Abstract
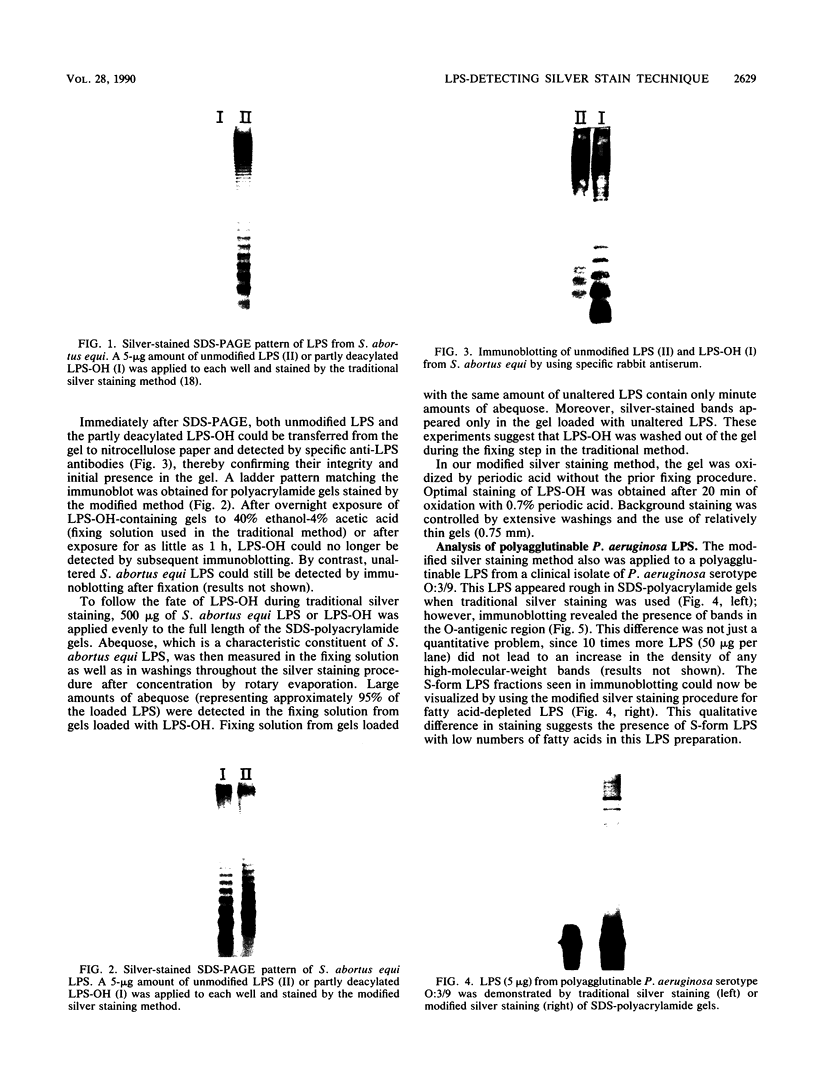
A silver staining method used routinely for detecting bacterial lipopolysaccharide (LPS) in sodium dodecyl sulfate-polyacrylamide gels (C. Tsai and E. Frasch, Anal. Biochem. 119:115-119, 1982) appeared to be inappropriate for visualizing certain LPS preparations. It did not stain S-form fractions of polyagglutinable Pseudomonas aeruginosa LPS or several partly deacylated (alkali-treated) S-form LPS after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. However, these LPS preparations could be detected by anti-LPS sera after electroblotting onto nitrocellulose, thereby confirming their integrity and presence in the polyacrylamide gel. This is because LPS fractions containing a low number of fatty acids are washed out of the gel during the initial fixing step (40% ethanol-4% acetic acid, overnight). By omitting this fixing step, which was originally developed for detecting proteins, and by increasing the LPS oxidation time (from 5 to 20 min), we restored the ability to detect LPS fractions that otherwise would not be stained. These modifications did not affect the detection of other S- and R-form LPSs. Thus, differences in the number of fatty acids present in polyagglutinable P. aeruginosa LPS may result in a selective loss of fatty acid-deficient S-form LPS in these apparent R-form LPS preparations. This modified procedure provides a fast, simple, and sensitive way to analyze LPS in polyacrylamide gels despite the number of acyl groups present.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baek L., Høiby N., Hertz J. B., Espersen F. Interaction between limulus amoebocyte lysate and soluble antigens from Pseudomonas aeruginosa and Staphylococcus aureus studied by quantitative immunoelectrophoresis. J Clin Microbiol. 1985 Aug;22(2):229–237. doi: 10.1128/jcm.22.2.229-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Conrad R. S., Galanos C., Shand G. H., Høiby N. Comparative immunochemistry of lipopolysaccharides from typable and polyagglutinable Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J Clin Microbiol. 1988 May;26(5):821–826. doi: 10.1128/jcm.26.5.821-826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Høiby N., Shand G. H., Conrad R. S., Galanos C. Longitudinal study of antibody response to lipopolysaccharides during chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. Infect Immun. 1988 Sep;56(9):2270–2278. doi: 10.1128/iai.56.9.2270-2278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Jiao B. H., Komuro T., Freudenberg M. A., Lüderitz O. Large-scale fractionation of S-form lipopolysaccharide from Salmonella abortus equi. Chemical and serological characterization of the fractions. J Chromatogr. 1988 May 25;440:397–404. doi: 10.1016/s0021-9673(00)94543-6. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N., Hertz J. B., Sompolinsky D. Antibody response in patients with Pseudomonas aeruginosa infection to a 'common antigen' from P. aeruginosa analysed by means of quantitative immunoelectrophoretic methods. Acta Pathol Microbiol Scand C. 1980 Jun;88(3):149–154. doi: 10.1111/j.1699-0463.1980.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Basu S., Mayer H. Comparison of enterobacterial common antigen from different species by serological techniques. Eur J Biochem. 1987 Jan 2;162(1):69–74. doi: 10.1111/j.1432-1033.1987.tb10543.x. [DOI] [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]








