Abstract
Context: Vitamin D deficiency is not adequately evaluated in older men.
Objective: The aim of the study was to determine the prevalence of vitamin D deficiency and identify risk factors for its occurrence.
Design and Setting: We conducted a cross-sectional evaluation of 1606 older men in the general community who were enrolled in the Osteoporotic Fractures in Men Study.
Participants: A randomly selected subcohort of a large population of men from six U.S. communities participated in the study.
Main Outcome Measures: Serum concentrations of 25-hydroxyvitamin D2 [25(OH)D2] and 25(OH)D3 were measured using mass spectrometry.
Results: Deficiency [25(OH)D <20 ng/ml] was present in 26%, and insufficiency (<30 ng/ml) was present in 72%. Deficiency was particularly common among men during the winter and spring (especially in the northern communities) and in the oldest and more obese men. For instance, in Caucasian men in winter or spring who were >80 yr old, did not engage in lawn/garden work, and had a body mass index greater than 25 kg/m2 and vitamin D intake below 400 IU/d, the prevalence of vitamin D deficiency was 86%. 25(OH)D2 levels were present in a small fraction of men and accounted for a low proportion of total 25(OH)D levels. The use of vitamin D supplements was reported by 58% of men, but supplement use had a small effect on total 25(OH)D levels and, despite supplement use, low levels remained frequent.
Conclusions: Vitamin D deficiency is common in older men and is especially prevalent in obese, sedentary men living at higher latitudes. Use of vitamin D supplements at levels reported here did not result in adequate vitamin D nutrition.
Vitamin D deficiency is highly prevalent in older men in the United States, despite the common use of vitamin D supplements.
Vitamin D is necessary for a wide variety of essential biological functions such as bone and mineral metabolism, muscle function, and immunity (1). Moreover, vitamin D insufficiency has been noted to be present in a variety of populations (2) and is implicated in the causation of common disorders including osteoporosis and fractures, falls, cancer, psoriasis, and others (3). Vitamin D deficiency is of particular interest because it is easily, safely, and inexpensively corrected with adequate supplementation. Although findings have been somewhat inconsistent, trials of the effects of vitamin D supplementation suggest beneficial effects on important clinical outcomes (2,4,5). As a result, increased attention has been directed at improving vitamin D nutrition, including recent recommendations to increase the routine intake of vitamin D (6).
Complicating the assessment of vitamin D status are concerns about measurement technique. Immunoassay and protein binding based methods have yielded discrepant results (7), and some of these assays may yield spurious levels when compared with liquid chromatography/mass spectrometry (8,9,10).
Despite concerns about vitamin D adequacy in the elderly, there remain too few large, community-based studies that use accurate and precise 25-hydroxyvitamin D [25(OH)D] assays to determine the extent of deficiency and identify those at risk. For instance, there are inadequate data about vitamin D levels in older men, a group commonly affected by disorders linked to vitamin D insufficiency (e.g. falls, fractures, cancer). To understand more completely the vitamin D status in a large population of elderly men and identify factors with potential for clinical intervention, we measured levels of 25(OH)D2 (a sterol produced in yeast and obtained primarily from nutritional supplements and supplemented foods) and 25(OH)D3 (obtained from nutritional supplements, supplemented foods, food, and UV irradiation of the skin).
Subjects and Methods
Study participants
Participants were enrolled in the Osteoporotic Fractures in Men Study (MrOS). From March 2000 through April 2002, a total of 5995 community-dwelling men at six clinical centers in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA) agreed to participate in a study of healthy aging, with a focus on osteoporosis and fractures. Eligible men were at least 65 yr old, without bilateral hip replacements, and able to walk without assistance from another person. Details of the MrOS design and cohort have been described (11,12). The Institutional Review Board at each center approved the study, and written consent was obtained from all participants.
Baseline serum for 25(OH)D assays were from 1608 participants who were randomly chosen from 5908 participants in the cohort who had at least one vial of serum available. One participant had insufficient serum to complete the assays. Another man had 25(OH)D levels more than 3 sd above the mean (75.6 ng/ml) and was omitted for analyses, leaving results from 1606 participants.
In addition, assays were performed on a second serum sample from men who volunteered for a follow-up visit an average of 1.9 yr after baseline. The results from those men who were also in the random sample selected at baseline (n = 352) were used to examine the stability of 25(OH)D levels over time.
25(OH)D assays
Fasting morning blood was collected, and serum was prepared immediately after phlebotomy and stored at −70 C in vials foil-wrapped to prevent light exposure. Samples remained frozen until assay. Measures for 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described (13). Deuterated stable isotope [d3-25(OH)D] was added to a 0.2-ml serum sample as internal standard. 25(OH)D2, 25(OH)D3, and the internal standard were extracted using acetonitrile precipitation. The extracts were further purified online and analyzed by liquid chromatography/tandem mass spectrometry using multiple reaction monitoring. 25(OH)D2 and 25(OH)D3 were reported individually. The minimum detectable limit was 4 ng/ml for 25(OH)D2 and 2 ng/ml for 25(OH)D3. Aliquots of a single serum pool were included in alternate assay runs. Using the pooled serum, the interassay coefficient of variation for 25(OH)D3 was 4.4%, and the intraassay coefficient of variation was 4.9%. In the study of stability of 25(OH)D levels over time, baseline and follow-up samples were run sequentially within an assay run, and the order of the pair in the run was randomly determined.
Total 25(OH)D was the sum of 25(OH)D3 and 25(OH)D2 levels. Baseline total 25(OH)D levels were categorized as normal, insufficient, or deficient according to the definitions proposed by Holick (3). Deficiency was defined as less than 20 ng/ml, insufficiency as 20–29 ng/ml, and sufficiency as 30–149 ng/ml. No participant had “toxic” levels (>150 ng/ml). We also report the proportion of participants with levels below 10 ng/ml.
Other measures
Demographic factors, smoking, alcohol consumption, and medications were determined by questionnaire. Physical activity was assessed with the Physical Activity Score for the Elderly (PASE) (14). In PASE, walking for exercise was estimated from the questions “Over the past 7 d, how often did you take a walk outside your home or yard for any reason? For example, for fun or exercise, walking to work, walking the dog, etc.?” During the clinic interview, an additional question was asked: “Do you take walks for exercise, daily or almost every day?” Outdoor activity was determined from the question “During the past 7 d, did you engage in any of the following activities: lawn work or yard care, including snow or leaf removal, wood chopping, etc.? Outdoor gardening?” A modified Block food frequency questionnaire was administered to assess usual dietary and supplement intake over the past year (Block Dietary Data Services, Berkeley, CA) (15). Vitamin D intake from food and supplements was determined, but information did not include whether vitamin D supplements included D3 or D2; hence, only total 25(OH)D content was considered. Participants brought in all medications they used within the last 30 d. A computerized dictionary, based on the original Established Populations for Epidemiologic Studies of the Elderly (EPESE) coding system (16) was used to categorize the medications. Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA). Height (centimeters) was measured on Harpenden stadiometers, and weight (kilograms) was measured on standard balance beam or digital scales. Body mass index (BMI) was calculated as kilograms per meter squared.
Statistical analyses
The distributions of 25(OH)D, 25(OH)D3, and detectable 25(OH)D2 levels were approximately normal. However, because the majority of 25(OH)D2 results were undetectable, the variable was examined both as dichotomous (detectable or not) and as continuous if in the detectable range (>4 ng/ml), and both results are reported. Analyses using 25(OH)D2 as a continuous variable used only participants with detectable levels. Pearson correlations were calculated for each 25(OH)D measure and continuous variables collected at baseline, including age, body size measures, and reported intake of vitamin D and calcium from diet and supplements. Means and sd values of baseline total 25(OH)D, 25(OH)D2, and 25(OH)D3 were calculated by participant characteristics, including demographic and physical activity variables, collected at the baseline exam. ANOVA and t tests were used to compare means, and χ2 tests were used to compare proportions.
Mean 25(OH)D concentrations were evaluated by season and clinic site. Season of baseline visit was coded as winter (January-March), spring (April-June), summer (July-September), and fall (October-December). Clinic sites also differed in their climate and latitude, which may affect participants’ exposure to sunlight; therefore, 25(OH)D levels were evaluated by site. Absolute change in 25(OH)D levels between baseline and a subsequent visit (1.9 ± 0.4 yr later) were calculated for 352 participants who had baseline and follow-up measures, and these levels were normalized for season of collection. Seasonal normalization was accomplished as follows. A five-category “season change” variable was created to describe whether a participant’s follow-up visit was: 1) in the same season as baseline; 2) in a “lighter” season than baseline (e.g. baseline visit was in winter and follow-up was in spring); 3) in a “much lighter” season (e.g. baseline visit was in winter and follow-up was in summer), and so on for “dark” and “much darker” season changes. Mean differences between baseline and follow-up in 25(OH)D and 25(OH)D3 were calculated for participants in each of the five categories of the season change variable. The mean differences were added to each follow-up value. For example, participants whose follow-up visit was in a much darker season had an average 5.55 ng/ml lower 25(OH)D3 value at follow-up than at baseline. Therefore, 5.55 ng/ml was added to each participant’s 25(OH)D3 value in that season change category. Values in other season-change categories were adjusted similarly.
Factors associated with vitamin D deficiency were identified using log-binomial models (PROC GENMOD). This method provides better estimates of prevalence ratios than does logistic regression for common outcomes, such as vitamin D deficiency (17). That is, logistic regression would be expected to overestimate strengths of association reported in this study (18). Prevalence ratio estimates for deficiency were calculated for each of several baseline characteristics alone and after adjustment for age, season, and race. Upon this basic model adjusted for these three variables, a multivariable model was constructed using each of the variables associated with vitamin D deficiency with a P < 0.10 in bivariate analysis. A likelihood ratio test was used to determine whether model fit improved significantly (P < 0.05) with the addition of new variables. Additional variables were added to the multivariate model in the order of lowest to highest P value (among those with P < 0.05) until no additional variable significantly improved the model fit as assessed by the likelihood ratio test.
All analyses were conducted using SAS version 9.1.2 (SAS Institute, Cary, NC).
Results
Characteristics of study participants are shown in Table 1. The average age was 73.8 ± 5.9 yr, with a range of 65–99. Most were Caucasian; 10.3% were from other racial and ethnic groups. The average serum total 25(OH)D level was 25.1 ± 7.9 ng/ml (range, 3.1–58.3 ng/ml). A total of 71.1% had 25(OH)D levels below 30 ng/ml, 25.7% had levels below 20 ng/ml, and 2.9% had levels below 10 ng/ml.
Table 1.
Baseline characteristics of participants (n = 1606)
| No. of participants (%) or mean ± sd | |
|---|---|
| Age category (yr) | |
| 65–69 | 474 (29.5) |
| 70–74 | 436 (27.2) |
| 75–79 | 408 (25.4) |
| 80–84 | 213 (13.3) |
| >85 | 75 (4.7) |
| BMI category (kg/m2) | |
| <25 | 424 (26.4) |
| 25–29 (overweight) | 858 (53.4) |
| 30–34 (obese) | 264 (16.4) |
| >35 | 60 (3.7) |
| Race | |
| White | 1441 (89.7) |
| Black | 54 (3.4) |
| Asian | 51 (3.2) |
| Hispanic | 42 (2.6) |
| Other | 18 (1.1) |
| Education | |
| Graduate school | 566 (35.2) |
| College | 642 (40.0) |
| High school | 288 (17.9) |
| <High school | 110 (6.9) |
| Season | |
| Winter (Jan-Mar) | 320 (19.9) |
| Spring (Apr-Jun) | 418 (26.0) |
| Summer (Jul-Sep) | 461 (28.7) |
| Fall (Oct-Dec) | 407 (25.3) |
| Site | |
| Birmingham | 258 (16.1) |
| Minneapolis | 239 (14.9) |
| Palo Alto | 278 (17.3) |
| Pittsburgh | 280 (17.4) |
| Portland | 276 (17.2) |
| San Diego | 275 (17.1) |
| Daily intake of vitamin D from diet (IU) | |
| <600 | 1574 (99.4) |
| >600 | 10 (0.6) |
| Daily intake of vitamin D from supplements (IU)a | |
| 0–199 | 730 (46.1) |
| 200–399 | 104 (6.6) |
| 400–599 | 644 (40.7) |
| 600 | 106 (6.7) |
| Daily intake of calcium from diet and supplements (mg) | 1153.1 ± 594.3 |
| Measures in serum | |
| Total 25(OH)D (ng/ml) | 25.1 ± 7.9 |
| 25(OH)D3 (ng/ml) | 22.9 ± 8.4 |
| 25(OH)D2, if detectable (ng/ml) | 8.4 ± 3.3 |
| 25(OH)D2 detectable, n (%) | 420 (26.2) |
No men reported the use of more than 600 IU/d.
A total of 352 men had measures of 25(OH)D in serum obtained on two occasions 1.9 ± 0.4 yr apart. After adjusting for season, there were no statistically significant differences in the means of the two measurements, and differences between measures in individuals were generally small. The partial correlation coefficient for baseline and second visit total 25(OH)D, adjusted for season change, was 0.72 (P < 0.0001). All the 151 men with two measures who were classified as deficient at baseline were also deficient at the second visit.
Almost all circulating 25(OH)D was derived from vitamin D3; only 26% of subjects had detectable levels of 25(OH)D2, and mean levels were low (8.4 ± 3.3 ng/ml) in those in whom it was detectable. The level of total 25(OH)D was weakly associated with the amount of vitamin D reported from supplement use (r = 0.28; P < 0.0001). Those who reported supplement use had slightly higher total 25(OH)D levels and more often had detectable levels of 25(OH)D2 (43.6% vs. 1.5%).
Age, obesity, and race
Total 25(OH)D levels tended to be lower in older men (r = −0.09; P = 0.0002) (Table 2). In men aged 80–84 yr, 31% had levels below 20 ng/ml, and in men at least 85 yr old, 40% were deficient (Fig. 1A). Levels of 25(OH)D3 were lower as age increased (r = −0.10; P < 0.0001), but older men tended to have slightly higher 25(OH)D2 levels (r = 0.11; P = 0.03).
Table 2.
25(OH)D concentrations by participant characteristics (n = 1606)
| Total 25(OH)D (ng/ml) | P | 25(OH)D3 (ng/ml) | P | |
|---|---|---|---|---|
| Age category (yr) | ||||
| 65–69 | 25.7 ± 8.3 | 23.8 ± 8.6 | ||
| 70–74 | 25.6 ± 8.2 | 23.4 ± 8.7 | ||
| 75–79 | 25.0 ± 7.2 | 22.4 ± 7.7 | ||
| 80–84 | 23.5 ± 7.8 | 21.3 ± 8.0 | ||
| >85 | 23.0 ± 7.2 | 0.001 | 20.9 ± 8.0 | 0.0004 |
| BMI category (kg/m2) | ||||
| <25 | 26.0 ± 8.0 | 23.4 ± 8.7 | ||
| 25–29 (overweight) | 25.3 ± 7.9 | 23.1 ± 8.3 | ||
| 30–34 (obese) | 24.0 ± 7.6 | 22.2 ± 8.1 | ||
| >35 | 20.2 ± 7.5 | <0.0001 | 18.7 ± 7.3 | 0.0003 |
| Race | ||||
| White | 25.5 ± 7.8 | 23.3 ± 8.2 | ||
| Black | 18.5 ± 8.8 | 15.8 ± 8.3 | ||
| Asian | 24.3 ± 6.7 | 21.7 ± 7.7 | ||
| Hispanic | 20.9 ± 7.7 | 19.3 ± 8.5 | ||
| Other | 24.4 ± 8.6 | <0.0001 | 23.8 ± 9.2 | <0.0001 |
| Education | ||||
| Graduate school | 25.6 ± 8.0 | 22.9 ± 8.5 | ||
| College | 25.1 ± 7.9 | 23.0 ± 8.4 | ||
| High school | 24.8 ± 7.5 | 23.2 ± 8.0 | ||
| <High school | 22.9 ± 8.3 | 0.01 | 21.2 ± 8.2 | 0.2 |
| Season | ||||
| Winter (Jan-Mar) | 22.2 ± 7.7 | 20.1 ± 8.0 | ||
| Spring (Apr-Jun) | 24.3 ± 7.4 | 21.7 ± 7.6 | ||
| Summer (Jul-Sep) | 27.5 ± 7.8 | 25.6 ± 8.4 | ||
| Fall (Oct-Dec) | 25.3 ± 7.9 | <0.0001 | 23.2 ± 8.4 | <0.0001 |
| Site | ||||
| Birmingham | 24.5 ± 7.5 | 22.9 ± 7.7 | ||
| Minneapolis | 23.9 ± 7.1 | 22.0 ± 7.5 | ||
| Palo Alto | 25.4 ± 7.6 | 22.9 ± 8.5 | ||
| Pittsburgh | 24.2 ± 7.3 | 22.2 ± 7.5 | ||
| Portland | 24.3 ± 8.7 | 21.9 ± 8.9 | ||
| San Diego | 28.0 ± 8.5 | <0.0001 | 25.3 ± 9.3 | <0.0001 |
| Alcoholic drinks per week | ||||
| 0 | 24.2 ± 7.8 | 22.3 ± 8.2 | ||
| 1–7 | 25.3 ± 7.4 | 22.9 ± 8.0 | ||
| >7 | 25.9 ± 8.8 | 0.005 | 23.6 ± 9.0 | 0.04 |
| Smoking status | ||||
| Never | 25.2 ± 7.7 | 23.0 ± 8.3 | ||
| Past | 23.0 ± 8.0 | 22.9 ± 8.4 | ||
| Current | 22.6 ± 8.9 | 0.05 | 21.4 ± 9.1 | 0.4 |
| Daily intake of vitamin D from diet and supplements (IU) | ||||
| <400 | 22.9 ± 8.1 | 22.6 ± 8.1 | ||
| ≥400 | 27.3 ± 7.2 | <0.0001 | 23.2 ± 8.6 | 0.2 |
| Daily intake of vitamin D from supplements (IU) | ||||
| 0–199 | 22.7 ± 8.0 | 22.5 ± 8.0 | ||
| 200–399 | 25.5 ± 7.6 | 24.3 ± 8.3 | ||
| 400–599 | 27.4 ± 7.2 | 22.8 ± 8.8 | ||
| 600 | 27.8 ± 7.3 | <0.0001 | 24.8 ± 8.1 | 0.02 |
| PASE score quartile | ||||
| 1 (lowest) | 23.6 ± 8.3 | 21.2 ± 8.5 | ||
| 2 | 25.2 ± 7.2 | 22.8 ± 8.0 | ||
| 3 | 25.4 ± 8.0 | 23.4 ± 8.2 | ||
| 4 | 26.1 ± 8.0 | <0.0001 | 24.2 ± 8.5 | <0.0001 |
| Walking | ||||
| Never | 23.5 ± 7.9 | 21.3 ± 8.0 | ||
| Rarely | 24.5 ± 8.3 | 22.2 ± 8.5 | ||
| Sometimes | 25.1 ± 7.4 | 23.0 ± 8.0 | ||
| Often | 25.7 ± 8.1 | 0.004 | 23.5 ± 8.5 | 0.007 |
| Daily walks for exercise | ||||
| No | 24.3 ± 7.8 | 22.3 ± 8.2 | ||
| Yes | 25.8 ± 8.0 | 0.0002 | 23.4 ± 8.5 | 0.009 |
| Lawn work and/or gardening | ||||
| No | 23.2 ± 8.2 | 20.3 ± 8.7 | ||
| Yes | 25.6 ± 7.8 | <0.0001 | 23.6 ± 8.1 | <0.0001 |
Data are expressed as mean ± sd. P values are for ANOVA or t test.
Figure 1.
A, Vitamin D deficiency and insufficiency by age group for three total 25(OH)D cut points. B, Vitamin D deficiency and insufficiency by BMI category for three total 25(OH)D cut points.
Obesity was also associated with lower total 25(OH)D levels (Table 2). Of those with BMI in the normal range (<25 kg/m2), 21% had 25(OH)D levels below 20 ng/ml; in overweight men (BMI = 25–29 kg/m2), 24.5% had vitamin D deficiency; and in obese men (BMI ≥ 30 kg/m2), 33.6% had vitamin D deficiency and 4.3% had levels below 10 ng/ml. Of those who were very obese (BMI ≥35 kg/m2), 53.3% were deficient (Fig. 1B).
Total 25(OH)D levels were lower in African-Americans (18.5 ± 8.8 ng/ml) than in Caucasians (25.5 ± 7.8 ng/ml). Whereas average 25(OH)D2 levels were similar (27.8% detectable, mean = 9.6 ng/ml, vs. 26.6% detectable, mean = 8.3 ng/ml, respectively), 25(OH)D3 levels were lower in African-Americans (15.8 vs. 23.3 ng/ml). A total of 64.8 and 22.2% of African-Americans had 25(OH)D levels below 20 and 10 ng/ml, respectively, whereas 23.2 and 1.8% of Caucasians were below those levels. Hispanics also had lower 25(OH)D levels (Table 2).
Geography and season
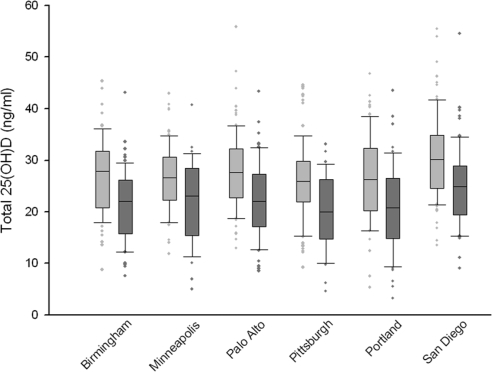
Geography and season did not have major effects on vitamin D levels. Slightly higher total 25(OH)D levels were present in San Diego and Palo Alto, whereas levels were slightly lower in Minneapolis. Moreover, mean total 25(OH)D levels were highest in summer and lowest in winter, although the mean differences between seasons were small (27.5 vs. 22.2 ng/ml, respectively) (Table 2 and Fig. 2). In men with two measurements who had the first one in summer and the second one during winter, there were no differences in mean 25(OH)D2 levels, but average 25(OH)D3 levels were 5.6 ng/ml lower during winter.
Figure 2.
Summer to winter comparison of total 25(OH)D by site.
Despite the modest seasonal and geographical differences, these factors affected the proportion of men identified with vitamin D deficiency. For instance, the proportion with vitamin D deficiency (<20 ng/ml) was higher in winter (30.9%) than in spring or fall (28.2% and 22.8%, respectively), and was lowest in summer (18.1%). Vitamin D deficiency was more common at the more northern clinical sites. In winter, the mean total 25(OH)D level in Minneapolis was 21.9 ng/ml (37.8% deficient) and in summer was 26.3 ng/ml (13.5% deficient). In contrast, for San Diego the winter mean was 25.0 ng/ml (26.8% deficient), and the summer mean was 30.8 ng/ml (5.0% deficient).
Physical activity
Higher activity level (PASE score) and amount of outside activity were associated with higher 25(OH)D levels (Table 2). For instance, for those who reported no lawn work or gardening, mean total 25(OH)D was 23.2 ng/ml, and 35.2% were deficient; whereas men who reported at least one outdoor yard activity had a mean 25(OH)D of 25.6 ng/ml (P < 0.0001), and 22.6% were deficient.
Vitamin D from diet and supplements
Diet supplied small amounts of vitamin D (mean intake, 163 ± 115 IU/d); only 10 subjects (0.6%) received the recommended daily allowance of 600 IU/d from diet alone (Table 1), and dietary intake correlated weakly with total 25(OH)D levels (r = 0.08). The use of vitamin D supplements was reported by 58.5% of subjects, but none reported taking more than 600 IU/d. Supplements were associated with higher total 25(OH)D levels (Table 2). The 15.5% who reported supplement use had 25(OH)D levels below 20 ng/ml, whereas 39.6% who did not report supplement use had concentrations below that level. Nevertheless, a high proportion had vitamin D insufficiency with all supplement doses (percent of subjects with 25(OH)D levels <30 ng/ml: supplement intake <200 IU/d, 83.6%; 200 to <400 IU, 68.3%; 400 to <600 IU, 69.3%, and 600 IU, 61.3%).
Multivariable analyses
In bivariate analyses, several factors were associated with vitamin D deficiency (<20 ng/ml) (Table 3). After adjustment for these factors, older age, obesity, Black or Hispanic race, and winter or spring season remained independently associated with a greater prevalence of deficiency (Table 4). For instance, the risk of vitamin D deficiency approximately doubled with age of at least 85 yr, BMI of at least 35 kg/m2, Black race, and winter season. Dietary intake of vitamin D of at least 400 IU and lawn work/gardening were both associated with a lower prevalence of deficiency. The additive impact of these risk factors was considerable. In Caucasian men who were older than 80 yr, with a BMI greater than 25 kg/m2, who had a vitamin D intake below 400 IU/d, did not engage in lawn/garden work, and who were sampled in winter or spring, the prevalence of vitamin D deficiency was 86%; whereas there was a prevalence of 24% in younger, thinner men who had higher vitamin D intakes, who were sampled in summer or fall, and who engaged in lawn/garden work.
Table 3.
Factors associated with vitamin D deficiency [total 25(OH)D <20 ng/ml] in bivariate analyses
| Prevalence ratio | 95% CI | Age-, season-, and race-adjusted prevalence ratio | 95% CI | |
|---|---|---|---|---|
| Age range (yr) | ||||
| 65–69 | ref | ref | ||
| 70–74 | 1.08 | 0.83–1.41 | 1.17 | 0.90–1.54 |
| 75–79 | 1.06 | 0.80–1.39 | 1.14 | 0.87–1.51 |
| 80–84 | 1.35 | 0.99–1.83 | 1.54 | 1.13–2.10 |
| >85 | 1.74 | 1.16–2.61 | 2.13 | 1.41–3.21 |
| BMI category (kg/m2) | ||||
| <25 | ref | ref | ||
| 25–29 (overweight) | 1.16 | 0.91–1.49 | 1.22 | 0.95–1.57 |
| 30–34 (obese) | 1.39 | 1.03–1.88 | 1.48 | 1.09–2.03 |
| >35 | 2.51 | 1.68–3.76 | 2.56 | 1.69–3.87 |
| Race | ||||
| White | ref | ref | ||
| Black | 2.76 | 1.95–3.90 | 3.16 | 2.21–4.51 |
| Asian | 1.08 | 0.62–1.89 | 1.26 | 0.72–2.20 |
| Hispanic | 1.83 | 1.13–2.93 | 1.85 | 1.15–2.98 |
| Other | 1.65 | 0.78–3.49 | 1.89 | 0.89–4.00 |
| Education | ||||
| Graduate school | ref | ref | ||
| College | 1.10 | 0.87–1.38 | 1.11 | 0.88–1.40 |
| High school | 1.16 | 0.87–1.53 | 1.17 | 0.89–1.55 |
| <High school | 1.61 | 1.13–2.29 | 1.32 | 0.92–1.90 |
| Season | ||||
| Winter (Jan-Mar) | 2.44 | 1.83–3.25 | 2.52 | 1.89–3.35 |
| Spring (Apr-Jun) | 1.69 | 1.26–2.26 | 1.69 | 1.27–2.27 |
| Summer (Jul-Sep) | ref | ref | ||
| Fall (Oct-Dec) | 1.43 | 1.06–1.94 | 1.37 | 1.01–1.86 |
| Site | ||||
| Birmingham | ref | ref | ||
| Minneapolis | 1.05 | 0.75–1.46 | 1.14 | 0.81–1.59 |
| Palo Alto | 0.88 | 0.64–1.24 | 0.79 | 0.55–1.13 |
| Pittsburgh | 1.00 | 0.72–1.38 | 1.16 | 0.83–1.62 |
| Portland | 1.19 | 0.87–1.63 | 1.15 | 0.84–1.59 |
| San Diego | 0.56 | 0.38–0.83 | 0.58 | 0.39–0.85 |
| Alcoholic drinks per week | ||||
| 0 | ref | ref | ||
| 1–7 | 0.78 | 0.63–0.98 | 0.80 | 0.64–1.01 |
| >7 | 0.87 | 0.68–1.11 | 0.89 | 0.70–1.14 |
| Ever smoked | ||||
| Never | ref | ref | ||
| Past | 1.00 | 0.82–1.23 | 1.04 | 0.85–1.28 |
| Current | 1.48 | 0.95–2.31 | 1.43 | 0.91–2.26 |
| Daily intake of vitamin D from diet and supplements (IU) | ||||
| <400 | ref | ref | ||
| >400 | 0.36 | 0.29–0.45 | 0.38 | 0.31–0.48 |
| Daily intake of vitamin D from supplements (IU) | ||||
| 0–199 | ref | ref | ||
| 200–399 | 0.67 | 0.45–0.99 | 0.69 | 0.46–1.03 |
| 400–599 | 0.33 | 0.26–0.42 | 0.35 | 0.27–0.44 |
| 600 | 0.32 | 0.18–0.55 | 0.36 | 0.21–0.63 |
| PASE score quartile | ||||
| 1 (lowest) | ref | ref | ||
| 2 | 0.73 | 0.56–0.95 | 0.77 | 0.59–1.00 |
| 3 | 0.71 | 0.55–0.93 | 0.78 | 0.60–1.01 |
| 4 | 0.65 | 0.50–0.85 | 0.74 | 0.56–0.98 |
| Walking | ||||
| Never | ref | ref | ||
| Rarely | 0.94 | 0.68–1.31 | 1.03 | 0.74–1.44 |
| Sometimes | 0.68 | 0.50–0.93 | 0.74 | 0.54–1.02 |
| Often | 0.69 | 0.52–0.91 | 0.76 | 0.57–1.01 |
| Daily walks for exercise | ||||
| No | ref | ref | ||
| Yes | 0.81 | 0.66–0.98 | 0.82 | 0.67–1.00 |
| Lawn work and/or outdoor gardening | ||||
| No | ref | ref | ||
| Yes | 0.65 | 0.53–0.80 | 0.73 | 0.59–0.90 |
CI, Confidence interval.
Table 4.
Factors independently associated with deficiency [total 25(OH)D <20 ng/ml]
| New model for clinical prediction use (AUC, 0.77)
|
||
|---|---|---|
| Relative risk | 95% CI | |
| Age (yr) | ||
| <80 | ref | |
| 80–84 | 1.30 | 0.99–1.71 |
| >85 | 1.99 | 1.35–2.94 |
| Race/ethnicity | ||
| White or Asian | ref | |
| Black | 2.40 | 1.66–3.45 |
| Hispanic or other | 1.78 | 1.18–2.69 |
| BMI (kg/m2) | ||
| <25 | ref | |
| 25–29 (overweight) | 1.14 | 0.88–1.46 |
| 30–34 (obese) | 1.34 | 0.98–1.82 |
| >35 | 2.10 | 1.38–3.20 |
| Season | ||
| Winter (Jan-Mar) | 2.19 | 1.64–2.92 |
| Spring (Apr-Jun) | 1.63 | 1.22–2.18 |
| Summer (Jul-Sep) | ref | |
| Fall (Oct-Dec) | 1.29 | 0.95–1.76 |
| Latitudea | ||
| Low | ref | |
| High | 1.37 | 1.12–1.67 |
| Vitamin D supplement use | ||
| Yes | ref | |
| No | 2.36 | 1.92–2.90 |
| Lawn work and/or gardening | ||
| Yes | ref | |
| No | 1.44 | 1.16–1.79 |
CI, Confidence interval.
For model fitting, ″high″ latitude clinic sites were Minneapolis (44°), Pittsburgh (40°), and Portland (45°). ″Low″ latitude sites were Birmingham (33°), Palo Alto (37°), and San Diego (32°).
Discussion
In a cohort of older men in the United States, vitamin D deficiency and insufficiency were common. Approximately one fourth had a 25(OH)D level below that commonly considered to represent frank deficiency (<20 ng/ml), and the majority had levels considered insufficient (<30 ng/ml). Deficiency was particularly common during the winter and spring months (especially in the northern communities) and in the oldest and more obese subjects. In fact, 86% with multiple risk factors were deficient. Because vitamin D deficiency has been linked to a variety of common diseases, these results may be of considerable public health relevance. Importantly, our data demonstrated that the level of 25(OH)D varied little in sera obtained approximately 2 yr apart, indicating that vitamin D status is stable over time and suggesting that many have longstanding vitamin D deficiency.
There is uncertainty concerning the levels of 25(OH)D levels that are adequate, but because measures of serum PTH tend to be higher in those with 25(OH)D levels below 30 ng/ml (19), that concentration is considered sufficient to prevent secondary hyperparathyroidism. Less than 30% of our population achieved that goal. One fourth of older men had 25(OH)D levels below 20 ng/ml, concentrations associated with more severe consequences (3). These results are similar to those reported in other studies (20,21,22) and confirm that vitamin D deficiency is widespread.
Considerable variation has been noted in measurements of 25(OH)D levels in multicenter comparisons of assay performance (7,8,9), apparently reflecting inconsistency between methods or laboratories. Thus, some of the difficulty in estimating what levels of 25(OH)D are adequate, and in surveying the population burden of vitamin D deficiency, may result from the lack of standardization of assay approaches. We measured 25(OH)D levels using liquid chromatography/mass spectrometry, and assay quality control revealed excellent performance.
25(OH)D3 was present in much higher concentrations than was 25(OH)D2. In fact, only one fourth of these men had detectable levels of 25(OH)D2, and few who did not take supplements had detectable 25(OH)D2. These results suggest that many older men in the United States have minimal exposure to vitamin D2. Vitamin D deficiency is thought to be less common in the United States because of vitamin D supplementation of dairy products (21,23,24); many of the men in the MrOS reported using daily vitamin D supplements, and in fact the levels of total 25(OH)D in these men were higher. Nevertheless, the prevalence of vitamin D deficiency remained high even in supplement users, and 39.6% of supplement users were deficient (<20 ng/ml). These results strengthen the impression (25) that commonly used supplement doses are inadequate to ensure vitamin D nutrition. To some extent this is not surprising because Holick et al. (26) recently reported that for every 100 IU of vitamin D2 or vitamin D3 ingested, there is an increase in circulating 25(OH)D levels of only 1 ng/ml, providing some explanation for why men reporting supplement use have marginally higher concentrations. In addition, we also estimated the intake of vitamin D from diet. On average, it was small and probably contributed little to overall vitamin D nutrition. In combination, the apparently small effects of supplements and diet support the fact that endogenous production from skin UV exposure is the primary source of vitamin D in older men.
Other studies reported that aging (2,27), obesity (28,29,30), and winter season (31) are associated with lower 25(OH)D levels. We demonstrate that those factors have important effects on the prevalence of vitamin D deficiency; 53.3% of the most obese men were deficient, and almost half of those 85 yr or older were deficient. The winter season considerably increased the proportion classified as deficient. Moreover, we showed that these are independent predictors of vitamin D deficiency and that the cumulative effects of these factors are impressive. The great majority with multiple risk factors were deficient. This information suggests that obese, older men may particularly benefit from vitamin D supplementation, especially in the winter and spring, and that trials are warranted to assess the potentially widespread benefits.
The study has several major strengths. To our knowledge this is the largest study of 25(OH)D levels in elderly men. It draws from a cohort of community-dwelling men from diverse geographical regions of the United States and includes the availability of information concerning relevant seasonal, nutritional, and lifestyle factors. In addition, 25(OH)D measurements were performed using mass spectrometry-based assays with high precision, thus avoiding potential artifacts associated with other approaches (8,9,32,33). These assay methods also allowed us to determine independently both 25(OH)D2 and 25(OH)D3 levels. On the other hand, study weaknesses included the volunteer nature of the participants, which limits the generalizability of the results. For instance, the prevalence of vitamin D deficiency would be expected to be higher in less healthy men (34). Nevertheless, the likelihood that our results apply to other older U.S. men is strengthened by the observation that means for height, weight, and BMI among Black, Hispanic, and White men studied here are nearly identical to the race/ethnicity-specific means from the National Health and Nutrition Examination Survey 1999–2000 reference data for men aged 60 yr or older (35). Finally, no women were studied, and the proportion of non-White participants was limited.
In summary, vitamin D deficiency is very common in older U.S. men, especially among the most elderly and obese, and during the winter in northern latitudes. In many, prevalent vitamin D deficiency appears to be a long-standing condition, rather than a recent occurrence. Because older men are commonly afflicted with disorders potentially linked to vitamin D deficiency and some studies have suggested benefit from supplementation, there is a need to further examine the usefulness of vitamin D supplements in clinical trials with adequate power to address multiple outcomes.
Acknowledgments
We acknowledge the expert assistance by Leslie Stonelake in the preparation of the manuscript, and of Catherine Pedersen, Jan Blank, and all MrOS staff in the outstanding conduct of these studies.
Footnotes
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 27, 2009
For editorial see page 1092
Abbreviations: BMI, Body mass index; 25(OH)D, 25-hydroxyvitamin D.
References
- DeLuca HF 2004 Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689S–1696S [DOI] [PubMed] [Google Scholar]
- Holick MF 2006 High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughs B, Bischoff-Ferrari HA 2007 Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res 65:501–506 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B 2005 Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA 2008 Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 624:55–71 [DOI] [PubMed] [Google Scholar]
- Hypponen E, Turner S, Cumberland P, Power C, Gibb I 2007 Serum 25-hydroxyvitamin D measurement in a large population survey with statistical harmonization of assay variation to an international standard. J Clin Endocrinol Metab 92:4615–4622 [DOI] [PubMed] [Google Scholar]
- Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF 1999 An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 9:394–397 [DOI] [PubMed] [Google Scholar]
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK 2004 Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89:3152–3157 [DOI] [PubMed] [Google Scholar]
- Jones G, Horst R, Carter G, Makin HLJ 2007 Contemporary diagnosis and treatment of vitamin D related disorders. J Bone Miner Res 22:V11–V15 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study. A large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR 2005 Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK 2006 C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Naughton D 1990 A reduced dietary questionnaire: development and validation. Epidemiology 1:58–64 [DOI] [PubMed] [Google Scholar]
- Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P 1994 Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E 2005 Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199–200 [DOI] [PubMed] [Google Scholar]
- Deddens JA, Petersen MR 2008Approaches for estimating prevalence ratios. Occup Environ Med 65:481, 501–506 [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ 1997 Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443 [DOI] [PubMed] [Google Scholar]
- Saquib N, Von Muhlen DG, Garland CF, Barret-Conner E 2006 Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: the Rancho Bernardo study. Osteoporos Int 17:1734–1741 [DOI] [PubMed] [Google Scholar]
- Lips P 2001 Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501 [DOI] [PubMed] [Google Scholar]
- Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF 2008 Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 93:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ 1992 Differences in vitamin D status between countries in young adults and the elderly. Am J Med 93:69–77 [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P 2008 Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 42:260–266 [DOI] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A 2007 The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85:649–650 [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD 2008 Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SS, Hollis BW, Tobin JD 1990 Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab 71:405–413 [DOI] [PubMed] [Google Scholar]
- Holick MF 2004 Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371 [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF 2000 Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr {lsqb;Erratum (2003) 77:1342{rsqb; 72:690–693 [DOI] [PubMed] [Google Scholar]
- Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P 2005 Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123 [DOI] [PubMed] [Google Scholar]
- Webb AR, Pilbeam C, Hanafin N, Holick MF 1990 An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 51:1075–1081 [DOI] [PubMed] [Google Scholar]
- Holick MF 2005 25-OH-vitamin D assays. J Clin Endocrinol Metab 90:3128–3129 [DOI] [PubMed] [Google Scholar]
- Fradinger EE 2005 Letter re: 25-OH-vitamin D assays. J Clin Endocrinol Metab 90:6337–6338; author reply, 90:6338 [DOI] [PubMed] [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS 1998 Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783 [DOI] [PubMed] [Google Scholar]
- McDowell MA, Fryar CD, Hirsch R, Ogden CL 2005 Anthropometric reference data for children and adults: U.S. population, 1999–2002. Adv Data 361:1–5 [PubMed] [Google Scholar]