuroprotective. The loading dose does not appear to play an important role in this neuroprotection. Our study also shows that only the selective ER-β agonist DPN attenuates brain injury in vulnerable brain regions in mice. The ER-α agonist PPT had no effect on neuronal survival in our experimental setting. Our results suggest that neuroprotective effects of estradiol are brain region-specific and are mediated through ER-β.
Many laboratory studies indicate that estrogen has beneficial effects on neuronal survival after focal cerebral ischemia. In experimental stroke, female animals have smaller infarct sizes than males (Alkayed et al, 1998). In estrogen-depleted (ovari-ectomized) female animals, neuroprotection was lost, whereas, chronic estrogen replacement administered before the insult restored neuroprotection (Rusa et al, 1999). Long-term administration of an estrogen antagonist for 7 days also correlated with greater brain injury in female mice (Sawada et al, 2000). Postischemic estrogen administration has also been shown to be effective after experimental focal ischemia. Estrogen infusion administered at 5 mins after reperfusion from focal ischemia reduced infarct size and restored cerebral blood flow (McCullough et al, 2001). Another study of E2 treatment after induction of focal ischemia decreased the size of the ischemic lesion, but only if E2 was administered within 3 h after the stroke onset (Yang et al, 2000). We recently showed that a single injection (intravenous) of E2 administered after CA/CPR reduced brain injury and that the effects are both dose-dependent and brain region-specific (Noppens et al, 2005). Only the administration of a low dose of E2 (0.5 µg) improved neuronal survival in the rostral and caudal CP, whereas higher E2 doses ( > 2.5 µg) had no effect on outcome. A recent review (Gibson et al, 2006) showed that the effect of estrogen treatment after transient brain ischemia decreased with increased quality score. Studies receiving the highest quality scores showed no beneficial effect of estrogen in models of cerebral ischemia. Estrogen was only effective in ovariectomized females and young adult male animals; however, very little is known about the effects of estrogen in aged animals (Gibson et al, 2006).
Although most studies show beneficial effects of estrogen on neuronal survival after cerebral ischemia, some using estrogen replacement found deleterious effects of estrogen. In an animal model of temporal global cerebral ischemia, using four-vessel occlusion, E2 treatment, or female sex resulted in an increased neuronal injury in the hippocampal CA1 region (Harukuni et al, 2001). Several animal studies examining estrogen as pre or posttreatment to focal ischemia report no effect on stroke size or even show an exacerbated ischemic injury (Bingham et al, 2005; Carswell et al, 2004a; Gordon et al, 2005; Theodorsson and Theodorsson 2005; Vergouwen et al, 2000). The optimal therapeutic dose, time point of administration, and the duration of treatment remain unclear, especially after global cerebral ischemia.
This study shows a beneficial effect of both, 0.5 and 2.5 µg (intravenous) loading E2 doses, when followed by long-term administration of E2 in a our model of transient global cerebral ischemia. According to these results, the initial dose does not appear to be a major factor for neuroprotection in our CA/CPR model. However, this study does not answer whether a chronic application of estrogen alone causes neuroprotection in this model. Thus, this study cannot exclude that chronic treatment after CA/CPR alone might act independently of an acute dose. Similar to our previous study, we found neuronal injury unaltered by E2 in the hippocampal CA1 region. In our mouse CA/CPR model, neuronal injury in the hippocampus is modest. In addition, hippocampal neurons in the CA1 field appear to be more resistant to cerebral ischemia in mice (Bottiger et al, 1999). It is well accepted that the development of neuronal injury in the hippocampus in mice is delayed compared with other vulnerable brain regions, such as the CP (Pulsinelli et al, 1982). Three-day survival after CA/CPR, such as that in our experiments, might not have allowed the full extent of neuronal injury to mature, and therefore might not reveal the neuroprotective effect of E2.
Effects of estrogen on behavioral outcome are conflicting. In a model of transient global ischemia in ovariectomized female rats, chronic E2 replacement before the ischemic insult improved visual memory and spatial memory using the object recognition and object placement test, as compared with vehicle-treated animals (Gulinello et al, 2006). When E2 was injected into the lateral ventricles immediately after the ischemic insult, visual memory was improved, whereas spatial memory was not altered, as compared with vehicle-treated animals. The same group showed no effect of either chronic or acute E2 treatment on locomotor activity or anxiety after transient global cerebral ischemia. E2 pretreatment in ovarectomized female mice before transient middle cerebral artery occlusion resulted in improved forelimb use assessed by the cylinder test (Li et al, 2004). In the same study, improved sensorimotor function was described with E2 pretreatment using the corner test. Effects of estradiol in intact animals on nonspatial working memory was dose-dependent (Wide et al, 2004). High-dose estradiol was associated with impaired working memory, whereas low levels facilitated memory in a T-maze, suggesting that supraphysiologic estrogen doses alone are able to influence behavior. Depletion of ER-α (ER-α knockout mice) resulted in impaired cognitive function in the passive avoidance test; however, ER-β knockout mice were not affected (Fugger et al, 2000). In our study, vehicle-treated animals recovered slower, reaching statistical significance on day 3 after CA/CPR, if compared with the 2.5 µg E2 + 12.6 µg group. We used a score that examines gross neurologic function such as consciousness and the presence of seizures. This score allows identifying animals with major neurologic deficit; however, it allows little information on functional outcome such as memory and cognitive function. Further study is needed using, for example, object recognition test and/or passive avoidance test to identify possible effects of estradiol on cognitive outcome and which ER might be involved.
Sex difference in survival after CA/CPR in humans has been shown in several studies (Tunstall-Pedoe et al, 1996; Wigginton et al, 2002). Cardiac arrest is rare at young age, especially before menopause in women. Therefore, it is difficult to evaluate the clinical effect of estrogen on survival and neurologic outcome in humans after cardiac arrest. Human data concerning the role of estrogen and survival after trauma or in the critically ill are conflicting. Higher estradiol serum levels were associated with higher mortality and the risk of death increased with serum levels in an adult critically ill population (May et al, 2008). However, two clinical studies have shown a higher survival rate after traumatic injury and traumatic shock in adolescent girls (Haider et al, 2007; Phelan et al, 2007). No difference in survival was found in prepubescent children, suggesting the potential involvement of female sex hormones in survival after traumatic injury. In an animal study examining the neuroprotective effects of estradiol in ovariectomized rats after middle cerebral artery occlusion, either 24-h pretreatment or 40-min posttreatment after the insult was associated with improved survival, as compared with vehicle-treated animals (Simpkins et al, 1997). We also showed an improved survival after E2 treatment; however, treatment with ER agonists showed no effect on mortality, although neuronal injury was reduced in the ER-β agonist-treated group. This indicates that animal survival is a multifactorial process, which is not related to brain injury alone. It is also possible that ERs play only a minor role in this observation.
Physiologic serum estradiol levels in female C57Bl/6 mice range from 10 to 50 pg/mL and may increase to approximately 300 pg/mL during pregnancy. Estrogen has shown in several studies a low therapeutic range when used for pretreating, with very low and very high levels failing to show an effect on neuronal survival (Noppens et al, 2005; Rusa et al, 1999). In this study, we used E2 implants that produced serum estradiol levels above those usually observed in normal cycling females, but well below levels described during mouse pregnancy. Most studies have been performed with estrogen pretreatment before the insult, but very little is known concerning the therapeutic range of estradiol when applied after brain ischemia. Our results show that chronic E2 treatment resulting in levels that are within the physiologic range is neuroprotective.
This study also shows, for the first time, effects of selective ER agonists after transient brain ischemia using CA/CPR on neuronal survival. Only the ER-β agonist DPN reduced neuronal injury in specific brain regions. In contrast, PPT (ER-α agonist) had no effects on brain injury in the CP, but increased neuronal injury in the hippocampal CA1 region, as compared with the DPN-treated group. The selective ER-α agonist PPT has a 410-fold binding affinity over ER-β (Stauffer et al, 2000). Systemically administered PPT, in a comparable dose used in this study, has been shown to induce specific ER-α-mediated effects on the brain, such as an increase in progesterone receptor mRNA (Harris et al, 2002). Furthermore, PPT has been shown to modify estrogen-deficient female rat behavior by increasing anxiogenic behavior (Lund et al, 2005). Diarylpropionitrile is an ER-β agonist with a 70-fold higher relative binding affinity and a 170-fold greater relative potency for ER-β than for ER-α (Meyers et al, 2001). Peripherally administrated DPN to ovariectomized rats increased (within 30 mins) occupied nuclear ER in the brain, showing that DPN affects neurons (Lund et al, 2005). Diarylpropionitrile was also found to reduce anxiety in estrogen-deficient female rats (Lund et al, 2005). Estrogen receptors are present throughout the brain and are brain region-specific. In the mouse brain, both subtypes of ER (α and β) express in the hippocampus (Mitra et al, 2003; Shughrue et al, 1997). Contradictory results have been reported concerning the expression of ER-α and ER-β in the CP (Kuppers and Beyer 1999). However, both ER isoforms have been identified in cerebral blood vessels, suggesting that direct vascular effects of estrogen may contribute to the effects of estrogen in the brain (Jesmin et al, 2003; Stirone et al, 2003). Some reports show increased cerebral blood flow during ischemia in the presence of estrogen, using models of temporal vessel occlusion (He et al, 2002; Hurn et al, 1995). We did not measure effects of E2 or ER agonists on cerebral blood flow in this study and cannot exclude the possibility that an improved reperfusion contributes to our results.
Little is known about the role of ER in neuroprotection and what is known is conflicting (Carswell et al, 2004b; Dubal et al, 2001; Sampei et al, 2000). In agreement with our findings is a study administering PPT and DPN for 1 week before experimental global ischemia using a two-vessel occlusion mouse model (Carswell et al, 2004b). Diarylpropionitrile provided neuroprotection, whereas PPT did not change injury. Another study using reversible middle cerebral artery occlusion in ER-α knockout mice showed no change in total stroke size, indicating that activation of this receptor subtype is not essential for neuroprotection (Sampei et al, 2000). In contrast, ER-α and not ER-β was identified to mediate neuroprotection in a model of permanent focal ischemia in knockout mice (Dubal et al, 2001). In a rat model of permanent focal ischemia, pretreatment with DPN did not influence survival, sensorimotor function, or infarct size (Farr et al, 2007). Studies showing involvement of ER-β in neuroprotection have in common that they all use models of reperfusion, whereas the study presenting opposing results used a model of nonreperfusion ischemia. Thus, severity of ischemia, brain region, and the character of ischemia may play important roles for potential beneficial effects of estrogen. Finally, we do not know whether the ER agonist dose used for these experiments is optimal. Further study whether needed to clarify if a dose response for neuroprotective effects exists and, if so by what mechanism.
Conclusion
In conclusion, long-term administration of E2 is neuroprotective in male mice when administered after transient brain ischemia caused by CA/CPR. The initial dose does not seem to play an important role in this neuroprotection. Most likely, the beneficial effect of E2 is mediated through ER-β in this mouse model of CA/CPR. However, improved neuronal survival with E2 was restricted to specific brain regions at the dose and time of administration used. These findings are of high clinical relevance, as identifying more selective pathways of estrogen-mediated neuroprotection might lead to the development of very specific drugs that mimic the beneficial effects of estrogen without potential side effects. This study identifies the ER-β as a promising target for further study.
Acknowledgments
This study was supported by National Institutes of Health Grants NS 46072, NS 20020, and NS 33368.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutheridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17beta-oestradiol after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2005;25:414–420. doi: 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass P. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Brain Res Mol Brain Res. 1999;65:135–142. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab. 2004a;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004b;287:H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Cobb LA, Fahrenbruch CE, Walsh TR. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular defbrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med. 2001;344:1304–1313. doi: 10.1056/NEJM200104263441707. [DOI] [PubMed] [Google Scholar]
- Farr TD, Carswell HV, Gsell W, Macrae IM. Estrogen receptor beta agonist diarylpropionitrile (DPN) does not mediate neuroprotection in a rat model of permanent focal ischemia. Brain Res. 2007;1185:275–282. doi: 10.1016/j.brainres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HV. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider AH, Efron DT, Haut ER, Chang DC, Paidas CN, Cornwell EE., III Mortality in adolescent girls versus boys following traumatic shock: an analysis of the National Pediatric Trauma Registry. Arch Surg. 2007;142:875–880. doi: 10.1001/archsurg.142.9.875. discussion 879–880. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Hurn PD, Crain BJ. Deleterious effect of beta-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and -independent mechanisms. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- Herlitz J, Rundqvist S, Bang A, Aune S, Lundstrom G, Ekstrom L, Lindkvist J. Is there a difference between women and men in characteristics and outcome after in hospital cardiac arrest? Resuscitation. 2001;49:15–23. doi: 10.1016/s0300-9572(00)00342-7. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34:338–341. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: effect of 17 beta-estradiol. J Cereb Blood Flow Metab. 1995;15:666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Hattori Y, Sakuma I, Liu MY, Mowa CN, Kitabatake A. Estrogen deprivation and replacement modulate cerebral capillary density with vascular expression of angiogenic molecules in middle-aged female rats. J Cereb Blood Flow Metab. 2003;23:181–189. doi: 10.1097/01.WCB.0000043341.09081.37. [DOI] [PubMed] [Google Scholar]
- Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–2703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, Popovsky KA, Sawyer RG. Estradiol is associated with mortality in critically ill trauma and surgical patients*. Crit Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Noppens RR, Kofler J, Hurn PD, Traystman RJ. Dose-dependent neuroprotection by 17beta-estradiol after cardiac arrest and cardiopulmonary resuscitation. Crit Care Med. 2005;33:1595–1602. doi: 10.1097/01.ccm.0000169884.81769.f7. [DOI] [PubMed] [Google Scholar]
- Phelan HA, Shafi S, Parks J, Maxson RT, Ahmad N, Murphy JT, Minei JP. Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J Trauma. 2007;63:1127–1131. doi: 10.1097/TA.0b013e318154c1b8. [DOI] [PubMed] [Google Scholar]
- Polentini MS, Pirrallo RG, McGill W. The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992–2002) Prehosp Emerg Care. 2006;10:52–60. doi: 10.1080/10903120500366961. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17Beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000;31:738–743. doi: 10.1161/01.str.31.3.738. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–E192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Theodorsson A, Theodorsson E. Estradiol increases brain lesions in the cortex and lateral striatum after transient occlusion of the middle cerebral artery in rats: no effect of ischemia on galanin in the stroke area but decreased levels in the hippocampus. Peptides. 2005;26:2257–2264. doi: 10.1016/j.peptides.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Morrison C, Woodward M, Fitzpatrick B, Watt G. Sex differences in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985 to 1991. Presentation, diagnosis, treatment, and 28-day case fatality of 3991 events in men and 1551 events in women. Circulation. 1996;93:1981–1992. doi: 10.1161/01.cir.93.11.1981. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Anderson RE, Meyer FB. Gender differences and the effects of synthetic exogenous and non-synthetic estrogens in focal cerebral ischemia. Brain Res. 2000;878:88–97. doi: 10.1016/s0006-8993(00)02713-x. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wigginton JG, Pepe PE, Bedolla JP, DeTamble LA, Atkins JM. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: a multiyear, prospective, population-based study. Crit Care Med. 2002;30:S131–S136. doi: 10.1097/00003246-200204001-00002. [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. [DOI] [PubMed] [Google Scholar]