Abstract
Context: Postmenopausal women have greater visceral adiposity compared with premenopausal women. Adipokines are associated with increased adiposity, insulin resistance, and atherosclerosis.
Objective: The objective of the study was to assess changes in adipokines and inflammatory markers through the menopausal transition and correlate them with changes in visceral adiposity.
Design and Setting: This was a prospective cohort study of women through the menopausal transition conducted at the University of Washington.
Participants: Sixty-nine healthy women were followed up longitudinally from premenopausal (aged 45–55 yr) to postmenopausal status (aged 49–60 yr).
Outcome: On premenopausal and postmenopausal visits, fasting blood was drawn for adiponectin, leptin, serum amyloid A (SAA), C-reactive protein (CRP), monocyte-chemotactic protein-1, tissue plasminogen activator antigen (tPA), IL-6, and TNF-α. Body composition measures were assessed by body mass index, whole-body dual x-ray absorptiometry scan, and computed tomography scan of the abdomen at the lumbar 4–5 level.
Results: Women had a statistically significant increase in SAA, tPA, monocyte-chemotactic protein-1, and adiponectin between the two measurement occasions (P = 0.04, P = 0.02, P = 0.001, and P < 0.001, respectively). The increase in intraabdominal fat was correlated positively with the change in SAA (r = 0.31, P = 0.02), CRP (r = 0.56, P < 0.001), tPA (r = 0.40, P = 0.002), and leptin (r = 0.41, P = 0.002) and negatively correlated with the change in adiponectin (r = −0.37, P = 0.005). After adjustment for change in sc abdominal fat, the correlation between change in CRP, tPA, leptin, and adiponectin remained significantly associated with change in intraabdominal fat.
Conclusions: Women going through the menopausal transition have deleterious changes in inflammatory markers and adipokines that correlate with increased visceral adiposity.
During the menopausal transition, women develop adverse alterations in adipokines and inflammatory markers in relation to increasing visceral adiposity.
Postmenopausal women have increased visceral adiposity in comparison with premenopausal women (1,2) that may contribute to the postmenopausal increase in cardiovascular risk (3). Central adiposity has been linked with insulin resistance, dyslipidemia, and increased risk for cardiovascular disease. The mechanism linking visceral adiposity to these downstream metabolic diseases remains an enigma.
There is a growing body of literature suggesting that visceral adipose tissue is an important source of inflammatory adipocytokines (4,5,6). Obese individuals with higher visceral adiposity have increased monocyte-chemotactic protein-1 (MCP)-1 expression and infiltration of macrophages in visceral fat compared with sc fat (7). Visceral adipose tissue from obese subjects has also been found to secrete higher levels of plasminogen activator inhibitor-1 (PAI)-1, IL-6, TNF-α, and leptin and lower levels of adiponectin compared with lean subjects (8,9,10). Circulating inflammatory markers elevated in obese subjects include C-reactive protein (CRP) and serum amyloid A (SAA) (11,12). The increase in visceral adiposity across the menopausal transition may result in the development of a proinflammatory adipokine changes (13). Associations between elevated CRP, IL-6, and tissue plasminogen activator antigen (tPA) with increased risk of coronary heart disease and diabetes in postmenopausal women have been shown (14,15,16).
Given that intraabdominal fat (IAF) increases across the menopausal transition, we hypothesize that deleterious changes in adipokines and inflammatory markers during this period will develop as a consequence. To our knowledge this is the first prospective paired study evaluating the changes in adipokines and measures of inflammation in relation to change in IAF across the menopausal transition.
Subjects and Methods
Subjects
One hundred twenty healthy, premenopausal women between the ages of 42–55 yr entered the study between 1997 and 2000. Fifty participants were recruited from the Seattle Midlife Women’s Health Study (17). Women in this study were enrolled using complete ascertainment of households in census tract areas with diverse ethnic and socioeconomic status in the Seattle area. A subsequent 70 participants also were recruited from the Seattle area by advertisement on radio and fliers.
Women were enrolled if they met the criteria for being premenopausal at study onset, defined as having at least one menstrual period within the 3 months before study enrollment. Menstrual histories were obtained every 4 months. Postmenopausal status was defined as the lack of menstrual periods for at least 12 continuous months. Women were excluded if they had a history of hysterectomy; a body mass index (BMI) greater than 40 kg/m2; history of diabetes mellitus; fasting glucose 110 mg/dl or greater; low-density lipoprotein cholesterol or triglycerides greater than the 95th percentile; active liver disease; or use of estrogen, β-blockers, or lipid-lowering agents. Written informed consent was approved by the University of Washington General Clinical Research Center and Institutional Review Board and signed by subjects.
During the study period 1997–2005, 69 women became postmenopausal, 32 women remained in the menopausal transition, and 19 women dropped out. This cohort of 69 women was comprised of 23 women recruited from the Seattle Midlife Women’s Health Study and 46 women recruited by advertisements on fliers/radio. There were no significant differences at baseline between women enrolled from the Seattle Women’s Midlife Health Study and those enrolled via advertisement, except the former group of women was slightly older (mean age 51.4 ± 2.3 vs. 49.0 ± 2.6 yr). The cohort for this analysis will include 69 of the 120 women who were followed up from premenopausal to postmenopausal status for a paired analysis.
Protocol
Subjects were admitted to the University of Washington General Clinical Research Center for a baseline premenopausal visit and a postmenopausal visit once they met criteria for being postmenopausal. On these study visits, participants had measures of weight, height, total percent body fat, and percent truncal fat by dual x-ray absorptiometry (DEXA) scan, and sc abdominal fat (SQAF) and IAF measurements by single-slice computed tomography (CT) scan. Fasting plasma was obtained for adiponectin, leptin, SAA, CRP, MCP-1, tPA, IL-6, and TNF-α concentrations.
Measurements of body composition
Weight in kilograms and height in centimeters were taken using the same scale and stadiometer, respectively. Total percent body fat and percent truncal fat were derived from DEXA scans performed on a QDR 1500 DEXA scanner (Hologic, Waltham, MA). The interassay coefficients of variation (CVs) for fat mass, lean mass, and percent body fat measured by the DEXA scans are 1.6, 1.3, and 1.3%, respectively. Single-slice CT scans were taken on inspiration at the level of lumbar 4–5 on a Highspeed Advantage CT scanner (GE, Milwaukee, WI). IAF and SQAF were measured by a single-blinded observer who analyzed cross-sectional fat areas using a density contour GE computer software program.
Laboratory measurements
For measurements of SAA, CRP, adiponectin, leptin, IL-6, TNF-α, and tPA, each participant had 40 ml of blood drawn into 0.1% EDTA tubes on ice after a 12-h fast. The plasma was centrifuged at 4 C at 3000 rpm for 15 min, frozen, and stored at −70 C. Assays for SAA, CRP, tPA, leptin, and adiponectin were performed on 58 stored pre- and postmenopausal samples at the same time. Assays for IL-6, MCP-1, and TNF-α were also run on 51 stored pre- and postmenopausal samples concurrently. CRP and SAA were measured by particle-enhanced immunonephelometry (Dade Behring Diagnostics, Deerfield, IL). Intra- and interassay CVs for CRP are 2.3–4.4 and 2.6–5.7%, respectively. Intra- and interassay CVs for SAA are 2–3 and 3–5.2%, respectively. MCP-1, IL-6, and TNF-α were measured by LINCOplex human cytokine kit (Millipore, Bedford, CT). Inter- and intraassay CVs for the cytokine kit measurements of IL-6, MCP-1, and TNF-α are approximately 10%. tPA was measured using ELISA (American Diagnostica Inc., Stamford, CT). Intra- and interassay CVs for tPA are 7 and 9%, respectively. Leptin was measured using ELISA (Diagnostic Systems Laboratories Inc., Webster, TX). Intra- and interassay CV are 1.5–6.2 and 3.3–5.3%, respectively. Adiponectin was measured by RIA (Linco Research, Inc., St. Charles, MO). Intra- and interassay CVs are 1.8–7.4% and 2.4–8.4%, respectively.
Data analysis
Statistical analyses were performed using STATA SE9 (STATA Corp., College Station, TX). Descriptions of quantitative variables measured on pre- and postmenopausal visits are shown with means and sds. For variables with a skewed distribution, medians and interquartile ranges were calculated. To detect a significant change between pre- and postmenopausal measures of inflammation and body composition, the paired t test was used and two-sided P < 0.05 was considered significant. The discrete value for change was calculated as the mean difference between pre- and postmenopausal values for body composition measures and measures of inflammation. The percent change from the premenopausal measurements was also calculated. To evaluate the correlation between change in IAF and change in markers of inflammation, Pearson’s correlation coefficients for change in IAF and the change in each measure of inflammation were calculated. Correlations between the change in markers of inflammation and change in SQAF and total percent body fat were shown for comparison. In addition, univariate linear regression analysis was performed and β-coefficients and confidence intervals are reported. Multivariate regression analysis was performed to adjust for SQAF, baseline age, and duration of study period for each subject.
Results
Our study cohort included 69 women followed up across the menopausal transition for a mean duration of 4.1 ± 1.4 yr. This is a predominantly Caucasian population (95.65%, n = 66) with the mean age of 50.6 ± 2.6 yr at baseline. The cohort was generally healthy, and only one subject was on aspirin. Only two women were smokers.
Changes in body composition occurred as women became postmenopausal and results are shown in Table 1. Whereas there was no significant change in total percent body fat across menopause, the women experienced a statistically significant increase in BMI, weight, percent truncal fat, IAF, and SQAF as they became postmenopausal. Of these measures of adiposity, IAF increased by the greatest proportion across the menopausal transition (21%).
Table 1.
Measures of body composition
| Measure | Premenopausal | Postmenopausal | Difference | Change, % | P value |
|---|---|---|---|---|---|
| Weight (kg) | 72.32 ± 14.33 | 73.66 ± 16.18 | 1.34 | 1.85 | 0.03 |
| BMI (kg/m2) | 26.42 ± 4.52 | 26.97 ± 5.24 | 0.56 | 2.12 | 0.01 |
| IAF (cm2) | 77.27 ± 42.31 | 93.78 ± 58.73 | 16.50 | 21.35 | <0.001 |
| SQAF (cm2) | 258.16 ± 133.69 | 296.39 ± 153.71 | 38.24 | 14.81 | <0.001 |
| Total body fat, % | 37.68 ± 7.33 | 38.32 ± 7.71 | 0.64 | 1.70 | 0.10 |
| Trunk fat, % | 34.10 ± 9.84 | 38.29 ± 9.27 | 4.19 | 10.94 | <0.001 |
Data are presented as mean ± sd.
Fifty-eight of 69 women had CRP, SAA, tPA, adiponectin, and leptin assayed on both the pre- and postmenopausal study visits (Table 2). Fifty-one of the 69 women had IL-6, MCP-1, and TNF-α measured for both study visits. There was a statistically significant increase in MCP-1, SAA, tPA, and adiponectin as women entered postmenopause. SAA had the greatest percent increase (28.65%). No significant changes were noted in the measures of TNF-α, IL-6, leptin, or CRP levels.
Table 2.
Measures of inflammation
| Measure | Premenopausal | Postmenopausal | Difference | Change, % | P value |
|---|---|---|---|---|---|
| CRP (mg/liter)a | 1.4 (0.5, 2.6) | 1.0 (0.6, 2.6) | 0.05 | 2.89 | 0.83 |
| SAA (mg/liter)a | 2.8 (2.1, 3.8) | 3.1 (2.4, 5.3) | 0.41 | 28.65 | 0.04 |
| IL-6 (pg/ml)a | 9 (6, 15) | 8 (6, 14) | 0.82 | 5.94 | 0.71 |
| TNF-α (pg/ml)a | 2 (0, 5) | 2 (0, 5) | −0.24 | −9.56 | 0.32 |
| MCP-1 (pg/ml) | 64.49 ± 26.60 | 73.65 ± 27.95 | 9.16 | 14.20 | 0.001 |
| Adiponectin (μg/ml) | 13.13 ± 4.98 | 14.44 ± 6.00 | 1.31 | 9.98 | <0.001 |
| Leptin (ng/ml)a | 38.2 (21.6, 57.6) | 36.8 (20.3, 64.8) | 0.24 | 0.51 | 0.95 |
| tPA (ng/ml) | 7.86 ± 2.72 | 8.69 ± 3.29 | 0.83 | 10.6 | 0.02 |
Data are presented as mean ± sd.
Data are presented as median (interquartile range).
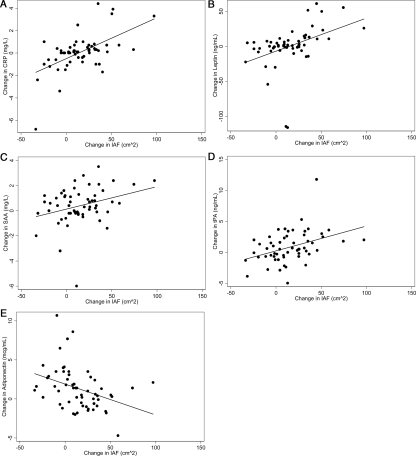
Correlation coefficients and P values between the change in IAF, SQAF, and total percent body fat and change in inflammatory markers were calculated (Table 3). Although there was not a statistically significant increase in CRP or leptin across menopause, the change in these two measures showed a significant positive correlation with the change in IAF (r = 0.56, r = 0.41, respectively) (Fig. 1, A and B). There was also a significant positive correlation between the change in IAF and the change in SAA and tPA (r = 0.31 and r = 0.40, respectively) (Fig. 1, C and D). Whereas adiponectin increased across the menopausal transition, there was a significant negative correlation between the change in adiponectin and the change in IAF (r = −0.37) (Fig. 1E). Whereas the change in IAF was significantly associated with changes in CRP, leptin, SAA, tPA, and adiponectin, the change in SQAF and total percent body fat correlated only with changes in CRP, leptin, and SAA.
Table 3.
Pearson’s correlation coefficient between change in measures of adiposity and change in measures of inflammation
| ΔIL-6 | ΔTNF-α | ΔMCP-1 | ΔSAA | ΔCRP | ΔtPA | ΔLeptin | Δadiponectin | |
|---|---|---|---|---|---|---|---|---|
| ΔIAF | −0.065 (0.65) | 0.15 (0.28) | −0.04 (0.78) | 0.31 (0.02) | 0.56 (<0.001) | 0.40 (0.002) | 0.41 (0.002) | −0.37 (0.005) |
| ΔSQAF | 0.05 (0.75) | 0.02 (0.90) | 0.20 (0.15) | 0.33 (0.02) | 0.29 (0.04) | 0.26 (0.06) | 0.35 (0.01) | −0.04 (0.80) |
| Δ %TBF | −0.02 (0.88) | 0.02 (0.87) | 0.12 (0.39) | 0.51 (<0.001) | 0.34 (0.01) | 0.16 (0.26) | 0.43 (0.002) | −0.12 (0.42) |
Data are presented with correlation coefficient (P value). %TBF, Percentage total body fat.
Figure 1.
A, Linear regression of ΔIAF (square centimeters) with ΔCRP (milligrams per liter; r = 0.56, P < 0.001). B, Linear regression of ΔIAF (square centimeters) with Δleptin (nanograms per milliliter; r = 0.41, P = 0.002). C, Linear regression of ΔIAF (square centimeters) with ΔSAA (milligrams per liter; r = 0.31, P = 0.02). D, Linear regression of ΔIAF (square centimeters) with ΔtPA (nanograms per milliliter; r = 0.40, P = 0.002). E, Linear regression of ΔIAF (square centimeters) with ΔAdiponectin (micrograms per milliliter; r = −0.37, P = 0.005).
Univariate regression analysis for the change in IAF as the dependent variable and change in CRP, leptin, adiponectin, SAA, and tPA was performed, and these results are shown using β-coefficients with confidence intervals (Table 4). After adjusting for the change in SQAF, the change in IAF remained significantly correlated with the change in CRP, leptin, tPA, and adiponectin but not with change in SAA. Results were unchanged when multivariate regression adjusted for changes in SQAF, time, and baseline age (results not shown). Similarly, univariate and multivariate regression analyses were performed for the change in SQAF and the change in CRP, leptin, adiponectin, SAA, and tPA (Table 4). The absolute value of the β-coefficients from univariate regression of the change in IAF with change in CRP, SAA, tPA, leptin, and adiponectin was greater than those from the univariate regression of the change in SQAF with those measures (0.36, 0.018, 0.04, 0.472, and −0.04 compared with 0.008, 0.008, 0.01, 0.157, and −0.003, respectively), suggesting that the relationship between inflammatory markers and adipokines was stronger with the visceral fat than sc fat depot. Furthermore, after adjustment for IAF, the correlation between change in SQAF and change in CRP, tPA, and adiponectin lost significance. The change in SAA and leptin remained positively correlated with the change in SQAF, even after adjustment for IAF, implying an independent contribution of SQAF accumulation to SAA and leptin levels. Results were unchanged when multivariate regression adjusted for changes in IAF, time, and baseline age (results not shown). As mentioned in our description of study subjects, women were recruited by two different methods, resulting in a baseline difference in age; however, adjustment for enrollment status in the multivariate regression analysis did not lead to changes in our results (results not shown).
Table 4.
Univariate and multivariate regression analysis between independent variables, ΔIAF, and ΔSQAF with dependent variables, ΔCRP, ΔSAA, ΔtPA, Δleptin, and Δadiponectin
| Independent variable | ΔCRP | ΔSAA | ΔtPA | Δleptin | Δadiponectin |
|---|---|---|---|---|---|
| ΔIAF | 0.036 (0.022, 0.051) | 0.018 (0.003, 0.033) | 0.04 (0.015, 0.065) | 0.472 (0.189, 0.855) | −0.040 (−0.066, −0.068) |
| ΔIAF multivariatea | 0.033 (0.019, 0.048) | 0.014 (−0.001, 0.028) | 0.035 (0.010, 0.060) | 0.391 (0.107, 0.675) | −0.040 (−0.068, −0.012) |
| ΔSQAF | 0.008 (0.002, 0.014) | 0.008 (0.002, 0.013) | 0.01 (0.001, 0.021) | 0.157 (0.047, 0.267) | −0.003 (−0.14, 0.008) |
| ΔSQAF multivariateb | 0.004 (−0.001, 0.010) | 0.006 (0.0004, 0.112) | 0.007 (−0.003, 0.017) | 0.118 (0.009, 0.226) | 0.001 (−0.010, 0.012) |
Data are presented as β-coefficients (95% confidence intervals).
Adjusted for ΔSQAF.
Adjusted for ΔIAF.
Discussion
Our data show that women gain predominantly IAF during the menopausal transition. Concurrently, SAA, tPA, MCP-1, and adiponectin levels increased significantly. The increase in IAF was positively correlated with changes in leptin, tPA, SAA, and CRP levels and negatively correlated with the change in adiponectin. These findings suggest that the increase in visceral adiposity across the menopausal transition results in adverse effects on adipokine and inflammation levels.
Our data do corroborate existing research showing higher IAF in postmenopausal women compared with premenopausal women (1,2). In addition to the increased visceral and sc abdominal fat, we saw small but significant increases in weight and BMI across the menopausal transition. Our results are also comparable with the changes in weight and BMI documented in prior longitudinal studies on women followed up across the menopausal transition (18,19). In contrast to the increase in total percent body fat by DEXA and bioelectrical impedance analysis demonstrated by these studies, we did not see an increase in total percent body fat by DEXA. The difference may be attributed to the longer duration of follow-up (8–9 yr) and chronological aging in comparison with the average follow-up time of 4.1 yr in our study. Whereas the current participants did not have increased total body fat, the increase in truncal fat we observed in the setting of no overall increase in body fat implies that there may have been an offsetting loss of fat in the extremities. The IAF (by CT scan) data confirm this increase in truncal fat. Whereas CT images demonstrated an increase in SQAF area, the women had a proportionally greater increase in visceral adiposity. This predominant increase in the visceral fat depot highlights the concern for the development of associated adipokine changes leading to an adverse metabolic profile in postmenopausal women.
Given that transcription of IL-6, TNF-α, PAI-1, and MCP-1 is increased in visceral adipose tissue of obese individuals, we measured these adipokines in this cohort of women who gained visceral adiposity across the menopausal transition (7,8,9,10). tPA was chosen as a surrogate for PAI-1 because this protein is less subject to fluctuations in secretion and clearance, and its levels tightly correlate with PAI-1 activity (20). SAA and CRP, markers of inflammation that correlate with visceral adiposity, were also measured. In this study, CRP, IL-6, and TNF-α did not change across the menopausal transition; however, SAA MCP-1, and tPA increased significantly. Whereas we expected an increase in IL-6 and TNF-α across the menopausal transition, there was no significant change in or correlation of these adipokines with the change in IAF. It is possible that IL-6 and TNF-α have paracrine effects and have undetectable changes in the systemic circulation. Our results differ from cross-sectional data reporting a difference in IL-1β, IL-6, IL-10, IL-18, TNF-α, and TNF-β in postmenopausal women compared with premenopausal women (21,22,23). However, cross-sectional studies may have detected significant differences in cytokines due to marked age gaps between cohorts (12–18 yr) or other comorbidities rather than due to the effects of menopause (21,23). This possibility is supported by the disappearance of a significant difference in IL-6 with age adjustment in one study (22). In contrast, our study of the same women prospectively is not subject to these differing comorbidities and large age gaps between two separate groups. Whereas CRP did not change significantly across the menopausal transition, we found that changes in its levels did correlate specifically with IAF.
tPA also demonstrated a similar relationship with the change in IAF. This is comparable with existing evidence that tPA increases across the menopausal transition (24) and further demonstrates that its increase may be related to the increase in IAF change. In contrast to tPA and CRP, the change in SAA appears to be independently associated with SQAF. This finding is supported by evidence that SAA expression is higher in sc white adipose tissue than visceral adipose tissue (25).
This association and its clinical implications are areas for further investigation. In contrast SAA, CRP, and tPA, the change in MCP-1 did not correlate with the change in IAF or SQAF; thus, its increase may be due to other effects of menopause and its source from other tissues. Whereas there has been evidence for differential expression of MCP-1 in visceral adipose tissue of obese vs. lean individuals, there are conflicting data on the correlation of systemic levels of MCP-1 with measures of adiposity (7). Our study parallels the lack of association between MCP-1 and adiposity found in other studies (26,27). Nevertheless, higher levels of MCP-1 relate to increased coronary artery calcification and cardiovascular mortality (27,28). Therefore, the increase in MCP-1 across the menopausal transition may reflect subclinical atherosclerosis and an increase in cardiovascular risk.
Other adipokines evaluated in this study included leptin and adiponectin. Smaller prospective paired studies found increased leptin in women transitioning to postmenopause (29,30). We expected an increase in leptin in conjunction with an increase in fat mass over the menopausal transition. Instead, we found no change in leptin. Other studies show that leptin is well correlated with measures of body fat (31). Given that we had no change in total percent body fat by DEXA, it is not surprising that leptin did not change across the menopausal transition. In univariate regression analysis, the change in leptin was positively correlated with both changes in SQAF and IAF separately. Leptin is produced by all adipocytes, and our results show that individuals with an increase in both SQAF and IAF also had an increase in leptin.
In contrast to leptin, we expected a decrease in adiponectin in conjunction with increased IAF over the menopausal transition. Adiponectin levels were not different in pre- and postmenopause in other studies (29,30). Surprisingly, we found a significant increase in adiponectin across the menopausal transition. The change in adiponectin was negatively correlated with IAF change; therefore, women who gained IAF appeared to develop an adverse metabolic profile in the transition to postmenopause. Decreases in adiponectin levels across the menopausal transition have been associated with increases in systolic blood pressure, insulin levels, and homeostasis model assessment for insulin resistance index and decreases in high-density lipoprotein cholesterol (30). In our data, the change in adiponectin was not associated with SQAF and appeared to be specific for IAF. This is supported by an ex vivo study that found 3-fold higher adiponectin secretion from visceral adipocytes in comparison with sc adipocytes (32). Whereas the negative correlation between adiponectin and IAF has been shown, prior studies have also found an independent positive correlation between adiponectin and age (33). Our data are also consistent with this increase in adiponectin level, potentially due to age. However, the strong negative correlation between adiponectin change and IAF change across the menopausal transition was also demonstrated by this study. Therefore, women who gained IAF had a decline in adiponectin levels.
To our knowledge, this is a unique study, given the paucity of prospective studies across the menopausal transition. It is challenging to study the menopausal transition given the wide variability in the age of onset and duration of the menopausal transition stages. In this study, the definition of premenopausal was presence of one menstrual period in the preceding 3 months, but defining premenopausal as no change in menstrual cycles in the preceding 12 months may be more specific (34). It is likely that women were in either the late reproductive or early menopausal transition stages at time of enrollment using our definition. Given this scenario, the current significant findings may have been even more pronounced if our participants had been studied at an earlier time point. Another challenge in conducting a prospective study across the menopausal transition is the inability to acquire age-matched controls to distinguish between the effects of age vs. menopause on our measurements. For our study population, even women of the same age who did not fully undergo menopause were still having hormonal changes and were not suitable controls. Without age-matched controls, we cannot fully attribute the changes to menopause. However, studies in postmenopausal women have shown a reversal in some of these adipocytokines with repletion of estrogen (35,36). In our study, no recommendations were made to the women regarding diet or exercise. The women may have adopted healthier lifestyles as participants in a clinical study. Despite this possibility, we still detected significant increases in weight, BMI, IAF, and SQAF. The measurements of IAF in this study included the retroperitoneal fat depot. It is not known whether this depot is more analogous to ip fat or sc fat with regard to cytokine and adipokine transcription and secretion. Whereas significant relationships between changes in adipokines and inflammatory markers were found with the change in IAF, these associations may have been attenuated by inclusion of the retroperitoneal depot if retroperitoneal fat is more similar to sc fat.
In conclusion, women may develop an adverse adipokine and inflammatory profile during the menopausal transition as a consequence of increased central adiposity. The increased IAF seen across the menopausal transition was associated with decreased adiponectin levels and increased levels of leptin, tPA, SAA, and CRP. After adjustment for change in SQAF, the changes in adiponectin, leptin, tPA, and CRP remained significantly correlated with the IAF changes, and we believe that this implies a greater contribution of the visceral fat depot on production of adipocytokines. However, definite conclusions regarding causal associations cannot be made based on these observational data. Previous studies have shown increased CRP and tPA are associated with increased risk for the development of coronary heart disease and diabetes mellitus in postmenopausal women (14,15,16), and these inflammatory changes may explain the increase in cardiovascular disease events after menopause. Larger studies are needed to determine whether these adipokines correlate with development of cardiovascular disease and insulin resistance in postmenopausal women. Our data suggest that the accumulation of IAF across the menopausal transition results in adverse alterations in adipokines and markers of inflammation.
Acknowledgments
The authors thank Linda Floyd for her assistance with participant recruitment and management and Steve Hashimoto and Alegria Aquino-Albers for their technical expertise. We are indebted to the participants for their dedication and contribution to this study. Laboratory assays performed at the University of Washington were processed by Dr. Wayne Chandler and Dr. Mark Wener (Department of Laboratory Medicine) and the Clinical Nutrition Research Unit Core Laboratory. Adiponectin assays were performed in the laboratories of Dr. Peter J. Havel, D.V.M., Ph.D. (University of California, Davis, Department of Nutrition).
Footnotes
This work was supported by National Institutes of Health Grants HL30086, HL64322, and NR04141, and K23 Grant RR16067 (to M.C.C.) and a grant from the Bristol Myers Squibb Foundation. These studies were performed with the support of the University of Washington Clinical Nutrition Research Unit (DK35816), the University of Washington General Clinical Research Center (M01-RR-00037), and the University of Washington Diabetes Endocrinology Research Center, which is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-17047.
Present address for M.C.C.: Wyeth Research, Collegeville, Pennsylvania.
Disclosure Summary: C.G.L., S.J.M., E.M., N.F.W., M.H.W., W.L.C., E.J.B., and J.D.B. have nothing to declare. M.C.C. worked on this study while employed at the University of Washington Medical Center.
First Published Online January 6, 2009
Abbreviations: BMI, Body mass index; CRP, C-reactive protein; CT, computed tomography; CV, coefficient of variation; DEXA, dual x-ray absorptiometry; IAF, intraabdominal fat; MCP, monocyte-chemotactic protein; PAI, plasminogen activator inhibitor; SAA, serum amyloid A; SQAF, sc abdominal fat; tPA, tissue plasminogen activator antigen.
References
- Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA, Weinstock RS 2001 Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age, and menopausal status. Metabolism 50:976–982 [DOI] [PubMed] [Google Scholar]
- Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rheaume C, Dupont P 2004 Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab 89:3425–3430 [DOI] [PubMed] [Google Scholar]
- Gohlke-Barwolf C 2000 Coronary artery disease—is menopause a risk factor? Basic Res Cardiol 95(Suppl 1):I77–183 [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR 2006 Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE 2005 Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949 [DOI] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Andersson CX, Smith U 2007 Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27:2276–2283 [DOI] [PubMed] [Google Scholar]
- Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A 2007 Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92:2240–2247 [DOI] [PubMed] [Google Scholar]
- He G, Pedersen SB, Bruun JM, Lihn AS, Jensen PF, Richelsen B 2003 Differences in plasminogen activator inhibitor 1 in subcutaneous versus omental adipose tissue in non-obese and obese subjects. Horm Metab Res 35:178–182 [DOI] [PubMed] [Google Scholar]
- Winkler G, Kiss S, Keszthelyi L, Sapi Z, Ory I, Salamon F, Kovacs M, Vargha P, Szekeres O, Speer G, Karadi I, Sikter M, Kaszas E, Dworak O, Gero G, Cseh K 2003 Expression of tumor necrosis factor (TNF)-α protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-α, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol 149:129–135 [DOI] [PubMed] [Google Scholar]
- Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM 2007 Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab 293:E656–E665 [DOI] [PubMed] [Google Scholar]
- Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW 2006 Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 3:e287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S 2007 The correlation between adiposity and adiponectin, tumor necrosis factor α, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest 30:210–214 [DOI] [PubMed] [Google Scholar]
- Carr MC 2003 The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, LaCroix AZ, Langer RD, Trevisan M, Lewis CE, Hsia JA, Oberman A, Kotchen JM, Ridker PM 2004 Tissue plasminogen activator antigen and D-dimer as markers for atherothrombotic risk among healthy postmenopausal women. Circulation 110:292–300 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM 2001 C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM 2002 Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. JAMA 288:980–987 [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES 1996 Patterns of depressed mood in midlife women; observations from the Seattle Midlife Women’s Health Study. Res Nurs Health 19:111–123 [DOI] [PubMed] [Google Scholar]
- Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG 2004 The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric 7:375–389 [DOI] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, Yosef M, Symons J 2007 Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab 92:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I 1997 Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation 96:761–768 [DOI] [PubMed] [Google Scholar]
- Cioffi M, Esposito K, Vietri MT, Gazzerro P, D'Auria A, Ardovino I, Puca GA, Molinari AM 2002 Cytokine pattern in postmenopause. Maturitas 41:187–192 [DOI] [PubMed] [Google Scholar]
- Giuliani N, Sansoni P, Girasole G, Vescovini R, Passeri G, Passeri M, Pedrazzoni M 2001 Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol 36:547–557 [DOI] [PubMed] [Google Scholar]
- Vural P, Akgul C, Canbaz M 2006 Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res 54:298–302 [DOI] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, Sowers MF 2007 Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom Med 69:124–130 [DOI] [PubMed] [Google Scholar]
- Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, Gouillon M, Bouillot JL, Oppert JM, Basdevant A, Clement K 2006 Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 14:309–318 [DOI] [PubMed] [Google Scholar]
- Herder C, Muller-Scholze S, Rating P, Koenig W, Thorand B, Haastert B, Holle R, Illig T, Rathmann W, Seissler J, Wichmann HE, Kolb H 2006 Systemic monocyte chemoattractant protein-1 concentrations are independent of type 2 diabetes or parameters of obesity: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Eur J Endocrinol 154:311–317 [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E 2003 Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 107:690–695 [DOI] [PubMed] [Google Scholar]
- Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA 2004 Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol 44:1812–1818 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Wildman RP, Mancuso P, Eyvazzadeh AD, Karvonen-Gutierrez CA, Rillamas-Sun E, Jannausch ML 2008 Change in adipocytokines and ghrelin with menopause. Maturitas 59:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman RP, Mancuso P, Wang C, Kim M, Scherer PE, Sowers MR 2008 Adipocytokine and ghrelin levels in relation to cardiovascular disease risk factors in women at midlife: longitudinal associations. Int J Obes (Lond) 32:740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua A, Hennes MI, Hoffmann RG, Maas DL, Krakower GR, Sonnenberg GE, Kissebah AH 1996 Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes 45:1635–1637 [DOI] [PubMed] [Google Scholar]
- Perrini S, Laviola L, Cignarelli A, Melchiorre M, De Stefano F, Caccioppoli C, Natalicchio A, Orlando MR, Garruti G, De Fazio M, Catalano G, Memeo V, Giorgino R, Giorgino F 2008 Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia 51:155–164 [DOI] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE 2003 Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469 [DOI] [PubMed] [Google Scholar]
- Cooper GS, Baird DD 1995 The use of questionnaire data to classify peri- and premenopausal status. Epidemiology 6:625–628 [DOI] [PubMed] [Google Scholar]
- Žegura B, Gužič-Salobir B, Šebeštjen M, Keber I 2006 The effect of various menopausal hormone therapies on markers of inflammation, coagulation, fibrinolysis, lipids, and lipoproteins in healthy postmenopausal women. Menopause 13:643–650 [DOI] [PubMed] [Google Scholar]
- Mueck OA, Genazzani AR, Samsioe G, Vukovic-Wysocki I, Seeger H 2007 Low-dose continuous combinations of hormone therapy and biochemical surrogate markers for vascular tone and inflammation: transdermal versus oral application. Menopause 14:978–984 [DOI] [PubMed] [Google Scholar]