Abstract
Context: Insulin-requiring diabetes affects 7–15% of teens and young adults, and more than 25% of older adults with cystic fibrosis (CF). Pancreatic exocrine disease caused by CF transmembrane conductance regulator (CFTR) dysfunction underlies the high rate of diabetes in CF patients; however, only a subset develops this complication, indicating that other factors are necessary.
Objective: Our objective was to estimate the relative contribution of genetic and nongenetic modifiers to the development of diabetes in CF.
Design/Patients: This was a twin and sibling study involving 1366 individuals at 109 centers in the CF Twin and Sibling Study, from which were derived 68 monozygous twin pairs, 23 dizygous twin pairs, and 588 sibling pairs, all with CF.
Main Outcome Measure: Chronic, insulin-requiring diabetes in the setting of CF, as established using longitudinal clinical and biochemical data, was studied.
Results: About 9% of this predominantly pediatric population (mean age = 15.8 yr) had diabetes. Key independent risk factors identified by regression modeling included having a twin or sibling with CF and diabetes, increasing age, pancreatic exocrine insufficiency or two mutations causing severe CFTR dysfunction, decreased lung function or decreased body mass index, and longer duration of glucocorticoid treatment. The concordance rate for diabetes was substantially higher in monozygous twins (0.73) than in dizygous twins and siblings with CF (0.18; P = 0.002). Heritability was estimated as near one (95% confidence interval 0.42–1.0).
Conclusions: Diabetes is a frequent complication of CF that is associated with worse outcomes. Although a nongenetic factor (steroid treatment) contributes to risk, genetic modifiers (i.e. genes other than CFTR) are the primary cause of diabetes in CF.
Genes other than CFTR contribute substantially to the risk of diabetes in cystic fibrosis patients.
Cystic fibrosis (CF) was first recognized as a disorder of malnutrition due to pancreatic exocrine deficiency, and the development of pancreatic enzyme replacement therapy was accompanied by dramatic improvement in survival from infancy to adolescence (1). Obstructive lung disease is currently the major cause of morbidity and mortality for CF patients, and pulmonary therapies have helped increase median life expectancy to 36 yr for a newborn with CF (2). The discovery that CF is caused by dysfunction of an epithelial chloride channel, the CF transmembrane conductance regulator (CFTR), provided a unifying molecular explanation for disturbances of electrolyte and fluid transport in the lungs, pancreas, and sweat gland (3). These successes have resulted in a larger fraction of CF patients entering into adulthood with the concomitant realization that diabetes is a frequent complication of this disorder (4).
Although diabetes affects few children with CF, its prevalence steadily increases in adolescence and adulthood, with at least 25% of those over 20 yr being affected (reviewed in Ref. 5). Diabetes in CF has features of both type 1 and 2 diabetes seen in the general population but has generally been considered to be a distinct entity (6). Diabetes in CF patients typically occurs in the absence of obesity and is associated with a significantly worse prognosis (7,8). Treatment of diabetes improves nutritional status and pulmonary function (9,10). Elucidating the mechanisms underlying this increasingly prevalent complication is essential to continued improvement in the survival of CF patients and may shed light on similar conditions in the general population. The CFTR gene itself plays a role because CF patients with mutations causing mild CFTR dysfunction are not at increased risk for diabetes (6). In addition, it has been suggested that other CFTR mutations may confer differential risk for diabetes (11,12), although these observations have not been replicated to date. However, other factors must be involved because diabetes affects only a fraction of patients with identical CFTR genotypes (e.g. Ref. 13). Although twin and other studies have established that type 1 and 2 diabetes have a strong genetic component (reviewed in Ref. 14), the extent to which genes (other than CFTR) and environmental factors modulate diabetes risk in CF patients is unknown. In the current study of affected twins and siblings, we derived the contribution of genes to the development of diabetes in CF patients.
Subjects and Methods
Study subjects
Clinical data and DNA samples were collected by the CF Twin and Sibling Study (15,16), with additional clinical data provided by the U.S. CF Foundation Patient Registry (Bethesda, MD). Enrollment was based on a diagnosis of CF (17) and having an affected twin and/or sibling. Informed consent was obtained from all subjects in the study. Isolation of patient DNA, identification of CFTR mutations, and zygosity testing have been previously described (15).
Phenotype definitions
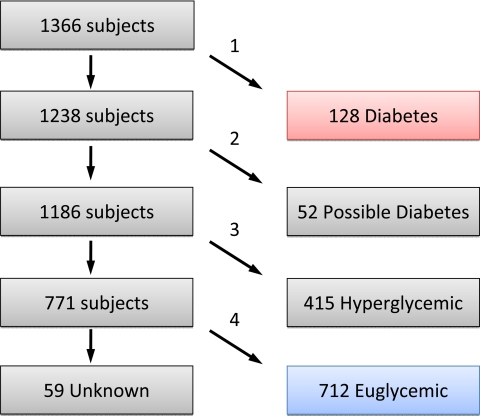
Recognizing that waxing and waning hyperglycemia in CF can lead to ambiguity in a diagnosis of diabetes, we used longitudinal data to identify patients with chronic, insulin-requiring diabetes as well as those with clearly normal glucose metabolism. Clinical information (e.g. age or date of diabetes diagnosis, clinician diagnoses, treatment modality, and diagnostic testing obtained as part of clinical management) was obtained from the medical record and from the CF Foundation (CFF) Patient Registry. Fasting hyperglycemia (≥126 mg/dl or 7 mm) as recorded in the CFF Registry was found to be an inconsistent indicator of diabetes after review of clinic notes for the 13 patients in question, so this particular parameter was not used to classify patients. The CFF Registry specifies for each encounter and/or at year’s end whether a patient had diabetes (or was euglycemic, starting with 2006 data), was treated chronically or intermittently with insulin, treated with oral agents, or with diet. Patients were classified as having diabetes, possible diabetes, hyperglycemia, and being euglycemic by the scheme shown in Fig. 1.
Figure 1.
Diabetes diagnostic flowchart. Selection processes: diabetes classification required a diagnosis of diabetes and treatment with insulin or oral agents for at least 1 yr (arrow labeled 1); possible diabetes required any of intermittent or previous diabetes and treatment (n = 34), current diabetes but no treatment (n = 15), glucose tolerance in diabetic range (6) (n = 2), or HbA1c more than 7% (n = 1), but no diabetes diagnosis and no treatment (arrow labeled 2); hyperglycemia classification required any of diagnosis of “impaired glucose tolerance,” diabetes, which resolved without treatment, insulin treatment without diabetes, impaired glucose tolerance or impaired fasting glucose by American Diabetes Association criteria (38), or HbA1c above the reference range (>6%) (arrow labeled 3); and euglycemia classification required diagnosis of “Normal,” at least two glucose measurements available, and all glucose and HbA1c levels within normal limits (arrow labeled 4). Classification as unknown included those with unknown diabetes diagnosis, unknown treatment, or fewer than two glucose measurements. “Treatment” refers to insulin or oral medication but does not include dietary treatment.
Patient age was as of the most recent clinical data. Steroid treatment was defined as use of systemic (i.e. oral or iv) glucocorticoids, excluding intranasal and inhaled steroids. Duration of steroid treatment and diagnosis of allergic bronchopulmonary aspergillosis (ABPA) were defined by CF clinicians. Other phenotype definitions [e.g. pancreatic insufficiency, lung function reported by CF-specific percentiles for the forced expiratory volume in 1 second (FEV1) for the most recent (MaxFEV1CF%) and all (AvgFEV1CF%) pulmonary function tests (16), and nutritional status reported by body mass index sd score (18)] are descrived elsewhere (15).
Statistical analysis
Statistical calculations were performed using Intercooled Stata 10 (StataCorp LP, College Station, TX). Heritability was estimated by the liability-threshold model (19) and by logistic regression (20). The logistic regression approach of Ramakrishnan et al. (20) was extended to the use of Cox regression for censored phenotypes (e.g. age of diabetes diagnosis). A P value of less than 0.05 was considered statistically significant. In case of small cell sizes, the Fisher exact test was used. Student’s t test was used to compare normally distributed continuous data.
Results
A substantial fraction of patients in the CF Twin and Sibling Study has abnormal glucose metabolism
Clinical information was collected for 1370 CF-affected individuals and 883 parents in 679 families in the CF Twin and Sibling Study. Four patients were excluded for antibody positive type 1 diabetes. Based on diagnostic and laboratory information in the medical record and CFF Patient Registry, the remaining 1366 patients were classified as euglycemic (n = 712), hyperglycemic (n = 415), possible diabetes (n = 52), diabetes (n = 128), or had insufficient information to be classified (unknown; n = 59) (Fig. 1).
There was general agreement between severity of diagnostic category and clinical parameters such as the patients’ stated diagnosis or their oral glucose tolerance test results (Table 1). Of the 128 patients categorized as having diabetes, 77 (60%) had at least two glucose tolerance tests in the diabetic range (fasting plasma glucose ≥126 mg/dl/7 mm or 2 h glucose ≥200 mg/dl/11.1 mm) or one glycosylated hemoglobin (HbA1c) measurement of 7% or greater, and 97 (76%) had at least one glucose measurement in the diabetic range. Most of those classified as diabetes without confirmatory glucose data were labeled as not having been tested due to already having diabetes; others included one patient with one recorded glucose measurement despite 7.5 yr daily insulin treatment, and four chronically insulin-treated patients with glucose data available only before diabetes diagnosis. The distribution of diabetes, impaired glucose tolerance (using hyperglycemia or possible diabetes as a proxy), and normal glucose tolerance (using euglycemia as a proxy) in this predominantly pediatric study group (mean age 15.8 yr) is comparable to that reported in other retrospective (2,13) and prospective (21,22,23,24) reports.
Table 1.
Glucose metabolism profiles of 1366 study subjects
| Euglycemic (n = 712) | Hyperglycemic (n = 415) | Possible diabetes (n = 52) | Diabetes (n = 128) | Unknown (n = 59) | |
|---|---|---|---|---|---|
| Reported diagnosisa | 712 normal | 327 normal | 2 normal | 128 diabetes | 59 none |
| 88 IGT | 1 IGT | ||||
| 34 intermittent | |||||
| DM 15 diabetes | |||||
| Use of insulin or oral hypoglycemic agentb | 712 none | 411 none | 18 none | 128 chronic | 59 none |
| 2 intermittent | 34 intermittent | ||||
| 2 chronic | |||||
| Glucose/HbA1c criteriac | 712 normal | 15 normal | 3 normal | 4 normal | 59 unknown |
| 392 IGT | 32 IGT | 46 IGT | |||
| 7 DM | 17 DM | 77 DM | |||
| 1 unknown | 1 unknown | ||||
| Ever had fasting glucose ≥ 126 mg/dl (7 mm) | 0/153 | 4/202 (2%) | 4/32 (12%) | 5/64 (8%) | 0/4 |
| Ever had random or 2 h OGTT >200 mg/dl (11.1 mm) | 0/710 | 93/414 (22%) | 34/52 (65%) | 97/127 (76%) | 0/59 |
| No. of glucose tests (mean ± sd) | 6.0 ± 2.4 (n = 683) | 8.7 ± 4.8 (n = 412) | 11.4 ± 7.9 (n = 48) | 10.0 ± 7.2 (n = 123) | 0.8 ± 0.4 (n = 46) |
| No. of tests in diabetic range (mean ± sd) | 0 | 0.2 ± 0.6 | 0.8 ± 1.0 | 2.0 ± 2.7 | 0 |
DM, Diabetes; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test.
As reported in medical records, including ″normal″ (normal glycemia), ″IGT″ (representing diagnoses of impaired glucose tolerance, impaired fasting glucose, or hyperglycemia), ″intermittent DM″ (intermittent or transient diabetes), or diabetes.
Diabetes medication treatment as grouped into none, intermittent, or chronic (>1 yr).
Classified as normal, impaired glucose tolerance, diabetes, or unknown based on glucose testing data interpreted by American Diabetes Association criteria (38), and HbA1c data (normal < 6%; diabetes ≥ 7%; see Fig. 1).
Risk factors for diabetes include pancreatic insufficiency, reduced lung function, steroid treatment, and diabetes in a CF-affected sibling
Clinical characteristics of the Twin and Sibling Study patients (Table 2) demonstrate that risk factors for diabetes in the Twin and Sibling Study are similar to what has been reported by others for unrelated CF patients. Diabetes was associated with increasing age, exocrine pancreatic insufficiency, and two “severe” CFTR mutations (e.g. nonsense or frameshift), reduced lung function, reduced body mass index, steroid use, and ABPA (13,25). The same risk factors were apparent either by comparing diabetic with euglycemic patients (e.g. Fisher exact or t test), or by comparing diabetic with all other patients (odds ratios by logistic regression). Age, sex, and pancreatic insufficiency rate differed between euglycemic and diabetes groups, so we derived a subset of euglycemic patients who were matched to the diabetes patients for the three variables (matched euglycemic). After matching, all variables except CFTR genotype, which is highly associated with pancreatic insufficiency (26), and longitudinal nutritional status remained different.
Table 2.
Comparison of clinical features of CF patients in the Twin and Sibling Study group with euglycemia, hyperglycemia or possible diabetes, or diabetes
| Euglycemic | Hyperglycemia or possible diabetes | Diabetes | Odds ratioa | Matched euglycemicb | Odds ratiob | |
|---|---|---|---|---|---|---|
| No. of subjects | 712 | 467 | 128 | 128 | ||
| Mean age (yr) | 13.6c | 16.9c | 24.2 | 1.08 per year (n = 1307)c | 24.3 | 1.0 (n = 256) |
| Female | 345/712 (48%) | 212/467 (45%) | 67/128 (52%) | 1.2 (n = 1307) | 67/128 (52%) | 1.0 (n = 256) |
| CFTR genotype | ||||||
| ΔF508 ×2d | 329/702 (47%)e | 237/465 (51%)f | 77/126 (61%) | 1.7 (n = 1293)e | 82/126 (65%) | 0.8 (n = 252) |
| Severe CFTR ×2g | 514/661 (78%)c | 393/434 (91%) | 112/118 (96%) | 3.9 (n = 1213)e | 118/124 (95%) | 0.9 (n = 242) |
| Pancreatic insufficiency | 534/619 (86%)c | 406/429 (95%)f | 115/116 (99%) | 13.9 (n = 1164)e | 120/120 (100%) | n/a |
| Lung function percentileh | ||||||
| Cross-sectional | 0.74 (n = 564)c | 0.66 (n = 429)c | 0.55 (n = 124) | 0.81 per 10% (n = 1117)c | 0.74 (n = 127)c | 0.78 per 10% (n = 251)c |
| Longitudinal | 0.65 (n = 368)c | 0.58 (n = 355)e | 0.51 (n = 117) | 0.83 per 10% (n = 840)c | 0.64 (n = 123)c | 0.80 per 10% (n = 240)c |
| Nutritional statusi | ||||||
| Cross-sectional | −0.04 (n = 695)c | −0.46 (n = 467)e | −0.74 (n = 126) | 0.68 per sd unit (n = 1288)c | −0.31 (n = 128)e | 0.7 per sd unit (n = 254)e |
| Longitudinal | −0.01 (n = 666)c | −0.30 (n = 452)f | −0.51 (n = 119) | 0.61 per sd unit (n = 1237)c | −0.36 (n = 123) | 0.8 per sd unit (n = 242) |
| Steroid treatment (≥30 d)j | 133/712 (19%)c | 105/467 (22%)c | 58/120 (45%) | 3.3 (n = 1307)c | 23/128 (18%)c | 3.8 (n = 256)c |
| Steroid treatment (d/yr)j | 17 (n = 416)c | 32 (n = 310)c | 87 (n = 74) | 1.2 per month (n = 800)c | 23 (n = 84)c | 1.3 per month (n = 158)c |
| ABPAj | 29/587 (5%)c | 42/401 (10%)c | 26/105 (25%) | 4.3 (n = 1093)c | 8/108 (7%)c | 4.1 (n = 213)c |
| Has an MZ twin with diabetes | 3/71 (4.2%)c | 1/43 (2.3%)c | 21/26 (81%) | 57.7 (n = 1307)c | 1/25 (4%)c | 25 (n = 256)e |
| Has a DZ twin or sibling with diabetes | 22/606 (3.6%)c | 26/410 (6.3%)c | 21/94 (22%) | 4.6 (n = 1307)c | 16/101 (16%) | 1.4 (n = 256) |
n/a, Not applicable.
Odds ratio for patients with diabetes vs. those in all other groups (euglycemic, hyperglycemic, and possible diabetes) pooled.
Matched euglycemic subjects were selected to match diabetic subjects for age, sex, and pancreatic insufficiency/sufficiency. Odds ratio is for patients with diabetes vs. matched euglycemic subjects.
P < 0.001 vs. diabetes.
Homozygosity for the ΔF508 CFTR mutation.
P < 0.01 vs. diabetes.
P < 0.05 vs. diabetes.
Both CFTR alleles with severe (e.g. frameshift or nonsense) mutations (list available upon request).
Cross-sectional and longitudinal measures of lung function were based on forced expiratory volume in 1 sec as defined previously (16).
Nutritional status was assayed by body mass index sd score, with longitudinal averages as defined previously (18). Cross-sectional body mass index sd score was calculated by averaging over the year before enrollment.
Oral or iv steroid use and diagnosis of ABPA were reported by either questionnaire or CFF Patient Registry (intranasal or inhaled steroid use was excluded).
Multivariate regression was used to examine interaction between risk factors. Confounding among correlated variables was evident when all factors were included (Model 1 in Table 3). Subsequent models were built both by stepwise addition of risk factors (keeping those that were significant) and by stepwise removal of the least significant risk factors. Patient age, pancreatic exocrine insufficiency, and having a twin or sibling with diabetes were found to be independent risk factors in all models. Sets of variables that were found to confound each other in multivariate models included: pancreatic exocrine insufficiency and having two severe CFTR mutations; cross-sectional and longitudinal lung function; all four measures of lung function and nutritional status; and steroid treatment and ABPA. Correlations among these sets of variables have been reported previously (16,26,27). In the second model shown in Table 3, we retained the member of each set of confounding variables with the greatest amount of available information. Female sex, identified by some as a risk factor for diabetes (discussed in Ref. 24), was not a statistically significant risk factor in this population, though the multivariate odds ratio of 1.6 in the second model was of borderline significance (P = 0.052). In summary, interactions among risk factors for diabetes in twins and siblings with CF are similar to those identified in unrelated CF patients.
Table 3.
Independent risk factors for diabetes identified by logistic regression analyses
| Model 1 | 95% CI | Model 2 | 95% CI | |
|---|---|---|---|---|
| Multivariate OR (n = 671) | Multivariate OR (n = 996) | |||
| Age at last diabetes screen | 1.1 per yeara | 1.08–1.18 | 1.1 per yeara | 1.07–1.13 |
| Female | 1.4 (NS) | 0.84–2.5 | 1.6 (NS) | 1.0–2.6 |
| Pancreatic insufficiencyb | 8.2c | 1.06–64 | 23.5a | 3.5–160 |
| Lung function percentiled | ||||
| Cross-sectional | 0.99 per 10% (NS) | 0.81–1.2 | 0.84 per 10%a | 0.78–0.91 |
| Longitudinal | 0.78 per 10%c | 0.62–0.99 | Not in model | |
| Nutritional statusd | ||||
| Cross-sectional | 0.84 per sd unit (NS) | 0.6–1.2 | Not in model | |
| Longitudinal | 1.5 per sd unit (NS) | 0.9–2.5 | Not in model | |
| Steroid treatment (≥30 d)e | 2.4f | 1.2–4.5 | 2.5a | 1.4–4.2 |
| ABPA | 1.5 (NS) | 0.7–3.0 | Not in model | |
| MZ twin with diabetes | 37.7a | 7.6–187 | 31.9a | 9.7–105 |
| DZ twin or sibling with diabetes | 3.1f | 1.5–6.4 | 3.5a | 1.8–7.0 |
NS, Not significant.
P < 0.001.
Pancreatic insufficiency, two severe CFTR mutations, and two ΔF508 mutations were risk factors for diabetes when individually included in multivariate models. Together, any two were correlated and confounded each other. The strongest and most significant correlation was with pancreatic insufficiency, which is included in the model shown.
P < 0.05.
These measures of lung function and nutritional status were risk factors for diabetes when individually included in multivariate models. Together, any two were correlated and confounded each other. The strongest and most significant correlation was with cross-sectional lung function, which is included in the model shown.
Increasing duration of steroid treatment, presence of prolonged steroid treatment (30 d or more), and presence of ABPA were risk factors for diabetes when individually included in multivariate models. Together, any two were correlated and confounded each other. The strongest and most significant correlation was with the presence of prolonged steroid treatment, which is included in the model shown.
P < 0.01.
Steroid treatment induces transient hyperglycemia and diabetes in CF (28) [though not in all studies (8,13)], and duration of steroid treatment was found to be a quantitative risk factor for diabetes in this study. Therefore, we assessed different lengths of treatment to determine the interval with the highest effect on diabetes risk in our study. Defining steroid treatment as any duration, for more than 7 d, or for more than 15 d (in the year before enrollment) were less potent risk factors and significant only in univariate regression. Steroid treatment for a minimum of either 30 or 60 d duration was a significant risk factor with similar odds ratios in all analyses (data not shown). Therefore, we defined “prolonged” exposure as more than 30 d steroid treatment in the year before enrollment.
Of note, relatedness (i.e. having a twin or sibling with diabetes) was found to be a substantial risk factor in all comparisons (Table 2). Even after adjusting for age, sex, pancreatic exocrine insufficiency, lung function, and prolonged steroid treatment, the odds ratio of a CF patient with diabetes having a monozygous (MZ) twin with diabetes was 31.9 [P < 0.001; 95% confidence interval (CI) 9.7–105], whereas the odds ratio for having a dizygous (DZ) twin or sibling with diabetes was 3.5 (P < 0.001; 95% CI 1.8–7.0). The odds ratios differed from each other (P < 0.001). Furthermore, Cox regression using age of diagnosis of diabetes as the phenotype and censoring at the current age (data not shown) also demonstrated that having a MZ twin or DZ twin/sibling with diabetes each conferred risks for diabetes that were different from each other [MZ twin with diabetes, hazard ratio (HR) = 3.6; DZ twin or sibling with diabetes, HR = 2.0; P < 0.001].
Concordance for diabetes correlates with the degree of gene sharing between CF patients
To quantify the contribution of genes other than CFTR to diabetes in CF, we determined concordance rates (i.e. how often both members of a pair have disease divided by the number of pairs in which at least one member has disease) in 68 pairs of MZ twins, 23 pairs of DZ twins, and 588 pairs of siblings with CF. Concordance for diabetes was 0.73 (11 of 15 pairs) in MZ twin pairs (Table 4), indicating the importance of shared genes and/or shared environment. The difference in concordance rates between MZ twins, sharing 100% of genes, and DZ twins, sharing 50% of genes on average, estimates the proportion of disease risk that can be attributed to genes (i.e. heritability) for diabetes (29). Because the few DZ twin pairs were too young, on average, to be at risk for diabetes, limited conclusions could be drawn from this group. Therefore, affected sibling pairs (who share 50% of genes on average, like DZ twins) were analyzed for concordance for diabetes. To account for the possibility that concordance was decreased in siblings due to differing age and sex (which could have led to the assignment of environmental effects as genetic), we selected same-sex sibling pairs and considered the diabetes status of the older sibling when he/she was the age of the younger sibling. To account for the dependence of diabetes prevalence on age (and possibly cohort), we selected a subset of siblings matched to the MZ twins for age, sex, and pancreatic insufficiency. Concordance for diabetes among the age and sex-matched siblings was 0.18 (four of 22 pairs; Table 4), which significantly differed from the concordance rate in MZ twins (0.73; P = 0.002) and yields heritability for diabetes in CF estimated at 0.98 (95% CI 0.42–1.0).
Table 4.
Diabetes concordance rates in pairs of MZ twins, DZ twins, and siblings with CF
| Median age (yr) | Diabetes status of pair
|
Concordancea | |||
|---|---|---|---|---|---|
| Both | One | Neither | |||
| MZ twin pairs | 18.3 (n = 68) | 11 | 4 | 53 | 0.73 |
| DZ twin pairs | 11.7 (n = 23) | 0 | 3 | 20 | 0.00 |
| Sibling pairs | 13.7 (n = 588) | 13 | 69 | 506 | 0.16 |
| DZ and sibling matched to MZb | 17.3 (n = 136) | 4 | 18 | 114 | 0.18 |
| Diabetes concordance rates after accounting for steroid use ≥ 30 dc | |||||
| MZ twin pairs | 18.3 (n = 66) | 8 | 1 | 47 | 0.89 |
| DZ and sibling pairs | 13.4 (n = 579) | 6 | 47 | 430 | 0.11 |
| DZ and sibling matched to MZb | 17.1 (n = 128) | 2 | 13 | 90 | 0.13 |
| Diabetes concordance rates after accounting for ABPAd | |||||
| MZ twin pairs | 17.9 (n = 57) | 8 | 1 | 48 | 0.89 |
| DZ and sibling pairs | 15.4 (n = 499) | 10 | 46 | 443 | 0.18 |
| DZ and sibling matched to MZb | 18.2 (n = 107) | 4 | 12 | 91 | 0.25 |
Six sibling pairs known to differ in CFTR genotype were excluded. Diabetes status of a pair was categorized as ″Both″ members having diabetes, ″One″ of two members having diabetes or ″Neither″ member having diabetes.
Concordance for diabetes was defined as the number of pairs in which both members had diabetes divided by the number of pairs in which one or both had diabetes.
Same-sex DZ twin pairs combined with same-sex, age-corrected sibling pairs. Age correction was done by considering the diabetes status of the older member at the age of the younger member. From this group were selected 136 pairs matched for age, sex, and pancreatic insufficiency to the 68 MZ pairs. Pairs did not differ in age (P = 0.4) or any other matched variable.
Pairs in which exactly one member had steroid treatment 30 d or more (i.e. discordant for steroid treatment) were excluded.
Pairs in which exactly one member had ABPA were excluded.
To account for the possibility that matched siblings were a biased sample, we evaluated the entire sibling group. Of 82 (16%) CF sibling pairs, 13 had both members affected with diabetes, significantly different from MZ twins (P = 1.9 × 10−5). Similar concordance rates were obtained when correcting only the diabetes status for differing ages of siblings (eight of 61; 0.13), when selecting same-sex pairs only (seven of 44; 0.16), or when doing both (six of 30; 0.20). Alternatively, sibling pairs were corrected for age by considering pairs differing by less than 3 yr of age, also yielding a low concordance rate (five of 44; 0.12). Thus, the wide gap in concordance between MZ twins and siblings could not be attributed to bias introduced by the matching process.
To assess whether the restrictive definition for diabetes strongly influenced the heritability estimate, a second analysis was performed, in which those in either the possible diabetes or diabetes categories were considered to be affected with diabetes. Heritability remained high but was slightly decreased (MZ concordance = 13 of 20, 0.65; matched DZ plus sibling concordance = nine of 36, 0.25; heritability = 0.8). Thus, the estimated effect of genetic modifiers on the development of diabetes in CF remained high, even when using a less restrictive definition of diabetes.
Factors correlated with diabetes in CF do not affect the estimate of genetic effect
Diabetes was correlated with other factors (e.g. pancreatic exocrine insufficiency, steroid treatment, and lung function), which, if influenced by genetic factors, could inflate the heritability estimate for diabetes. However, estimates of genetic effect (independent of the CFTR gene) were low for both pancreatic insufficiency (h2 = 0) and steroid treatment of 30 d or more (h2 = 0.3) (data not shown). Neither result is unexpected because pancreatic exocrine insufficiency is known to be determined primarily by CFTR genotype (26), and steroid treatment is an environmental factor. Furthermore, when considering only twins and siblings who both had exocrine pancreatic insufficiency, essentially identical concordance rates for diabetes were derived (data not shown). The same was true for twins and siblings who were homozygous for the common CF mutation, ΔF508, or who had two mutations causing severe CFTR dysfunction (data not shown). Likewise, accounting for systemic steroid treatment (≥30 d) had little effect on concordance rates that remained markedly different between MZ twins and DZ twins plus siblings (Table 4). Although ABPA, a severe form of asthma treated with steroids, was not an independent risk factor in this regression analysis, others have found ABPA to be associated with diabetes (e.g.13). Adjustment for ABPA had a negligible effect on concordance rates for diabetes (Table 4). Diabetes was also correlated with lung function, itself a heritable phenotype (16). To test whether this interaction inflated the estimate of diabetes heritability, concordance rates were compared between 64 MZ twin pairs and 128 sibling pairs matched both for pair averages of cross-sectional (MaxFEV1CF%) and longitudinal (AvgFEV1CF%; see Subjects and Methods section for definitions) measures of CF lung function (to enable comparison between MZ and sibling pairs) and for intrapair differences in these phenotype measures (so that MZ and matched sibling pairs have similar degrees of concordance for lung function). No significant differences were seen in matched variables (P = 0.2–0.9). Concordance rates for diabetes were essentially unchanged after accounting for lung function [0.73 (11 of 15) in MZ twins compared with 0.21 (four of 19) in sibling pairs; P = 0.005, Fisher exact]. Thus, these important risk factors for diabetes in CF do not confound the estimated genetic contribution to diabetes risk.
To estimate genetic effect while simultaneously accounting for multiple covariates, heritability was estimated by regression analysis (20). Using diabetes as a dichotomous variable, multivariate logistic regression (including age and sex as covariates) estimated heritability as approaching 1.0 (0.99; 95% CI 0.98–1.0). Extending the regression approach to the use of a time-censored variable (age of diabetes diagnosis), multivariate Cox regression (with the same covariates) yielded similarly high heritability estimates for age of onset of diabetes (0.99; 95% CI 0.92–1.0). Essentially identical results were obtained when including prolonged steroid treatment and cross-sectional or longitudinal lung function in the regression model (data not shown). Thus, the high heritability estimate for diabetes in CF could not be attributed to correlation with other traits that are under genetic control.
Discussion
Pancreatic exocrine insufficiency, which is highly correlated with specific CFTR mutations (26), was a near-universal prerequisite for developing diabetes in this study. However, not all patients with pancreatic insufficiency develop this complication. We demonstrate here that the risk of diabetes correlates with the degree of gene sharing among related CF patients, indicating that genes other than CFTR contribute substantially to diabetes complicating CF. Three analyses were performed to quantify the effect of these genetic modifiers of diabetes in CF. First, multivariate analysis revealed that having a MZ CF twin with diabetes (100% gene sharing) independently conferred a high risk of diabetes (similar to that of pancreatic insufficiency) and that having a sibling or DZ twin with diabetes (50% gene sharing) also conferred a significant, though, lower risk. Second, comparison of MZ twin pairs and age- and sex-matched DZ twins and siblings revealed that MZ twins had substantially higher rates of concordance for diabetes. Third, accounting for important diabetes risk factors by matching or by regression had negligible effect on the estimate of a strong genetic component (i.e. heritability) for diabetes risk in CF.
A major strength of this study is that recruitment of subjects in the CF Twin and Sibling Study was based on a diagnosis of CF and not diabetes. Thus, biases of ascertainment or phenotype definition (when the second member of a pair receives greater scrutiny for the diagnosis of diabetes) were minimized. Furthermore, the high density of clinical data available (standard of care is to see CF patients every 3 months) allowed for detailed characterization of diabetes phenotype in a longitudinal manner, making it possible to distinguish between intermittent and chronic diabetes. Weaknesses of the study included the small number and young age of DZ twins that necessitated the use of siblings as proxies for individuals sharing 50% of their genes. However, the high estimate of heritability is primarily due to the high MZ twin concordance rate. This study was not able to distinguish between the effects of shared genes and shared intrauterine environment (or features thereof, e.g. the placenta), which is a recognized limitation even of classical twin studies (25). The absence of concordant DZ twin pairs suggests that such unaccounted sources of between-sibling variability are not likely to affect the conclusion that genetic modifiers are largely responsible for diabetes in CF. In addition, we did not have complete retrospective data for fasting glycemia, which would have allowed classifying patients strictly by CF consensus criteria (6). However, treatment with insulin or oral agents is a reasonable proxy for fasting hyperglycemia for patients treated according to those same guidelines. Furthermore, the insidious onset of diabetes in CF can lead to misdiagnoses, especially if only a single point in time is considered. Our use of longitudinal data should have reduced misclassification of euglycemic patients by requiring multiple normal screening tests. These data also allowed us to distinguish those with intermittent hyperglycemic episodes (e.g. during disease exacerbations and/or treatment with systemic steroids) from those with chronic diabetes. Finally, our criteria may have placed some CF patients with chronic diabetes in the hyperglycemic or possible diabetes categories. However, this approach should have minimized the false-positive classifications in the euglycemic and diabetes categories, albeit at the cost of reducing study power.
Although genetic modifiers are an important cause of diabetes in CF, nongenetic factors (such as treatment with steroids) also play a role. There is significant agreement between risk factors found in the CF families in the Twin and Sibling Study and what has been seen for unrelated CF patients. As noted here, diabetes has been associated with increasing age, pancreatic insufficiency, reduced lung function, poor nutritional status, and ABPA and/or systemic steroid treatment (13,25,30). We found that increasing duration of steroid treatment and prolonged steroid treatment (over a threshold of 30 d) were more potent risk factors for diabetes in CF than steroid treatment of no specific duration. Treatment duration may be a proxy for cumulative steroid dose, which has been reported to be a risk factor for steroid-induced diabetes in the general population (31). Female sex has been noted to be a significant risk factor for diabetes in CF, and we saw evidence of this association in this study, although significance was not achieved, which may be due to insufficient power (13). Other associated CF complications such as lung transplantation (32), markers of chronic inflammation (24), and liver disease (33) were not evaluated.
Results of this study enable a comparison of genetic contribution to diabetes in CF to type 1 and 2 diabetes in the general population. The concordance rate of diabetes among CF siblings (0.18) divided by the frequency of diabetes in this family study (0.09) estimates the sibling recurrence risk ratio at 2.0. When adjusting for pertinent covariates, the sibling odds ratio was estimated at 4.6 (logistic regression) and 2.0 (HR; Cox regression). Of note, the estimates of sibling recurrence risk for diabetes in CF patients are more comparable to recent estimates for type 2 diabetes [sibling recurrence risk ranging from 1.2–1.8 (34)] than to type 1 diabetes [sibling recurrence risk of ∼15 (35)], whereas heritability estimates are higher than both type 1 and 2 diabetes [0.72–0.88 (36) and 0.26–0.61 (37), respectively]. Thus, diabetes complicating CF demonstrates a degree of genetic control, at least as high as other forms of diabetes occurring in the general population.
Acknowledgments
The authors gratefully acknowledge the many cystic fibrosis patients, families, research coordinators, and clinicians in the Cystic Fibrosis Twin and Sibling Study. We thank Lindsay B. Henderson, B.S., Deanna Green, M.D., Patrick Sosnay, M.D., and Kathryn McDougal, Ph.D., for helpful discussions, Patricia Cornwall for administrative help, and David J. Cutler, Ph.D., for guidance in data analysis.
Footnotes
Funding was provided by National Institutes of Health Grants DK076446 (to S.M.B.), DK044003 (to G.R.C), and HL068927 (to G.R.C.), and Cystic Fibrosis Foundation Grant CUTTIN06P0 (to G.R.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 6, 2009
Abbreviations: ABPA, Allergic bronchopulmonary aspergillosis; CF, cystic fibrosis; CFF, Cystic Fibrosis Foundation; CFTR, cystic fibrosis transmembrane conductance regulator; CI, confidence interval; DZ, dizygous; HbA1c, glycosylated hemoglobin; HR, hazard ratio; MZ, monozygous.
References
- Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR 2001 Cystic fibrosis. In: Scriver CR, Beaudet AL, Valle D, Sly WS, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 5121–5188 [Google Scholar]
- Cystic Fibrosis Foundation 2005 Cystic Fibrosis Foundation Patient Registry Annual Data Report 2005. Bethesda, MD: Cystic Fibrosis Foundation [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC 1989 Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073 [DOI] [PubMed] [Google Scholar]
- Mackie AD, Thornton SJ, Edenborough FP 2003 Cystic fibrosis-related diabetes. Diabet Med 20:425–436 [DOI] [PubMed] [Google Scholar]
- Brennan AL, Geddes DM, Gyi KM, Baker EH 2004 Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros 3:209–222 [DOI] [PubMed] [Google Scholar]
- Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, Brunzell C, Campbell III PW, Chesrown SE, Duchow C, Fink RJ, Fitzsimmons SC, Hamilton N, Hirsch I, Howenstine MS, Klein DJ, Madhun Z, Pencharz PB, Quittner AL, Robbins MK, Schindler T, Schissel K, Schwarzenberg SJ, Stallings VA, Tullis DE, Zipf WB 1999 Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 45:61–73 [DOI] [PubMed] [Google Scholar]
- Lanng S, Thorsteinsson B, Nerup J, Koch C 1992 Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr 151:684–687 [DOI] [PubMed] [Google Scholar]
- Milla CE, Warwick WJ, Moran A 2000 Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 162(3 Pt 1):891–895 [DOI] [PubMed] [Google Scholar]
- Nousia-Arvanitakis S, Galli-Tsinopoulou A, Karamouzis M 2001 Insulin improves clinical status of patients with cystic-fibrosis-related diabetes mellitus. Acta Paediatr 90:515–519 [PubMed] [Google Scholar]
- Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ 2008 Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration 76:181–186 [DOI] [PubMed] [Google Scholar]
- Cucinotta D, De Luca F, Scoglio R, Lombardo F, Sferlazzas C, Di Benedetto A, Magazzù G, Raimondo G, Arrigo T 1999 Factors affecting diabetes mellitus onset in cystic fibrosis: evidence from a 10-year follow-up study. Acta Paediatr 88:389–393 [DOI] [PubMed] [Google Scholar]
- Cotellessa M, Minicucci L, Diana MC, Prigione F, Di Febbraro L, Gagliardini R, Manca A, Battistini F, Taccetti G, Magazzù G, Padoan R, Pizzamiglio G, Raia V, Iapichino L, Cardella F, Grinzich G, Lucidi V, Tuccio G, Bignamini E, Salvatore D, Lorini R 2000 Phenotype/genotype correlation and cystic fibrosis related diabetes mellitus (Italian Multicenter Study). J Pediatr Endocrinol Metab 13:1087–1093 [DOI] [PubMed] [Google Scholar]
- Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ 2005 Epidemiology of cystic fibrosis-related diabetes. J Pediatr 146:681–687 [DOI] [PubMed] [Google Scholar]
- Florez JC, Hirschhorn J, Altshuler D 2003 The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet 4:257–291 [DOI] [PubMed] [Google Scholar]
- Blackman SM, Deering-Brose R, McWilliams R, Naughton K, Coleman B, Lai T, Algire M, Beck S, Hoover-Fong J, Hamosh A, Fallin MD, West K, Arking DE, Chakravarti A, Cutler DJ, Cutting GR 2006 Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology 131:1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanscoy LL, Blackman SM, Collaco JM, Bowers A, Lai T, Naughton K, Algire M, McWilliams R, Beck S, Hoover-Fong J, Hamosh A, Cutler D, Cutting GR 2007 Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med 175:1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein BJ, Cutting GR 1998 The diagnosis of cystic fibrosis: a consensus statement. J Pediatr 132:589–595 [DOI] [PubMed] [Google Scholar]
- Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR 2008 Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 299:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS 1965 Inheritance of liability to certain diseases estimated from incidence among relatives. Ann Hum Genet 29:51–71 [Google Scholar]
- Ramakrishnan V, Meyer JM, Goldberg J, Henderson WG 1996 Univariate analysis of dichotomous or ordinal data from twin pairs: a simulation study comparing structural equation modeling and logistic regression. Genet Epidemiol 13:79–90 [DOI] [PubMed] [Google Scholar]
- Lanng S, Thorsteinsson B, Lund-Andersen C, Nerup J, Schiotz PO, Koch C 1994 Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late diabetic complications. Acta Paediatr 83:72–77 [DOI] [PubMed] [Google Scholar]
- Moran A, Doherty L, Wang X, Thomas W 1998 Abnormal glucose metabolism in cystic fibrosis. J Pediatr 133:10–17 [DOI] [PubMed] [Google Scholar]
- Yung B, Hodson ME 1999 Diabetes in cystic fibrosis. J R Soc Med 92(Suppl 37):35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, de Blic J, Lenoir G, Robert JJ 2008 Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr 152:540–545 [DOI] [PubMed] [Google Scholar]
- Adler AI, Gunn E, Haworth CS, Bilton D 2007 Characteristics of adults with and without cystic fibrosis-related diabetes. Diabet Med 24:1143–1148 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Corey M, Forstner G, Zielenski J, Tsui LC, Ellis L, Tullis E, Durie P 2003 Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut 52:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla CE 2004 Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med 10:505–509 [DOI] [PubMed] [Google Scholar]
- Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV 1995 A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. J Pediatr 126:515–523 [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC 1996 Heritability. In: Introduction to quantitative genetics. 4th ed. Essex, UK: Longman; 160–183 [Google Scholar]
- Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C 1995 Glucose tolerance in patients with cystic fibrosis: five year prospective study. Br Med J 311:655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisaeter AV, Hartmann A 2001 Risk factors and incidence of posttransplant diabetes mellitus. Transplant Proc 33(Suppl):8S–18S [DOI] [PubMed] [Google Scholar]
- Hadjiliadis D, Madill J, Chaparro C, Tsang A, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S, Elizabeth Tullis D 2005 Incidence and prevalence of diabetes mellitus in patients with cystic fibrosis undergoing lung transplantation before and after lung transplantation. Clin Transplant 19:773–778 [DOI] [PubMed] [Google Scholar]
- Minicucci L, Lorini R, Giannattasio A, Colombo C, Iapichino L, Reali MF, Padoan R, Calevo MG, Casciaro R, De Alessandri A, Haupt R 2007 Liver disease as risk factor for cystic fibrosis-related diabetes development. Acta Paediatr 96:736–739 [DOI] [PubMed] [Google Scholar]
- Weijnen CF, Rich SS, Meigs JB, Krolewski AS, Warram JH 2002 Risk of diabetes in siblings of index cases with type 2 diabetes: implications for genetic studies. Diabet Med 19:41–50 [DOI] [PubMed] [Google Scholar]
- Field LL 2002 Genetic linkage and association studies of type I diabetes: challenges and rewards. Diabetologia 45:21–35 [DOI] [PubMed] [Google Scholar]
- Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J 2003 Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 52:1052–1055 [DOI] [PubMed] [Google Scholar]
- Vaag A, Poulsen P 2007 Twins in metabolic and diabetes research: what do they tell us? Curr Opin Clin Nutr Metab Care 10:591–596 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2008 Diagnosis and classification of diabetes mellitus. Diabetes Care 31(Suppl 1):S55–S60 [DOI] [PubMed] [Google Scholar]