Abstract
Objective: Linkage to type 2 diabetes (T2D) is well replicated on chromosome 1q21-q23. Within this region, T2D was associated with common single nucleotide polymorphisms that marked an extended linkage disequilibrium block, including the liver pyruvate kinase gene (PKLR), in several European-derived populations. In this study we sought to determine the molecular basis for the association and the phenotypic consequences of the risk haplotype.
Research Design and Methods: Genes surrounding PKLR were resequenced in European-American and African-American cases and controls, and association with T2D was tested. Copy number variants (CNVs) were tested for four regions with real-time PCR. Expression of genes in the region was tested in adipose and muscle from nondiabetic subjects with each genotype. Insulin secretion, insulin sensitivity, and hepatic glucose production were tested in nondiabetic individuals with each haplotype combination.
Results: No coding variant in the region was associated with T2D. CNVs were rare and not associated with T2D. PKLR was not expressed in available tissues, but expression of genes HCN3, CLK2, SCAMP3, and FDPS was not associated with haplotype combinations in adipose or muscle. Haplotype combinations were not associated with insulin secretion or peripheral insulin sensitivity, but homozygous carriers of the risk haplotype had increased hepatic glucose production during hyperinsulinemia.
Conclusions: Noncoding variants in the PKLR region likely alter gene expression of one or more genes. Our extensive physiological and molecular studies suggest increased hepatic glucose production and reduced hepatic insulin sensitivity, thus pointing to PKLR itself as the most likely candidate gene in this population.
Noncoding DNA polymorphisms near the liver pyruvate kinase gene are associated with reduced insulin suppression of hepatic glucose production in humans.
Multiple studies in populations of European descent from England and the United States and Chinese, Pima Indian, and African-American populations have shown linkage of type 2 diabetes (T2D) to chromosome 1q over a broad region (1,2). The region encompasses a very large number of strong candidate genes, and fine mapping suggested at least three possible linkage peaks (3), the most proximal of which includes the liver pyruvate kinase gene (PKLR). An early analysis of that gene by our lab showed a significant association with T2D in European-Americans drawn from families contributing to the linkage (4). Subsequent studies suggested replication in Amish families (5) and in individuals of European descent from the International Type 2 Diabetes 1q Consortium (6). Initial analyses of the PKLR region including HapMap data (7) and 1q Consortium genotypes (our unpublished data) suggested an extended region of strong linkage disequilibrium (LD). Such strong LD raised challenges for the identification of the causative variant using traditional genetic approaches. Indeed, this region is well characterized for LD, extending centromeric from the PKLR locus to the Gaucher’s Disease gene GBA (8).
The structure of the PKLR region further complicated the interpretation of the association. Just centromeric to PKLR is the gene HCN3, a widely expressed sodium and potassium channel gene. Further centromeric is CLK2, a dual specificity kinase that acts to activate the phosphatase PTP1B (9), itself a diabetes-associated candidate gene that in turn dephosphorylates and thus inactivates the insulin receptor (10). Just centromeric and nearly overlapping CLK2 is SCAMP3, which encodes a vesicular transport protein that is predicted by informatics approaches (11) to be a strong T2D candidate gene based on function and tissue distribution. The final centromeric gene before the gene encoding Gaucher’s disease (GBA) is C1orf2, an open reading frame and cDNA about which little is known. Telomeric to PKLR and on the opposite strand (Fig. 1) is FDPS, which is under the control of the liver X receptor and encodes the enzyme that synthesizes the formation of farnesyl pyrophosphate in the cholesterol synthesis pathway. FDPS is widely expressed including in liver, muscle, and pancreas. Telomeric on the same strand is RUSC1, another widely expressed protein that may be involved in signaling pathways, although function has been attributed to neuronal growth (12). Farthest telomeric is the large gene ASH1L, a widely expressed histone methyltransferase (13). Thus, this relatively small region includes at least seven genes that are expressed in diabetes-related tissues and may be relevant to diabetes pathogenesis. Furthermore, transcripts overlap on opposite strands in configurations that have been shown by the ENCODE project to affect gene regulation (14). Finally, this region of chromosome 1q has known copy number variants (CNVs) that could potentially alter gene dosage or regulation and, given the gene dense nature of the region, might contribute to T2D.
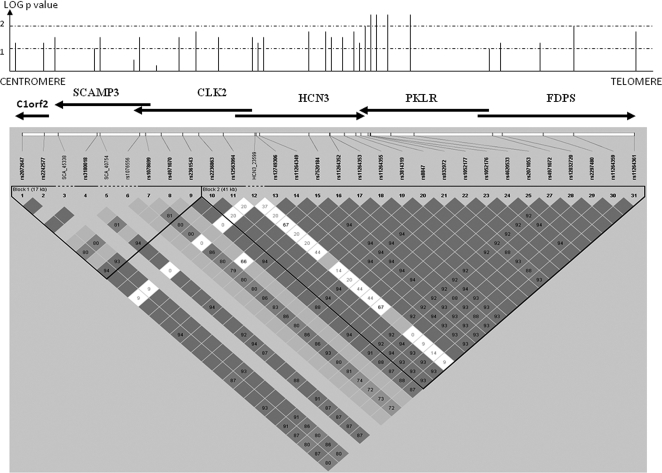
Figure 1.
Association and LD map in European-American samples. The association in European ancestry cases and controls from Utah is shown on the log scale as the height of the line. Note that lines indicating the association are not shown exactly above the genes for purposes of clarity. Genes in the region are shown as arrows pointing in the direction of transcription, and SNP or insertion/deletion markers are shown in their approximate locations on the LD map. Dark squares represent D′ values; dark squares without values represent 1.0, values under 1.0 are presented as a percent (1 = 100%), and light colored squares have insufficient data to be significant (low frequency SNPs).
Based on our prior data and the strength of the preliminary findings from the International Type 2 Diabetes Chromosome 1q Consortium, we undertook a multifaceted investigation of this region. We searched for novel variants that might explain the association, determined the role of reported CNV, examined expression of genes and transcripts in the PKLR region in adipose and muscle, and finally determined the physiological impact of the extended haplotypes in this region.
Subjects and Methods
Human subjects
Multiple populations were examined for this study. Case/control association studies in European-Americans were conducted in 384 individuals as described previously (15). Cases included individuals from families with linkage to chromosome 1q. Individuals were of predominately Northern European Ancestry by history. Association studies in African-American individuals were conducted in 184 nondiabetic individuals and 352 individuals with T2D who had a first-degree relative with T2D (15). All African-American individuals were ascertained in Arkansas, and on average show 20% Caucasian admixture. Screening studies for new variants were conducted in individuals representing each extended haplotype and comprised 16 European-American and 16 African-American individuals with T2D and a family history of T2D, and eight European-American and eight African-American control individuals. To determine the physiological consequences of haplotypes in this region, we examined 255 nondiabetic, European-American individuals (90 males, 165 females) who had undergone iv glucose tolerance tests as described in detail elsewhere (15,16). Details of this population are shown in Table 1. To estimate hepatic glucose production along with insulin sensitivity and secretion, we tested 28 nondiabetic, European-American individuals recruited by genotype (13 homozygous common, 10 heterozygous, 5 homozygous rare; 8 males, 20 females) using the euglycemic-hyperinsulinemic clamp technique, as described below (see Table 3). All study subjects signed written, informed consent forms under protocols approved by the University of Arkansas for Medical Sciences and Central Arkansas Veterans Healthcare System Institutional Review Boards.
Table 1.
Metabolic traits by PKLR region haplotype combination
| Trait | Hom-C | Heterozygous | Hom-R | P value |
|---|---|---|---|---|
| n (M/F) | 128 (45/83) | 113 (43/70) | 14 (2/12) | |
| Age (yr) | 38.2 (9.6) | 36.9 (9.3) | 39.3 (5.6) | 0.4 |
| BMI (kg/m2) | 30.0 (6.3) | 29.2 (5.6) | 32.9 (5.0) | 0.067 |
| % Body fat | 35.6 (9.0) | 33.9 (9.6) | 40.9 (6.3) | 0.021 |
| Waist-hip ratio | 0.89 (0.08) | 0.89 (0.08) | 0.90 (0.08) | 0.91 |
| Systolic BP (mm Hg) | 117 (12) | 117 (12) | 115 (11) | 0.88 |
| Diastolic BP (mm Hg) | 77 (12) | 77 (8) | 75 (9) | 0.67 |
| Total cholesterol (mmol/liter) | 4.68 (4.53, 4.86) | 4.50 (3.44, 4.68) | 4.81 (4.34, 5.35) | 0.24 |
| Triglycerides (mmol/liter) | 1.21 (1.11, 1.32) | 1.21 (1.11, 1.32) | 1.34 (1.04, 1.73) | 0.64 |
| HDL cholesterol (mmol/liter) | 1.25 (1.20, 1.31) | 1.23 (1.71, 1.29) | 1.20 (1.06, 1.36) | 0.72 |
| LDL cholesterol (mol/liter) | 2.74 (2.59, 2.87) | 2.90 (2.64, 3.18) | 3.08 (2.38, 3.96) | 0.12 |
| SI (× 10−4 min−1 [μU/ml]−1) | 3.18 (2.84, 3.56) | 3.43 (3.00, 3.91) | 3.09 (2.15, 4.44) | 0.65 |
| AIRg (pmol-min/liter) | 2190 (1908, 2514) | 2100 (1824, 2418) | 2070 (1254, 3414) | 0.90 |
| Sg | 0.0173 (0.006) | 0.0183 (0.007) | 0.0172 (0.007) | 0.54 |
| Disposition index | 1131 (981, 1304) | 1199 (1030, 1394) | 1064 (582, 1944) | 0.80 |
| AIRmax (pmol/liter) | 1470 (1260, 1710) | 1164 (984, 1386) | 1380 (762, 2508) | 0.27 |
Traits are shown for combinations of extended haplotypes: Hom-C, common homozygotes; Heterozygotes; and Hom-R, rare haplotype homozygotes. Recombinant haplotypes were assigned to the closest common haplotype. Data are shown as mean (sd) for normal or near normal data, or as geometric mean (95% confidence interval) for nonnormal data. AIRmax was available in only 106 subjects. M, Males; F, females; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SI, minimal model-derived insulin sensitivity; AIRg, insulin area under curve from 2 to 20 min after glucose bolus; Sg, glucose effectiveness; AIRmax, insulin area under curve after maximally stimulated arginine bolus.
Table 3.
Euglycemic clamp studies by PKLR region haplotype
| Trait | Homozygous common | Heterozygous | Homozygous rare | P value |
|---|---|---|---|---|
| Gender (M/F) | 6/7 | 2/8 | 0/5 | |
| Age (yr) | 40.1 (6.8) | 39.8 (8.4) | 43.8 (2.39) | 0.5 |
| BMI (kg/m2) | 35.1 (3.1) | 34.6 (5.4) | 31.0 (4.6) | 0.2 |
| % Fat | 42.2 (6.4) | 42.4 (4.7) | 41.5 (6.0) | 0.8 |
| Waist-hip ratio | 0.956 (0.075) | 0.924 (0.074) | 0.921 (0.99) | 0.5 |
| Systolic BP (mm Hg) | 120.0 (11.4) | 119.6 (12.9) | 119.4 (11.3) | 0.9 |
| Diastolic BP (mm Hg) | 79.6 (8.0) | 73.7 (9.1) | 75.7 (8.4) | 0.07 |
| Basal HGP (μmol/kg-FFM/min) | 31.8 (7.1) | 29.1 (6.6) | 32.5 (8.9) | 0.6 |
| Insulin-sup. HGP (μmol/kg-FFM/min) | 14.9 (9.9) | 13.6 (7.4) | 40.3 (20.1) | 0.023 |
| GD (μmol/kg -FFM/min) | 35.6 (19.3) | 36.4 (15.9) | 76.6 (25.0) | 0.014 |
| Ox GD (μmol/kg-FFM/min) | 22.3 (5.8) | 23.5 (6.9) | 28.2 (4.2) | 0.09 |
| Nonox GD (μmol/kg-FFM/min) | 13.2 (14.6) | 12.9 (9.7) | 48.5 (22.9) | 0.008 |
Demographic and phenotypic data for 28 individuals recruited for euglycemic clamp studies. P values are based on ANOVA, with natural or common logarithmic transformation where required. All significant values were also significant without transformation. M, Male; F, female; BP, blood pressure; HGP, hepatic glucose production; Insulin-sup. HGP, hepatic glucose production during hyperinsulinemic portion of clamp; GD, glucose disposal, shown normalized for fat free mass (FFM); Ox GD, oxidative glucose disposal; Nonox GD, nonoxidative glucose disposal. All measures of glucose disposal and hepatic glucose production are shown normalized to fat free mass (FFM: μmol/kg-FFM/min).
Euglycemic-hyperinsulinemic clamp study
Insulin-mediated glucose disposal was assessed using the euglycemic-hyperinsulinemic clamp technique (17,18). A primed continuous infusion (priming dose, 18 μmol · kg−1; infusion, 0.22 μmol · kg−1 · min−1) of [6,6-2H2]glucose (Cambridge Isotope Labs, Andover, MA) was administered for the duration of the clamp to allow measurement of endogenous glucose production at baseline and during hyperinsulinemia. After 150 min of [6,6-2H2]glucose infusion, insulin (Humulin; Eli Lilly, Indianapolis, IN) was infused at 240 pmol · m−2 · min−1, and 20% glucose was infused to maintain a plasma glucose at 5 mmol/liter ± 4% (range, 2–7%). A spike of [6,6-2H2]glucose (44.4 mmol) was added to the exogenously administered glucose to maintain a constant plasma glucose isotopic enrichment. Glucose was determined every 5 min, and plasma insulin, C-peptide, and glucose isotope enrichment was determined every 15 min. Mean glucose disposal (μmol · kg FFM−1 · min−1), calculated as glucose infusion plus isotopically derived endogenous glucose production during the final 30 min of the clamp, was used to assess insulin-mediated glucose disposal, and measurement of substrate oxidation was performed by continuous, open-circuit indirect calorimetry (Vmax 29N; SensorMedics Corp., Yorba Linda, CA) during this same time period. Nonoxidative glucose disposal was calculated by subtracting carbohydrate oxidation rates from the sum of exogenous glucose infusion plus endogenous glucose production rates. For two individuals, measured oxidative glucose disposal slightly exceeded the measured total glucose disposal, and the two measures were set to be equal with nonoxidative glucose disposal set to 0. Plasma [6,6-2H2] glucose isotopic enrichment was measured by gas chromatography-mass spectrometry (model 6890N; Agilent Technologies, Palo Alto, CA) after plasma deproteinization and purification (19). Basal endogenous glucose production rate was determined using standard steady-state calculations (20), and the rate of endogenous glucose production during hyperinsulinemia was calculated using a modification of the Steele equation (21).
Mutation detection
The region from 153,567,583 bp centromeric to RUSC1 telomeric (153,488,566 bp) was screened for novel variants in 16 individuals with T2D and eight controls of both European-American and African-American descent using either denaturing HPLC (Wave System 3500; Transgenomic, Inc., Omaha, NE) (22,23) or bidirectional resequencing (Polymorphic DNA, Inc., Alameda, CA). Bidirectional resequencing was the primary method for gene HCN3; all other genes were screened primarily by denaturing HPLC, which in our hands is more sensitive, unless the particular fragment had an unfavorable melting profile. Diabetic individuals chosen for screening had a family history of T2D and represented each haplotype combination. Fragments showing altered migration were confirmed by bidirectional resequencing. Screening included individuals homozygous for each extended haplotype as well as heterozygous individuals. Screened regions included all exons, conserved regions, promoter sequences up to 1000 bp, and at least 100 bp flanking each exon. In total, we directly screened or resequenced 58 kb of the 79-kb region of LD. The PKLR gene, which was screened previously (4), was not rescreened for the current study, and we did not extend screening into the large ASH1L gene.
Genotyping
Initial single nucleotide polymorphism (SNP) genotyping for newly identified SNPs was performed in pooled case and control samples using Pyrosequencing (Biotage AB, Uppsula, Sweden). Pools were constructed in triplicate and measured in duplicate. SNPs with a minor allele frequency over 0.02 were typed in 48 nondiabetic individuals of European ancestry and 48 nondiabetic African-American individuals to establish LD. We subsequently typed individual samples to define the association using either Pyrosequencing or Taqman assays. We selected SNPs for individual typing in both European-American and African-American populations, providing the SNP was not in complete LD (r2 > 0.9) with a SNP already typed in individual samples, and had a minor allele frequency over 5%. SNPs were advanced to second-stage individual typing if the allele frequency difference in pools exceeded 5%. All nonsynonymous SNPs were typed in individual samples. SNPs that could not be typed by Taqman or Pyrosequencing were examined by restriction digest. Primer sequences and methods are provided in Supplemental Table 1S (which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Methods for typing of each SNP are provided along with allele frequencies in Supplemental Table 2S. For ASH1L, SNPs were chosen based on allele frequency, distribution, and ability to tag the gene using the TAGGER program (24). We chose six SNPs that had frequencies over 10% and tagged 82% of all alleles. The six SNPs tagged all six observed haplotypes based on HapMap European (CEU) data.
CNV
Five CNVs were selected from the Database of Genomic Variants (http://projects.tcag.ca/variation/) and tested with four chromosome 1 primer sets (A, 153,407,866-153,407,975; B, 153,464,362-153,464,455; C, 153,504,305-153,504,420; D, 153,514,225-153,514,331; Supplemental Fig. 1S) by quantitative real-time PCR. Primer sets C and D overlapped genes CLK2 and HCN3. We tested 256 individuals for each primer set, comprising 64 diabetic and 64 control individuals in both African-American and European-American individuals. Briefly, we amplified 16 ng of genomic DNA with 1X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) for 40 cycles with 0.3 μmol/liter forward and reverse primers using a Rotorgene 2000 Real time-PCR system (Corbett Life Science, Sydney Australia). Results were normalized to the diploid SPRY3 gene (X chromosome, 154,654,501-154,654,628; and Y chromosome, 57,513,701-57,513,828) (25). Additionally, we validated the methods using X chromosome gene TEX11 (69, 761, 280-69, 761, 379). Samples showing CNVs were validated in three additional DNA aliquots along with control samples.
Gene expression studies
Total RNA was extracted from adipose tissue using the RNA Easy Lipid Tissue Mini kit (QIAGEN Inc., Valencia, CA), and from muscle using the Ultraspec RNA kit (Biotecx Laboratories Inc., Houston, TX). Total RNA (1 μg) was reverse transcribed using random hexamers (TaqMan Reverse Transcription reagents; Applied Biosystems), and expression quantified by real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems) with 0.3 μmol/liter of gene-specific forward and reverse primers. Primers were designed to cross intron:exon boundaries. Expression levels were normalized to 18S RNA, and standard curves were generated using pooled RNA.
Statistical analysis
SNP and DNA variant association with T2D was tested separately for each ethnic group using the Fisher Exact test. Pairwise LD (D′ and r2) was calculated, and haplotypes were constructed with the Expectation Maximization algorithm in the combined case and control population data using Haploview 4.1 (Whitehead Institute, Cambridge, MA). Correlations of genotype with physiological traits and gene expression were conducted using ANOVA or general linear models in SPSS version 12.0 for Windows (SPSS, Inc., Chicago, IL). Nonnormal variables were logarithmically transformed to normality. We considered P < 0.05 to be significant given strong prior hypotheses and lack of independence between tests.
Results
Genetic screening and CNVs
We screened known genes and putative transcripts SCAMP3, CLK2, HCN3, FDPS, C1ORF104, and RUSC1; PKLR was reported previously (4). An additional six SNPs were chosen to tag common variants in the very large gene, ASH1L, and two additional SNPs in the transcript C1ORF2 based on HapMap data. The association with T2D was restricted to European-Americans and to SNPs or Insertion-Deletion (InDel) variants in nearly complete LD (r2 > 0.9) with previously described PKLR SNPs. We examined 87 variants, including six intronic InDel variants, 66 noncoding SNPs, three synonymous SNPs, and 12 nonsynonymous SNPs (Fig. 1 and Supplemental Table 2S). All nonsynonymous SNPs were rare (minor allele frequency under 5%), none was observed in both European-American and African-American populations, and most were newly described (Supplemental Table 1S). Only P55S in SCAMP3 (exon 3) showed any trend to significance, but no nonsynonymous SNP could explain the association of common haplotypes with T2D in European-Americans. Most common noncoding variants in European-Americans fell on one of two common haplotypes (Table 2) that fully explained the observed association, but these common variants showed little or no trend to association with T2D in African-Americans. In European-Americans, the associated SNPs extended from C1ORF2 centromeric through the 3′ (centromeric) end of ASH1L near the RUSC1 gene telomeric. No common variants were found in RUSC1, and only the most centromeric SNPs in ASH1L showed any association. These findings were consistent with the break in LD structure between RUSC1 and ASHL1. Thus, extensive resequencing suggested that common haplotypes of noncoding variants, most probably altering gene expression, were responsible for the observed association of the PKLR region with T2D in European-derived populations.
Table 2.
Six locus haplotypes in the PKLR region
| SNP | RS3180018 | RS6836863 | RS8847 | RS1052177 | RS11264359 | RS11264351 | Frequency |
|---|---|---|---|---|---|---|---|
| Location | 153,496,775 | 153,507,985 | 153,525,947 | 153,526,974 | 153,549,453 | 153,556,169 | |
| 1 | C | G | G | T | T | A | 0.71 |
| 2 | T | A | A | C | G | G | 0.24 |
| 2a | C | A | A | C | G | G | 0.01 |
| 2b | T | A | A | C | T | A | 0.01 |
Shown are four observed haplotypes with over 1% allele frequency estimated using the expectation maximization algorithm in HaploView 4.1. Locations of the six tag SNPs are given in base pairs based on build 35. For purposes of this study, haplotypes 2, 2a, and 2b were considered the same. Recombinant bases are marked in bold.
The chromosome 1q21-23 region encompasses multiple known CNVs that might influence gene expression. We tested four primer sets covering five CNVs over the region from 153,407,866 to 153,514,331, extending from centromeric of our associated haplotype to the region of genes CLK2 and HCN3. No CNV was identified in the CLK2 or HCN3 region, but both centromeric CNVs showed copy number variation with loss of one copy for the most centromeric primer set (A, 153,407,866-153,407,975) in two European-American controls and gain of one copy at the next telomeric set (B, 153,464,362-153,464,455) in 11 of 63 diabetic and 6 of 56 control samples from African-Americans (P = 0.3; Supplemental Fig. 1S). Hence, copy number variation did not explain the association of the region with T2D.
Physiological studies of subjects by PKLR region haplotypes
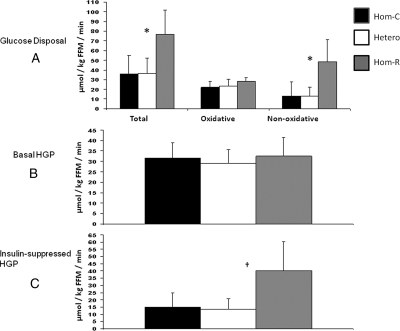
Because common SNPs in the region surrounding the PKLR locus could be reduced to two extended haplotypes, we examined the physiological impact of the three common haplotype combinations in 255 European-American individuals who had undergone insulin-modified, frequently sampled iv glucose tolerance tests (FSIGT) (Table 1). Individuals homozygous for the risk haplotype were more obese by dual-energy x-ray absorptiometry-determined percentage fat, but insulin sensitivity (SI), insulin secretion (acute insulin response to glucose, disposition index, or maximally potentiated arginine-stimulated insulin secretion), lipids, and blood pressure did not differ by haplotype combination. To test directly the role of hepatic glucose production, we recruited 28 additional European-American individuals in a separate study for the three haplotype combinations (13 common haplotype homozygotes, 10 heterozygotes, and 5 rare haplotype homozygotes). In contrast to the larger FSIGT study, both total and nonoxidative glucose disposal were increased significantly in individuals homozygous for the T2D-associated haplotype, as was the insulin-suppressed hepatic glucose production (Table 3 and Fig. 2). These data suggested a recessive effect on hepatic insulin resistance to suppression of glucose output.
Figure 2.
Euglycemic, hyperinsulinemic clamp studies by genotype. A, Glucose disposal. Total, oxidative, and nonoxidative glucose disposal are shown for each haplotype combination. Error bars represent sd values. Asterisks represent significant differences (P < 0.02). B, Basal hepatic glucose production. Noninsulin stimulated (basal) hepatic glucose production (HGP); no differences were significant. Error bars show sd values. C, Insulin-suppressed hepatic glucose production (insulin-suppressed HGP). Error bars show sd values, and the dagger represents a significant difference (P = 0.02). For all panels, Hom-C are carriers of two copies of the common haplotype, Hetero represents individuals with one copy of each haplotype, and Hom-R represents carriers of two copies of the rare (risk) haplotype. All values are shown normalized to fat-free mass (FFM).
PKLR region gene expression studies
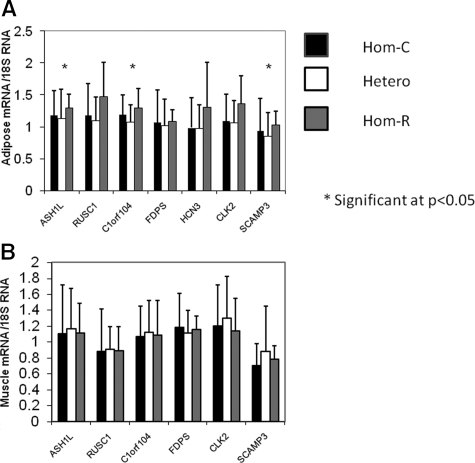
We examined gene expression in sc adipose tissue from 105 individuals and muscle from 100 individuals who were studied by either clamp or FSIGT. Expression of ASHL1, RUSC1, C1orf104, FDPS, CLK2, and SCAMP3 was all detectable in muscle, but did not differ significantly by genotype (Fig. 3). In adipose, only transcript C1orf104 was significant by ANOVA (P < 0.04), albeit with modestly reduced expression in heterozygotes compared with either homozygote. Gene expression of ASH1L, RUSC1, and SCAMP3, but not CLK2, was modestly increased in adipose tissue from rare haplotype homozygotes and reached nominal significance under a recessive model (Fig. 3; P < 0.05). PKLR expression was undetectable in adipose and muscle.
Figure 3.
Gene expression in adipose and muscle by genotype. Gene expression levels normalized to 18S RNA are shown for each haplotype combination. A, Adipose gene expression. C1orf104 was significant on ANOVA (P = 0.036), whereas genes ASH1L and SCAMP3 showed significant differences (P = 0.023 and P = 0.014, respectively) under a recessive model by independent t test. B, Muscle gene expression. No differences were significant. Means and error bars represent sd values on the linear scale; significance was determined using ln-transformed values. Significance is marked by an asterisk.
Given the hypothesis that SCAMP3, FDPS, and CLK2 are involved in insulin action, we examined the correlation of gene expression in muscle and adipose tissue with SI corrected for age, gender, body mass index (BMI), and ethnicity, irrespective of genotype. Among 79 individuals who underwent adipose biopsies, RUSC1 but not CLK2, SCAMP3, or FDPS expression was significantly correlated with corrected SI (P < 0.01; r = 0.26). No transcript levels were correlated with SI among 75 individuals for whom we had muscle samples (P > 0.1; data not shown).
Discussion
We undertook the current study based on the considerable support for an association of SNPs in an extended region of LD with T2D. These findings were originally reported by us in Utah residents of northern European descent (4) and subsequently in the Amish (5), and more recently by the International Type 2 Diabetes Chromosome 1q Consortium (6), where this was one of two regions that showed an association. In contrast, recent studies expanding beyond families with chromosome 1q linkage have been less consistent (26). Furthermore, no region on chromosome 1q has appeared in recent genome-wide association studies despite the large LD block (27,28). These recent findings suggest either that the original reports, based on relatively small samples, were spurious or that gene-environment interactions obscured the association in large-scale follow-up studies. With considerable data supporting common regulatory variants in the genome (29) and the large number of candidate genes including PKLR, a role for this region in diabetes pathogenesis remains plausible.
Based on such considerations, we undertook a comprehensive analysis of the region surrounding the PKLR gene to identify any coding variants that might explain the association, to examine the role of CNVs, to explore the physiological consequences of carrying the putative risk haplotype, and finally to examine gene expression among individuals carrying that haplotype. This study thus took a unique approach to understand how a region of extended LD might influence disease risk. We identified no coding variant or CNV that was likely to explain the association, consistent with findings in most confirmed diabetes susceptibility loci including TCF7L2 (30). Such data suggest that the risk haplotype is most likely to regulate mRNA levels. Our most striking finding was increased hepatic glucose production in individuals homozygous for the risk allele, suggesting a recessive physiological effect. Consistent with this recessive model, we found modestly increased expression of genes ASH1L, RUSC1, and SCAMP3 in sc adipose. Unfortunately, we could not evaluate the PKLR gene in available tissues. Furthermore, although PKLR is known to be expressed in pancreatic β-cells where it is under control of HNF1α (31), we could find no evidence for altered insulin secretion in a reasonably large and carefully phenotyped population. An unexpected and puzzling finding was the increased glucose disposal in homozygous carriers of the risk haplotype, with most of this glucose uptake being nonoxidative. This increased glucose disposal might be expected to balance the reduced sensitivity to insulin suppression of hepatic glucose production. Perhaps this finding was the result of our selection of homozygous risk carriers who were glucose tolerant rather than a direct manifestation of the risk haplotype.
Considered together, our results suggest that altered expression of PKLR is the most likely mechanism by which this region would alter T2D risk. We base this argument on the apparent hepatic insulin resistance in homozygous carriers of the risk haplotype and the relative lack of altered gene expression for the strongest candidate genes, particularly CLK2, in available tissues although we cannot exclude a role for ASH1L, RUSC1, and SCAMP3 in adipose. Recently, Schadt et al. (32) examined gene expression for 39,000 transcripts in 400 human liver samples and mapped the transcripts to over 780,000 SNPs. Interestingly, SNP rs2277872, approximately 1 Mb from the SNPs studied here, showed cis-acting effects on PKLR expression (P = 0.0014), thus providing some support for cis-acting regulatory variants altering hepatic PKLR expression.
Our studies have several inherent limitations. Our intent for the genotyping studies was to identify coding variants that might explain the associated haplotypes, not to directly test the association. We recognize that our sample was significantly underpowered to detect novel associations based on effect sizes identified in recent genome-wide association studies. Furthermore, we did not attempt to test the association with more distant SNPs such as rs2277872 and thus cannot formally exclude the possibility that a variant outside of the LD block was in fact driving the apparent association with this region. Given the relatively low frequency of rare haplotype homozygotes (<10%), our sample size under a recessive model was limited for physiological and gene expression studies despite attempts to target recruitment based on genotype. Hence, the findings we report might be the result of statistical fluctuations, and our failure to find greater evidence for cis-acting effects could be the result of low power under a recessive model. Finally, we could not directly observe liver, nor could we examine tissue-specific effects in the pancreatic β-cells or visceral fat. We cannot exclude undetected, tissue-specific cis-acting effects of SNPs in tissues not available for study. Variants in this region might have altered expression of strong candidates CLK2 or SCAMP3 in tissues not available to us for study.
In summary, we have thoroughly explored the role of a region with multiple excellent diabetes candidate genes and significant prior evidence for an association with T2D in T2D pathogenesis. Our data suggest recessive effects of an extended haplotype, likely resulting from noncoding, regulatory variants, with the strongest effect to reduce insulin suppression of hepatic glucose production. Such data would suggest primary defects in hepatic gluconeogenesis as early contributors to the pathogenesis of type 2 diabetes. That hypothesis would be consistent with the expression of monogenic causes of T2D (glucokinase, HNF1α, HNF4α) in the liver as well as the pancreatic β-cell.
Supplementary Material
Acknowledgments
We thank Terri Hale and Oksana Hackney for assisting with subject recruitment. We thank the Clinical Research Center staff, particularly S. Ranganathan for insulin measurements; Cynthia Witkowski, R.N., and Carol Smith, R.N., for support of the clinical studies; and Richard Harris for assistance with database design and data management.
Footnotes
This work was supported by a research grant from the American Diabetes Association. Parts of the work presented were also supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK039311, and by the Research Service of the Department of Veterans Affairs. Human physiology studies were supported by General Clinical Research Center Grant M01RR14288 from the National Center for Research Resources, National Institutes of Health (to the University of Arkansas for Medical Sciences).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 13, 2009
Abbreviations: BMI, Body mass index; CNV, copy number variant; FSIGT, frequently sampled iv glucose tolerance test; LD, linkage disequilibrium; PKLR, liver pyruvate kinase gene; SI, insulin sensitivity; SNP, single nucleotide polymorphism; T2D, type 2 diabetes.
References
- Das SK, Elbein SC 2007 The search for type 2 diabetes susceptibility loci: the chromosome 1q story. Curr Diab Rep 7:154–164 [DOI] [PubMed] [Google Scholar]
- McCarthy MI 2003 Growing evidence for diabetes susceptibility genes from genome scan data. Curr Diab Rep 3:159–167 [DOI] [PubMed] [Google Scholar]
- Das SK, Hasstedt SJ, Zhang Z, Elbein SC 2004 Linkage and association mapping of a chromosome 1q21–q24 type 2 diabetes susceptibility locus in northern European Caucasians. Diabetes 53:492–499 [DOI] [PubMed] [Google Scholar]
- Wang H, Chu W, Das SK, Ren Q, Hasstedt SJ, Elbein SC 2002 Liver pyruvate kinase polymorphisms are associated with type 2 diabetes in northern European Caucasians. Diabetes 51:2861–2865 [DOI] [PubMed] [Google Scholar]
- Fu M, Sabra M, Damcott C, Pollini TI, Ott S, Tanner K, Wang J, Shi X, Connell JM, Mitchell BD, Shuldiner AR, Strong association of intragenic SNPs with type 2 diabetes in the 152 megabase region of chromosome 1q21 in the Old Order Amish. Proc of the 54th Annual Meeting of the American Society of Human Genetics, Toronto, Canada, 2004 (Abstract 347) [Google Scholar]
- Zeggini E, Rayner W, Groves CJ, Hanson RL, Mitchell BD, Osapos J, Connell MV, Jia W, Ng MCY, Knowler WC, Baier LJ, Froguel P, Xiang K, Chan JCN, Deloukas P, Cardon L, Bogardus C, Elbein SC, Shuldiner A, McCarthy M 2005 Meta-analysis of 3000 single nucleotide polymorphisms from chromosome 1q in samples from seven linked populations reveals shared type 2 diabetes susceptibility variants. Diabetes 54(Suppl 2):A33 [Google Scholar]
- Gibbs RA, Belmont JW, Hardenbol P, Willis TD, Yu F, Yang H, Ch’ang LY, Huang W, Liu B, Shen Y, Tam PK, Tsui LC, Waye MM, Wong JT, Zeng C, Zhang Q, Chee MS, Galver LM, Kruglyak S, Murray SS, Oliphant AR, Montpetit A, Hudson TJ, Chagnon F, Ferretti V, Leboeuf M, Phillips MS, Verner A, Kwok PY, Duan S, Lind DL, et al. 2003 The International HapMap Project. Nature 426:789–796 [DOI] [PubMed] [Google Scholar]
- Mateu E, Perez-Sezaun A, Martinez-Arias R, Andres A, Valles M, Bertranpetit J, Calafell F 2002 PKLR-GBA region shows almost complete linkage disequilibrium over 70 kb in a set of worldwide populations. Hum Genet 110: 532–544 [DOI] [PubMed] [Google Scholar]
- Moeslein FM, Myers MP, Landreth GE 1999 The CLK family kinases, CLK1 and CLK2, phosphorylate and activate the tyrosine phosphatase, PTP-1B. J Biol Chem 274:26697–26704 [DOI] [PubMed] [Google Scholar]
- Palmer ND, Bento JL, Mychaleckyj JC, Langefeld CD, Campbell JK, Norris JM, Haffner SM, Bergman RN, Bowden DW 2004 Association of protein tyrosine phosphatase 1B gene polymorphisms with measures of glucose homeostasis in Hispanic Americans: the insulin resistance atherosclerosis study (IRAS) family study. Diabetes 53:3013–3019 [DOI] [PubMed] [Google Scholar]
- Rayner W, Zeggini E, Groves CJ, Mitchell BD, Sabra M, Hanson RL, Vaxillaire M, Weiping J, Ng MCY, Knowler WC, Baier LJ, Froguel P, Xiang K, Chan JCN, Cardon L, Bogardus C, Fu M, Elbein S, Deloukas P, McCarthy MI 2005 Bioinformatic-based positional candidate selection on chromosome 1q and large scale association analysis. Diabetes 54(Suppl 1):A33 [Google Scholar]
- MacDonald JI, Kubu CJ, Meakin SO 2004 Nesca, a novel adapter, translocates to the nuclear envelope and regulates neurotrophin-induced neurite outgrowth. J Cell Biol 164:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory GD, Vakoc CR, Rozovskaia T, Zheng X, Patel S, Nakamura T, Canaani E, Blobel GA 2007 Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol 27:8466–8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, et al. 2007 Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447:799–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WS, Das SK, Wang H, Chan JC, Deloukas P, Froguel P, Baier LJ, Jia W, McCarthy MI, Ng MC, Damcott C, Shuldiner AR, Zeggini E, Elbein SC 2007 Activating transcription factor 6 (ATF6) sequence polymorphisms in type 2 diabetes and pre-diabetic traits. Diabetes 56:856–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein SC, Chu WS, Das SK, Yao-Borengasser A, Hasstedt SJ, Wang H, Rasouli N, Kern PA 2007 Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia 50:1621–1630 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Hays NP, Starling RD, Sullivan DH, Fluckey JD, Coker RH, Evans WJ 2006 Comparison of insulin sensitivity assessment indices with euglycemic-hyperinsulinemic clamp data after a dietary and exercise intervention in older adults. Metabolism 55:525–532 [DOI] [PubMed] [Google Scholar]
- Tserng KY, Kalhan SC 1983 Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol 245:E308–E311 [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL 2005 Isotope tracers in metabolic research: principles and practice of kinetic analysis. Hoboken, NJ: John Wiley, Sons [Google Scholar]
- Toth MJ, Sites CK, Cefalu WT, Matthews DE, Poehlman ET 2001 Determinants of insulin-stimulated glucose disposal in middle-aged, premenopausal women. Am J Physiol Endocrinol Metab 281:E113–E121 [DOI] [PubMed] [Google Scholar]
- Das SK, Chu W, Zhang Z, Hasstedt SJ, Elbein SC 2004 Calsquestrin 1 (CASQ1) gene polymorphisms under chromosome 1q21 linkage peak are associated with type 2 diabetes in northern European Caucasians. Diabetes 53:3300–3306 [DOI] [PubMed] [Google Scholar]
- Das SK, Chu WS, Hale TC, Wang X, Craig RL, Wang H, Shuldiner AR, Froguel P, Deloukas P, McCarthy MI, Zeggini E, Hasstedt SJ, Elbein SC 2006 Polymorphisms in the glucokinase-associated, dual-specificity phosphatase 12 (DUSP12) gene under chromosome 1q21 linkage peak are associated with type 2 diabetes. Diabetes 55:2631–2639 [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Weksberg R, Hughes S, Moldovan L, Bassett AS, Chow EW, Squire JA 2005 A method for accurate detection of genomic microdeletions using real-time quantitative PCR. BMC Genomics 6:180.:180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko I, Zeggini E, Rayner NW, Groves CJ, Hanson RL, Mitchell BD, Jia W, Ng MC, Froguel P, Chan JC, Bogardus C, Elbein SC, Shuldiner AR, McCarthy MI 2008 Putative association signals identified through high-density LD mapping of the replicated T2D linkage region on chromosome 1q are not confirmed in large scale follow-up studies. Diabetes 57(Suppl 1):A332 [Google Scholar]
- Frayling TM 2007 Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 8:657–662 [DOI] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Bostrom KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, et al. 2008 Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastinen T, Ge B, Hudson TJ 2006 Influence of human genome polymorphism on gene expression. Hum Mol Genet 15(Spec no. 1):R9–R16 [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K 2006 Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323 [DOI] [PubMed] [Google Scholar]
- Boj SF, Parrizas M, Maestro MA, Ferrer J 2001 A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci USA 98:14481–14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, Kasarskis A, Zhang B, Wang S, Suver C, Zhu J, Millstein J, Sieberts S, Lamb J, Guha Thakurta D, Derry J, Storey JD, Avila-Campillo I, Kruger MJ, Johnson JM, Rohl CA, van Nas A, Mehrabian M, Drake TA, Lusis AJ, Smith RC, Guengerich FP, Strom SC, Schuetz E, Rushmore TH, Ulrich R 2008 Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6:e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.