Abstract
Context: Polycystic ovary syndrome (PCOS) is a common endocrine disorder characterized by chronic oligo/anovulation, hyperandrogenemia, infertility, and metabolic alterations related to insulin resistance. These abnormalities in PCOS may have complex effects on pathophysiology of the endometrium, contributing to infertility and endometrial disorders.
Objective: The objective of this study was to examine dysregulated signaling pathways in the endometrium of patients with PCOS (PCOSE) by analyzing expression profiles with a pathway-oriented method.
Design: Microarrays, RT-PCR, laser capture microdissection, and immunohistochemistry were performed with endometrial tissues.
Setting: This study was performed at a university hospital laboratory.
Patients: This study comprised 12 regularly cycling women and 12 PCOS patients.
Main Outcome Measure: Dysregulated signaling pathways in PCOSE were identified as a gene set.
Results: Hierarchical clustering revealed distinct expression profiles for PCOSE and the endometrium of normal cycling women. Gene sets associated with androgen signaling were not enriched in PCOSE, although they affect ovarian physiology of PCOS patients. Several biological pathways including cell cycle, apoptosis, glycolysis, and integrin-Rho-cytoskeleton network were aberrantly down-regulated in PCOSE. Expression of genes constituting these gene sets enriched in normal cycling women was systemically down-regulated in PCOSE. Laser capture microdissection-coupled real-time RT-PCR and immunohistochemistry further demonstrated that cell proliferation in the stroma, but not the epithelium, is significantly reduced in PCOSE.
Conclusions: Systemic down-regulation of various signaling pathways in PCOSE with extremely prolonged proliferative phase provides insight into the abnormal phenotypes that reflect pathophysiology of PCOS in the endometrium, possibly leading to increased risks of endometrial disorders.
Genome-wide transcriptional profiling showed systemic down-regulation of various signaling pathways, including cell cycle and glucose metabolism in the endometrium of patients with PCOS.
In women of reproductive age, polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder characterized by chronic anovulation, hyperandrogenism, and frequently accompanying insulin resistance and hyperinsulinemia (1,2). Insulin resistance/hyperinsulinemia is associated in 75% of the patients (3) and high risks of metabolic alteration such as type 2 diabetes mellitus have been reported (4,5). In ovaries of patients with PCOS, growth of early antral follicles is typically arrested at the 5- to 10-mm stage, resulting in ovaries with multiple follicular structures less than 10 mm in diameter (6). The theca cell layers are prominent in these arrested follicles and represent the major source of the increased circulating androgens in women with PCOS (7).
The endometrium is a unique organ that undergoes a rapid cycling process of proliferation, differentiation, and cell death under the influence of ovarian hormonal milieu (8). Thus, aberrant hormonal contexts and metabolic disorders in patients with PCOS could alter endometrial homeostasis via dysregulated expression of gene clusters. In fact, patients with PCOS are at increased risk of recurrent spontaneous abortion (9,10), endometrial hyperplasia, and adenocarcinoma (11,12,13). They are closely related to unopposed estrogen, hyperandrogenemia, hyperinsulinemia, and obesity (14). It was also suggested that an altered intrafollicular microenvironment due to enhanced theca cell androgen biosynthesis increases a risk of implantation failure in patients with PCOS undergoing in vitro fertilization (15). The expression of steroid receptors and apoptosis-related proteins in the endometrium is also altered in patients with PCOS (16,17). However, a comprehensive investigation of the consequence of PCOS on endometrial homeostasis has not been undertaken.
Microarray analysis for cultured theca cells from patients with PCOS has demonstrated distinct biochemical and molecular phenotypes different from the cells of regularly cycling women (18). Another expression profiling for PCOS ovaries identified a collection of genes whose expression is dysregulated (19). Their expression profiles showed considerable overlap with those of ovaries from long-term androgen-treated female-to-male transsexuals. These works strongly suggest that androgens play a key role in the pathogenesis of PCOS (18,19,20). A recent work revealed that oocytes from women with PCOS have molecular abnormalities compared with those of normal healthy women although they appear to be morphologically normal (2). Although several studies have shown that PCOS affects oocyte and ovarian physiology via dysregulated gene expression, genome-wide understanding of altered signaling pathways in the endometrium of patients with PCOS (PCOSE) has not been investigated. The aim of this study was to understand dysregulated signaling pathways in PCOSE by analyzing expression profiles with a pathway-oriented method. By applying gene set enrichment analysis (GSEA), a knowledge-based supervised computational analysis tool (21), we first demonstrated that a variety of signaling pathways such as the cell cycle, DNA replication, apoptosis, glycolysis, and integrin-actin cytoskeleton-Rho network are dysregulated in PCOSE.
Subjects and Methods
Subjects
This study was approved by the Institution Review Board at Cheil General Hospital before sample collection, and all women signed an informed consent form before participating in the study. Study subjects included 12 normal cycling women and 12 patients with PCOS. Normal cycling women enrolled as the control group in this study had regular menstrual cycles occurring every 21 to 35 d without any sign of infertility. Normal ovarian morphology and ovulation of these women were assessed by ultrasonogram (2). According to the revised 2003 Rotterdam consensus diagnostic criteria for PCOS (22), the diagnosis of PCOS was based on the association of at least two of the following criteria: 1) oligomenorrhea with intermenstrual intervals of more than 60 d; 2) clinical or biochemical sign of hyperandrogenism; and 3) at least one ovary with polycystic morphology.
The control endometrium was selected in the early proliferative phase for the following reasons: 1) histological morphology of PCOSE is similar to that of the endometrium of normal cycling women (NE) in this phase (17); and 2) relatively constant circulating levels of 17β-estradiol (E2) resulting from persistent anovulation in patients with PCOS are comparable to those of regularly cycling women in this phase (7). The oral contraceptive or other hormonal medications were not administered within 3 months in all the patients. Patients with other potential causes of anovulation or hyperandrogenemia were excluded.
Hormone assays
Basal serum levels for E2, LH, FSH, TSH, prolactin, total testosterone, free testosterone, SHBG, dehydroepiandrosterone sulfate (DHEA-S), and 17-hydroxyprogesterone (17-OHP) were determined on menstrual cycle d 2 or 3 in control and PCOS patients by appropriate laboratory assays, such as electrochemiluminescence assay and RIA. Intraassay coefficients of variation of total testosterone, free testosterone, and SHBG were 2.5, 5.2, and 3.8%, respectively. Interassay coefficients of variation of total testosterone, free testosterone, and SHBG were 5.6, 8.5, and 7.9%, respectively. Sensitivity of total testosterone, free testosterone, and SHBG was 0.08 ng/ml, 0.15 pg/ml, and 0.04 nmol/liter, respectively. No patients with PCOS in this study had typical hirsutism (Ferriman-Gallwey score >8) as a clinical sign of hyperandrogenism. Biochemical sign of hyperandrogenism was documented with a total testosterone greater than the 90th percentile for normal cycling women (n = 350) (0.52 ng/ml). To evaluate insulin resistance and metabolic syndrome, a 75-g oral glucose tolerance test with insulin level measurement was done in all patients with PCOS. One patient showed impaired glucose tolerance, and another patient showed high 2-h insulin level (>150 μU/ml) suggesting insulin resistance.
Endometrial tissue biopsy
Endometrial biopsies were performed with endometrial sampler (SureSample, Wallace, Kent, UK) or curettage from the uterine corpus under sterile condition. After menstruation had occurred, ultrasonogram was performed to examine endometrial thickness in all patients, and endometrial tissues with 6- to 9-mm thickness were biopsied. Only the endometrial samples of patients with PCOS, which show similar morphology to those of NE in early proliferative phase by routine histological evaluation, were included in this study. The tissue samples were divided into two pieces. One was fixed in 10% formaldehyde solution for routine histological evaluation and immunohistochemistry. The other was snap-frozen in liquid nitrogen and stored in deep freezer at −70 C until laser capture microdissection (LCM) or RNA extraction.
RNA isolation
Total RNA was isolated from each biopsied endometrial sample using Trizol reagent (Life Technologies, Carlsbad, CA) following the manufacturer’s protocol. The integrity of RNA samples was assessed by using a 2100 Bioanalyzer, running an aliquot of the RNA samples on the RNA 6000 Nano Labchip (Agilent Technologies, Rockville, MD). Four endometrial RNAs were randomly selected from both groups and used to synthesize probes for microarray hybridization. Other RNA samples were used in semiquantitative and/or real-time RT-PCR to validate the results of data analysis.
Semiquantitative and real-time RT-PCR
One microgram RNA of human endometrium was subjected to reverse transcription using M-MuLV reverse transcriptase (Roche Applied Science, Indianapolis, IN) for cDNA synthesis. Synthesized cDNA was used for PCR with specific primers at optimized cycles. Real-time RT-PCR was performed by monitoring in real time the increase in fluorescence of the SYBR Green dye as previously described (23). Real-time RT-PCR was performed using DNA Engine Opticon 2 fluorescence detection system (MJ Research, Waltham, MA) and Dynamo SYBR green qPCR Kit (Finnzymes Oy, Espoo, Finland). For comparison of transcript levels between samples, a standard curve of cycle thresholds for several serial dilutions of a cDNA sample was established and then used to calculate the relative abundance of each gene. Values were then normalized to the relative amounts of 18S cDNA. All PCRs were performed in duplicate.
Microarray hybridization and data analysis of expression profiling
Affymetrix Human Genome U133A 2.0 Genechip (Affymetrix, Santa Clara, CA) containing approximately 22,000 human genes were hybridized with cRNA probes at the core facility of Digital Genomics (Seoul, Korea). The expression value and detection calls were computed from the raw data by following the procedures outlined for the Affymetrix MAS 5.0 analysis software.
GSEA version 1.0 (Broad Institute, Cambridge, MA) was applied to interpret expression profiles from microarrays of PCOSE (21). GSEA was originally developed to identify cohorts of genes whose functions are integrated into a certain biological process and/or signaling pathways. Pathways were ranked according to the significance of enrichment, and the validation mode measure of significance was used to identify pathways of greatest enrichment. Significance was tested by comparing the observed enrichment with the enrichment seen in data sets in which sample labels were randomly permutated (n = 1000). Gene sets consisting of less than 20 or more than 500 genes were filtered out by gene set size filters. Gene set size filters resulted in filtering out 358 from 522 gene sets. The remaining 164 gene sets were used in the analysis.
LCM
LCM was performed with snap-frozen tissue specimens to isolate each cell type in human endometrium using Veritas (Arcturus, Mountain View, CA). Detailed procedures of LCM were performed as described previously (24). Briefly, several LCM caps were pooled into a single tube containing 200 μl of denaturing buffer guanidium isothiocyanate with β-mercaptoethanol. Cell type-specific total RNA from each LCM procedure was resuspended in 10 μl of RNase-free water and used for real-time RT-PCR.
Immunohistochemistry
Immunohistochemistry for estrogen receptor α (ERα), progesterone receptor (PR), Ki-67, and Bcl-2 antigen was performed on 6-μm sections of formalin-fixed, paraffin-embedded endometria. Diaminobenzidine (Sigma, St. Louis, MO) was used as the chromogen. Paraffin sections were deparaffinized in xylene and hydrated in a series of graded ethanols. After a PBS rinse, the endogenous peroxidase activity was quenched on incubation for 30 min with 0.3% (vol:vol) hydrogen peroxide in absolute ethanol. Assessment of staining intensity and distribution was made using a semiquantitative analysis of HSCORE scoring system as described elsewhere (25). In all cases, 300 cells were evaluated by two independent observers.
Statistical analysis
Student t test was performed to examine statistical significance (*, P < 0.05; **, P < 0.01).
Results
Characteristics and hormonal profiles of patients with PCOS
The mean age, body mass index, and levels of basal E2 and FSH were similar and not statistically different between control women and patients with PCOS. However, as expected, levels of serum LH, total testosterone, free testosterone, DHEA-S, and 17-OHP were significantly higher in patients with PCOS compared with those of control women. SHBG was significantly lower in patients with PCOS compared with those of control women (Table 1).
Table 1.
Characteristics of control cycling women and patients with PCOS
| Control (n = 12) | PCOS (n = 12) | P values | |
|---|---|---|---|
| Age (yr) | 31.3 ± 4.1 | 30.3 ± 2.5 | NS |
| BMI (kg/m2) | 20.6 ± 2.2 | 22.5 ± 3.3 | NS |
| E2 (pg/ml) | 23.1 ± 10.7 | 23.8 ± 11.0 | NS |
| LH (mIU/ml) | 1.92 ± 0.76 | 6.98 ± 3.18 | <0.01 |
| FSH (mIU/ml) | 7.27 ± 1.08 | 6.07 ± 0.93 | NS |
| Total testosterone (ng/ml) | 0.23 ± 0.12 | 0.50 ± 0.14 | <0.01 |
| Free testosterone (pg/ml) | 0.43 ± 0.22 | 1.97 ± 0.83 | <0.01 |
| SHBG (nmol/liter) | 105.7 ± 49.0 | 42.1 ± 21.8 | <0.01 |
| DHEA-S (ng/ml) | 1385.0 ± 554.7 | 2427.5 ± 1154.7 | <0.05 |
| 17-OHP (ng/ml) | 0.66 ± 0.23 | 1.39 ± 0.97 | <0.05 |
Values are mean ± sd. NS, Not significant; BMI, body mass index.
Genome-wide comparison of expression profiles between endometria of healthy women and patients with PCOS
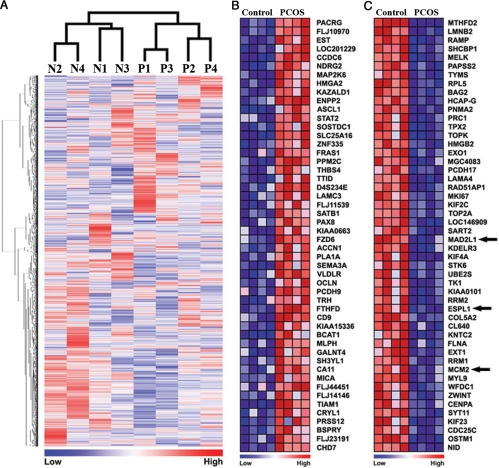
To define how PCOS affects endometrial homeostasis and physiology, the genome-wide expression profiles of PCOSE and NE were compared. dCHIP analysis (26) indicated that 12,491 probe sets from approximately 22,000 probes in Affymetrix Human Genome U133A 2.0 had a 50% presence call throughout the eight samples. Unsupervised hierarchical clustering with 12,491 probes showed that the global expression patterns of PCOSE are distinctly different from those of NE (Fig. 1A). Multiple probes for a single gene were collapsed, resulting in a total of 8754 genes for use in GSEA. GSEA with 8754 genes provided heatmaps representing lists of the top 50 most increased and decreased genes in PCOSE. Tumor suppressor, signal transducer, and bone morphogenetic protein antagonist, such as NDRG2 (N-myc down-regulated gene family 2), STAT2 (signal transducer and activator of transcription 2), and SOSTDC1 (sclerostin domain containing 1) were included in the list of the 50 most increased genes in PCOSE (Fig. 1B). KIF2C, KIF4A, and KIF23, members of kinesin family and key players in the intracellular transport system essential for cellular function and morphology, were included in the list of the 50 most decreased genes in PCOSE (Fig. 1C). In addition, mRNAs for many enzymes, such as MTHFD2, MELK, TYMS, TOP2A, and TK1, were significantly reduced in PCOSE.
Figure 1.
Global expression profiles of the endometrium of patients with PCOS are distinctly different from those of regularly cycling women. A, Unsupervised hierarchical clustering analysis for microarray data from PCOSE and NE using dCHIP. N and P represent NE and PCOSE, respectively. B and C, Heatmaps for the 50 most increased and decreased genes in PCOSE. The color spectrum from blue to red indicates low to high expression. Control and PCOS represent NE and PCOSE, respectively. Arrows indicate genes that are included in the Cell Cycle gene set in Fig. 2.
Identification of dysregulated signaling pathways in PCOSE
To understand underlying mechanisms by which PCOS affects endometrial homeostasis, it is critical to identify significantly dysregulated signaling pathways and biological processes. Supervised analyses, such as GSEA, provide insight into aberrantly regulated signaling pathways or biological processes (Table 2) as well as a list of differentially expressed genes (Fig. 1). Table 2 shows that 44 gene sets were enriched in NE with a false discovery rate at 25%, whereas only two gene sets were enriched in PCOSE (Hox_List and glycerolipid metabolism). This finding suggested that enriched gene sets representing signaling pathways and biological processes are less active in PCOSE. Six of 44 enriched gene sets in NE were directly associated with cell cycle regulation and cell proliferation. They are cell_cycle, cancer_ cell_cycle, cell_proliferation, cell_cycle_checkpoint, prolif_ genes, and cell_cycle_regulator. Eight of 44 gene sets were directly associated with metabolic pathways. They are Glut_down, Leu_down, MAP00240_Pyrimidine_metabolism, MAP00230_ Purine_metabolism, Glucose_down, MAP00010_Glycolysis_ Gluconeogenesis, Insulin_2F_up, Leu_up, and MAP00620_ Pyruvate_metabolism. Furthermore, four gene sets associated with integrin-mediated signaling pathway connected to actin cytoskeleton networks, and two apoptosis-related gene sets (Fas pathway and TNFR1 pathway) were also down-regulated in PCOSE. Interestingly, gene sets known to be regulated by androgen signaling were not dysregulated in PCOSE.
Table 2.
The list of 44 down-regulated gene sets in the endometrium of patients with PCOS
| Name | Size | Nominal P | FDR (%) |
|---|---|---|---|
| CELL_CYCLE | 66 | 0 | 0 |
| CR_CELL_CYCLE | 67 | 0 | 0 |
| GLUT_DOWN | 271 | 0 | 0 |
| LEU_DOWN | 163 | 0 | 0 |
| RAP_DOWN | 201 | 0 | 0 |
| G1PATHWAY | 22 | 0 | 0.7 |
| MAP00240_PYRIMIDINE_METABOLISM | 39 | 0 | 0.6 |
| CELL_PROLIFERATION | 138 | 0 | 0.7 |
| MAP00230_PURINE_METABOLISM | 66 | 0 | 0.6 |
| CELL_CYCLE_CHECKPOINT | 24 | 0 | 0.8 |
| P53_UP | 32 | 0 | 0.9 |
| EMT_UP | 47 | 0 | 0.8 |
| PROTEASOMEPATHWAY | 21 | 0 | 0.7 |
| DNA_DAMAGE_SIGNALLING | 78 | 0 | 1.1 |
| SIG_REGULATION_OF_THE_ACTIN_CYTOSKELETON_BY_RHO_GTPASES | 24 | 0 | 1.2 |
| PROTEASOME_DEGRADATION | 31 | 0 | 1.3 |
| INTEGRINPATHWAY | 28 | 0 | 1.3 |
| PROLIF_GENES | 246 | 0 | 2.1 |
| ELECTRON_TRANSPORT_CHAIN | 81 | 0 | 2.3 |
| RADIATION_SENSITIVITY | 26 | 0 | 2.4 |
| FASPATHWAY | 25 | 0 | 2.7 |
| BIOPEPTIDESPATHWAY | 28 | 0 | 2.7 |
| PGC | 336 | 0 | 2.8 |
| MRNA_PROCESSING | 42 | 0.01 | 2.8 |
| TNFR1PATHWAY | 22 | 0 | 3.0 |
| CELL_CYCLE_REGULATOR | 22 | 0.03 | 3.0 |
| GLUCOSE_DOWN | 121 | 0 | 3.8 |
| MAP00010_GLYCOLYSIS_GLUCONEOGENESIS | 33 | 0 | 4.3 |
| KRAS_TOP100_KNOCKDOWN | 76 | 0 | 4.3 |
| HTERT_UP | 104 | 0 | 4.4 |
| ST_INTEGRIN_SIGNALING_PATHWAY | 60 | 0 | 4.7 |
| ST_T_CELL_SIGNAL_TRANSDUCTION | 25 | 0.01 | 6.9 |
| INSULIN_2F_UP | 182 | 0 | 9.3 |
| RHOPATHWAY | 23 | 0.06 | 9.2 |
| VOXPHOS | 80 | 0.01 | 8.9 |
| CR_CAM | 59 | 0.06 | 13.1 |
| LEU_UP | 90 | 0.02 | 16.9 |
| HUMAN_MITODB_6_2002 | 354 | 0 | 18.6 |
| CHEMICALPATHWAY | 21 | 0.03 | 18.9 |
| TGF_BETA_SIGNALING_PATHWAY | 22 | 0.10 | 20.9 |
| MAP00620_PYRUVATE_METABOLISM | 24 | 0.04 | 21.1 |
| CR_REPAIR | 35 | 0.05 | 21.0 |
| UPREG_BY_HOXA9 | 30 | 0.06 | 22.3 |
| SIG_CHEMOTAXIS | 29 | 0.09 | 24.4 |
Size indicates the number of genes in each gene set. A brief description of each gene set is summarized in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). FDR, False discovery rate.
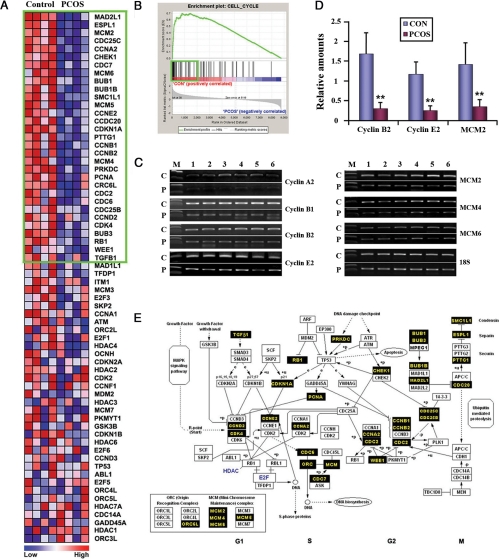
Genes associated with cell cycle are collectively down-regulated in PCOSE
Figure 2A shows a heatmap for the cell cycle gene set comprising 66 genes. The highly ranked 31 genes in the green box, which contribute to the establishment of the enrichment score for the gene set, were consistently down-regulated in PCOSE. Figure 2B showed the enrichment plot of genes in the cell cycle gene set. Most hits in ranking order were enriched in the area of NE (CON), suggesting that many genes are highly expressed in NE. Thirty-one genes in the green box of Fig. 2A are also represented as black boxes in the interactive map to demonstrate how they work cooperatively to drive cell cycle progression (Fig. 2E). Three of these genes (MAD2L1, ESPL1, and MCM2) were listed in the top 50 most decreased genes in PCOSE (Fig. 1C). Semiquantitative and real-time RT-PCR have shown that minichromosome maintenance proteins (MCMs) important for DNA replication, such as MCM2, -4, and -6, and cyclins (A2, B1, B2, and E2) are collectively down-regulated in PCOSE (Fig. 2, C and D).
Figure 2.
Cell cycle regulators are collectively down-regulated in PCOSE. A, A heatmap of genes in the Cell Cycle gene set. The color spectrum from blue to red indicates low to high expression. B, Enrichment plot of the Cell Cycle gene set. Genes within the green boxes in A and B are leading characters for building enrichment scores in the NE. C, Results of semiquantitative RT-PCR for members of MCM and Cyclin family at optimized cycles. C and P represent NE and the PCOSE, respectively (n = 6 each). D, Real-time RT-PCR was performed to measure the relative difference of gene expression between PCOSE and NE. CON and PCOS represent NE and PCOSE, respectively (n = 6 each). E, An interactive map of cell cycle progression. This map was originally adapted from GenMAPP.org and was modified to illustrate genes that are down-regulated in PCOSE in concert. Black boxes point out leading factors for enrichment scores in NE. **, P < 0.01.
Metabolic pathways are systemically down-regulated in PCOSE
Another interesting pattern observed in Table 2 is that many gene sets associated with metabolism are enriched in NE over PCOSE. Gene sets associated with amino acid (3 of 44), nucleotide (2 of 44), or glucose metabolism (3 of 44) were systemically down-regulated in PCOSE. This suggests that the total cellular activity/metabolism of PCOSE is lower and/or slower than that of NE. Concurrent down-regulation of purine and pyrimidine metabolism in PCOSE strongly suggests that DNA replication in PCOSE is less active than that in NE. This is consistent with reduced expression of cell cycle regulators, such as MCMs and cyclins in PCOSE (Fig. 2). Interestingly, enrichment of three gene sets associated with glucose metabolism and insulin signaling (Glucose_down, MAP00010_Glycolysis_Gluconeogenesis, and Insulin_2F_up) in NE suggest that glucose metabolism in the endometrium as well as the endocrine system is also affected by PCOS.
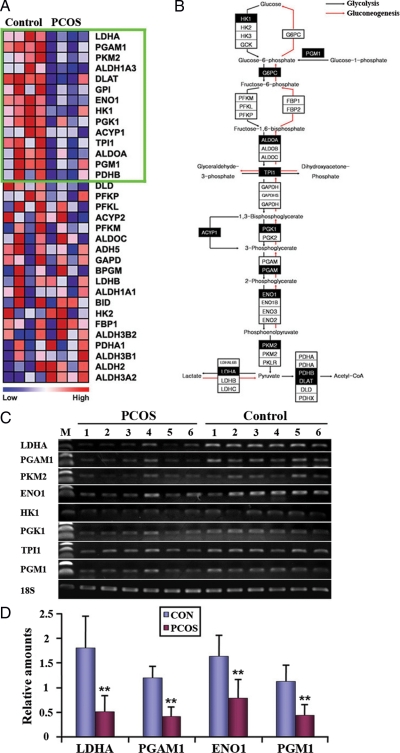
Glycolysis, but not gluconeogenesis, is cooperatively down-regulated in PCOSE
Glycolysis/gluconeogenesis is a bidirectional metabolic pathway that contains a series of enzymes regulating levels of glucose and its derivatives. Figure 3A shows a heatmap of Glycolysis/Gluconeogenesis gene set comprising 33 genes. Many enzymes regulating glucose synthesis/consumption were down-regulated in PCOSE (Fig. 3B). Interestingly, a few enzymes that work for glycolysis, but not gluconeogenesis, such as HK1 (Hexokinase 1), PGM1 (Phosphoglucomutase 1), ACYP1 (Acrylphosphatase 1), were cooperatively down-regulated in PCOSE, suggesting that glycolysis in PCOSE is reduced compared with that of NE. RT-PCR with additional endometrial samples confirmed that the expression of a collection of genes involved in glycolysis/gluconeogenesis is systemically down-regulated in PCOSE (Fig. 3C). Furthermore, real-time RT-PCR validated collective down-regulation of genes that encode enzymes regulating glucose consumption in PCOSE with 50–70% reduction (Fig. 3D).
Figure 3.
Enzymes for glycolysis are systemically down-regulated in PCOSE. A, A heatmap of genes in the Glycolysis and Gluconeogenesis gene set. The color spectrum from blue to red indicates low to high expression. Genes within the green box are leading characters for building enrichment scores in normal endometrium (NE). B, An interactive map of Glycolysis and Gluconeogenesis that was originally adapted from GenMAPP.org and modified to illustrate genes that are down-regulated in the PCOSE in concert. Black boxes point out leading factors for enrichment scores in NE. Note that enzymes that work in glycolysis, but not gluconeogenesis, such as HK1, PGM1, ACYP1, and PKM2, are consistently down-regulated in PCOSE. C and D, Semiquantitative and real-time RT-PCR of glycolysis-related genes. Control (CON) and PCOS represent NE and PCOSE, respectively (n = 6 each). Relative amounts of LDHA, PGAM1, ENO1, and PGM1 in panel D were normalized by 18S. **, P < 0.01.
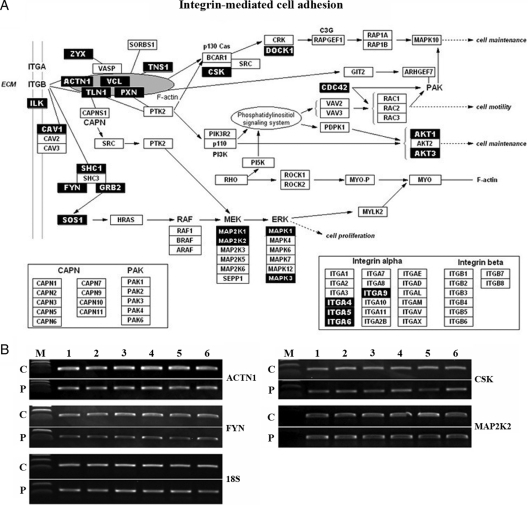
Integrin-rho-cytoskeleton network is cooperatively down-regulated in PCOSE
The integrin signaling pathway is also down-regulated in PCOSE. Two gene sets associated with integrin mediated signal transduction (Integrin pathway and St_Integrin_Signaling_Pathway) were enriched in NE. Two other gene sets enriched in NE (Sig_regulation_of_the_actin_cytoskeleton_by_Rho_GTPase and Rho pathway) shared several numbers of genes with the integrin signaling pathway, suggesting that they are connected with each other by sharing these factors. Thus, these four gene sets integrating to Integrin-Actin cytoskeleton-Rho signaling networks were also down-regulated in PCOSE. An interactive map with a collection of genes contributing enrichment scores for the Integrin pathway and/or St_Integrin_Signaling_Pathway is shown in Fig. 4A. RT-PCR for ACTN1, FYN, CSK, and MAP2K2 validated that the integrin-mediated cell adhesion signaling pathway is down-regulated in PCOSE (Fig. 4B).
Figure 4.
Integrin-Rho-cytoskeleton network is cooperatively down-regulated in PCOSE. A, An interactive map of integrin-mediated cell adhesion that was originally adapted from GenMAPP.org and was modified to illustrate genes that are down-regulated in the PCOSE in concert. Black boxes point out leading factors for enrichment scores in the NE. B, Semiquantitative RT-PCR of genes associated with integrin-mediated cell adhesion. C and P represent NE and PCOSE, respectively (n = 6 each).
Cell type-specific expression of genes in enriched gene sets validates interpretation of GSEA for PCOSE
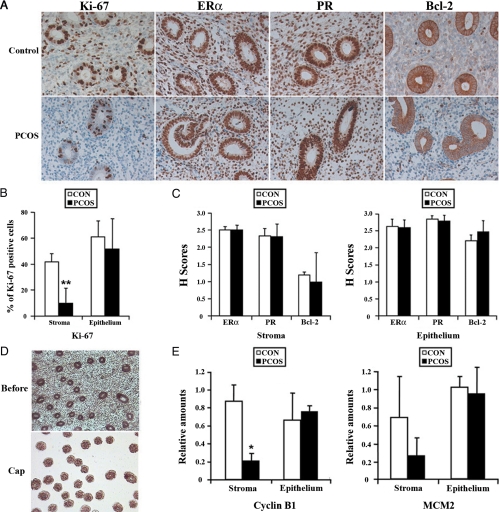
To examine whether maturation state of the endometrium with patients with PCOS is similar to that of normal cycling women, protein expression of ER, PR, and Bcl-2 was examined. Immunohistochemistry and HSCORE analysis showed strong nuclear staining for ER and PR in both glandular epithelial and stromal cells of PCOSE and NE (Fig. 5, A and C). Bcl-2 was also similarly expressed in PCOSE and NE. These results suggest that the underlying cellular environments in PCOSE and NE are similar. However, immunostaining and semiquantitative analysis for Ki-67, a proliferation marker, showed that cell proliferation in the stromal compartment, but not the epithelial compartment, was dramatically reduced in PCOSE (Fig. 5, A and B). To further validate the cell type-specific aberration of cell cycle progression in PCOSE, LCM was performed (Fig. 5D). Consistent with the reduced number of Ki-67 positive cells in stromal cells of PCOSE, LCM-coupled real-time RT-PCR confirmed that cell cycle regulators are similarly down-regulated in stromal, but not in epithelial cells of PCOSE (Fig. 5E). These results suggest that cell cycle progression in the stroma of PCOSE is severely impaired.
Figure 5.
Cell proliferation in the stromal compartment, but not in the epithelial compartments, is severely impaired in PCOSE. A, Immunohistochemical analysis for expression of Ki-67, ERα, PR, and Bcl-2 proteins in the endometrium. Note that the number and intensity of Ki-67-positive cells were significantly reduced in the stroma, but not in the glands in the PCOSE. B, Semiquantitative analysis of Ki-67-positive cells in PCOSE. Note that cell proliferation in stromal compartment, but not in epithelial compartment, is statistically different (P < 0.01) between PCOSE and the NE. C, HSCORE analysis for immunohistochemistry of ERα, PR, and Bcl-2. D, Representative images of LCM procedures. Light microscopic images were taken at 100× before and after LCM (on cap) on glandular epithelial cells. E, Real-time RT-PCR for Cyclin B1 and MCM2 with RNA isolated from LCM-captured homogenous stromal and epithelial cells. Control (CON) and PCOS represent NE and PCOSE, respectively (n = 5 for each in immunostaining, n = 3 for each in LCM). *, P < 0.05; **, P < 0.01.
Discussion
PCOS is a complex endocrine and metabolic disease commonly manifesting as infertility due to chronic anovulation. These phenotypes of PCOS have brought a caution that it may disrupt the physiology of the endometrium (14,27), possibly leading to implantation failure, spontaneous pregnancy loss, and endometrial hyperplasia and adenocarcinoma (13). However, mechanisms underlying the pathophysiology of PCOS in the endometrium have not yet been thoroughly explored. Our study was the first attempt to understand the pathophysiology of PCOSE by analyzing genome-wide expression profiles with a pathway-oriented analysis method. The gene sets representing 46 biological pathways including cell cycle, apoptosis, nucleotide and glucose metabolism, and integrin signaling were found to be dysregulated in PCOSE. Our study provided valuable information as to how PCOS affects endometrial homeostasis with respect to identification of dysregulated signaling pathways.
Proliferation, differentiation, and/or apoptosis of endometrial cells in women with PCOS can be aberrantly influenced by various factors, such as androgens, insulin, and unopposed estrogens. In the absence of ovulation, continuous exposure to the stimulatory and mitogenic effects of estrogens in PCOSE could result in endometrial overgrowth, possibly leading to hyperplasia and cancer (7,28). However, we observed a puzzling molecular phenotype that DNA replication and cell proliferation in PCOSE are largely reduced in stromal cells of PCOSE. This abnormality could potentially provide a chance for epithelial cells to exit the controlled cell cycle and become hyperplastic at a later stage. In fact, a recent study demonstrated that cell proliferation is persistently active in the epithelial compartment of PCOSE during the secretory phase while it is very rare in this phase of NE (29). This may explain why patients with PCOS have a higher risk of endometrial hyperplasia and adenocarcinoma.
In contrast, recent reports suggested that the apparent association between PCOS and endometrial adenocarcinoma could be due to specific endocrine and metabolic abnormalities occurring as a result of PCOS (12,28). Because obesity itself increases the risk of endometrial adenocarcinoma and diabetes mellitus is well recognized as a risk factor for endometrial adenocarcinoma, anovulation may not be an independent risk factor for endometrial adenocarcinoma in patients with PCOS. It has been suggested that hyperinsulinemia could increase endometrial hyperplasia by affecting the level of SHBG and IGF-I (30). Aberrant down-regulation of glycolysis enzymes in PCOSE in our study also demonstrated that glucose imbalance in the endometrium as well as the endocrine system is a phenotypic alteration that occurs in PCOS patients. However, considering that the body mass index of patients with PCOS in our study was similar to that of controls and patients with severe insulin resistance were not included, reduced expression of genes responsible for glucose consumption in PCOSE could be simply a consequence of reduced stromal cell proliferation in PCOSE because DNA synthesis and amino acid metabolism are concomitantly down-regulated in PCOSE. Therefore, further studies are required to examine whether reduced stromal cell proliferation and/or imbalanced glucose metabolism in PCOSE affects aberrant cell proliferation in the epithelium.
Abnormal endometrial development and uterine receptivity in patients with PCOS might be related to decreased expression of genes involved in integrin-mediated cell adhesion pathways. Integrins are cell-surface receptors that mediate intracellular signals in response to the extracellular matrix, including cellular shape, mobility, and progression through the cell cycle (16,31). They play an important role in embryo implantation by mediating the organization of extracellular matrix proteins. Expression of integrin αvβ3, one of the uterine receptivity markers, is delayed or absent in PCOSE (16). In this study, integrin α4, α5, α6, and α9 were down-regulated in PCOSE, suggesting that aberrantly down-regulated expression of various integrins might affect mechanisms that regulate uterine receptivity in PCOSE. This could lead to higher risk of implantation failure and pregnancy loss in these patients.
Expression profiling experiments using theca cells and oocytes of PCOS patients provided evidence that androgens play a key role in the ovarian pathogenesis of PCOS (2,18,19,20). Whereas androgen has a critical role in the ovary, we did not find that aberrantly up-regulated androgen signaling affects endometrial homeostasis in these patients. A gene set comprising genes whose expression is up-regulated by androgen (Androgen_up_genes) was not significantly enriched in PCOSE. In addition, gene sets associated with Wnt signaling, which are mainly dysregulated by androgen signaling in the ovary of PCOS patients (19), were not enriched in PCOSE. This could be due to similar expression of androgen receptor (AR) between the endometria of normal cycling women and PCOS patients in the proliferative phase (16). However, during the secretory phase, the expression of AR, which is persistently low in NE, increases significantly in PCOSE (16,31), suggesting possible influences of high androgen levels on pathophysiology of PCOSE in this period.
Elevated levels of LH in patients with PCOS could directly affect the pathophysiology of the endometrium as well as the ovary because LH receptor (LHR) is also expressed in the endometrium (32,33). However, our results from microarrays suggest that elevated LH levels do not have critical actions on the expression profiles in PCOSE. It is supported by previous studies that LHR expression is higher during secretory phase than proliferative phase in many species including humans (32,33). In fact, mRNA of LHR was very low to undetectable in endometrial samples of this study (data not shown), suggesting that the levels of LHR in the endometrium seem to be more important than the concentration of LH in the plasma. Further works on PCOSE in secretory phase with elevated levels of AR and LHR are needed for understanding definitive actions of elevated levels of androgens and LH in PCOSE. In conclusion, by applying GSEA, a pathway-oriented supervised computational analysis, we demonstrated that a variety of dysregulated signaling pathways such as the cell cycle and glycolysis were aberrantly down-regulated in PCOSE. Systemic down-regulation of various signaling pathways in PCOSE with extremely prolonged proliferative phase provides insight into the abnormal phenotypes that reflect pathophysiology of PCOS in the endometrium.
Supplementary Material
Acknowledgments
We thank Drs. In Ok Song and Kwang Moon Yang, who recruited patients for this study, and all the patients who consented to donate their tissues. We also appreciate technical help from members of the infertility center and the laboratories of reproductive biology and infertility, endocrinology, and pathology at Cheil General Hospital and Women’s Healthcare Center.
Footnotes
Current address for J.H.J.: Department of Bio-Medical Laboratory Science, Eulji University, Kyoung-gi, Korea.
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-E00290).
Disclosure Summary: All authors have nothing to declare.
First Published Online January 13, 2009
For editorial see page 1084
Abbreviations: AR, Androgen receptor; DHEA-S, dehydroepiandrosterone sulfate; E2, 17β-estradiol; ER, estrogen receptor; GSEA, gene set enrichment analysis; LCM, laser capture microdissection; LHR, LH receptor; MCM, minichromosome maintenance protein; NE, endometrium of normal cycling women; 17-OHP, 17-hydroxyprogesterone; PCOS, polycystic ovary syndrome; PCOSE, endometrium of patients with PCOS; PR, progesterone receptor.
References
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Wood JR, Dumesic DA, Abbott DH, Strauss III JF 2007 Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab 92:705–713 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A 1989 Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J 1999 Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- Pache TD, Hop WC, de Jong FH, Leerentveld RA, van Geldrop H, Van de Kamp TM, Gooren LJ, Fauser BC 1992 17 β-Oestradiol, androstenedione and inhibin levels in fluid from individual follicles of normal and polycystic ovaries, and in ovaries from androgen treated female to male transsexuals. Clin Endocrinol (Oxf) 36:565–571 [DOI] [PubMed] [Google Scholar]
- Giudice LC 2006 Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 20:235–244 [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le SN, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC 2006 Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- Carmina E, Lobo RA 1999 Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 84:1897–1899 [DOI] [PubMed] [Google Scholar]
- Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, Beard RW, Franks S 1988 Recurrent early miscarriage and polycystic ovaries. BMJ 297:1027–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balen A 2001 Polycystic ovary syndrome and cancer. Hum Reprod Update 7:522–525 [DOI] [PubMed] [Google Scholar]
- Hardiman P, Pillay OC, Atiomo W 2003 Polycystic ovary syndrome and endometrial carcinoma. Lancet 361:1810–1812 [DOI] [PubMed] [Google Scholar]
- Niwa K, Imai A, Hashimoto M, Yokoyama Y, Mori H, Matsuda Y, Tamaya T 2000 A case-control study of uterine endometrial cancer of pre- and postmenopausal women. Oncol Rep 7:89–93 [PubMed] [Google Scholar]
- Okon MA, Laird SM, Tuckerman EM, Li TC 1998 Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril 69:682–690 [DOI] [PubMed] [Google Scholar]
- Ludwig M, Finas DF, al-Hasani S, Diedrich K, Ortmann O 1999 Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod 14:354–358 [DOI] [PubMed] [Google Scholar]
- Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA 2002 Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod 66:297–304 [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Clementi M, Gabler F, Johnson MC, Palomino A, Sir-Petermann T, Vega M 2003 Expression of steroid receptors and proteins related to apoptosis in endometria of women with polycystic ovary syndrome. Fertil Steril 80(Suppl 2):812–819 [DOI] [PubMed] [Google Scholar]
- Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss III JF 2003 The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem 278:26380–26390 [DOI] [PubMed] [Google Scholar]
- Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, Westland J, Mosselman S, Fauser BC 2004 Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol 18:3050–3063 [DOI] [PubMed] [Google Scholar]
- Diao FY, Xu M, Hu Y, Li J, Xu Z, Lin M, Wang L, Zhou Y, Zhou Z, Liu J, Sha J 2004 The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol 33:59–72 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP 2005 Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- Koo TB, Song H, Moon I, Han K, Chen C, Murphy K, Lim H 2005 Differential expression of the PEA3 subfamily of ETS transcription factors in the mouse ovary and peri-implantation uterus. Reproduction 129:651–657 [DOI] [PubMed] [Google Scholar]
- Ehrig T, Abdulkadir SA, Dintzis SM, Milbrandt J, Watson MA 2001 Quantitative amplification of genomic DNA from histological tissue sections after staining with nuclear dyes and laser capture microdissection. J Mol Diagn 3:22–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani JG, Johnston SR, Salter J, Detre S, Dowsett M 1994 Comparison of new immunohistochemical assay for oestrogen receptor in paraffin wax embedded breast carcinoma tissue with quantitative enzyme immunoassay. J Clin Pathol 47:900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Li C, Wong WH 2003 ChipInfo: software for extracting gene annotation and gene ontology information for microarray analysis. Nucleic Acids Res 31:3483–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balen AH, Schachter ME, Montgomery D, Reid RW, Jacobs HS 1993 Polycystic ovaries are a common finding in untreated female to male transsexuals. Clin Endocrinol (Oxf) 38:325–329 [DOI] [PubMed] [Google Scholar]
- Navaratnarajah R, Pillay OC, Hardiman P 2008 Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med 26:62–71 [DOI] [PubMed] [Google Scholar]
- Avellaira C, Villavicencio A, Bacallao K, Gabler F, Wells P, Romero C, Vega M 2006 Expression of molecules associated with tissue homeostasis in secretory endometria from untreated women with polycystic ovary syndrome. Hum Reprod 21:3116–3121 [DOI] [PubMed] [Google Scholar]
- Gibson M 1995 Reproductive health and polycystic ovary syndrome. Am J Med 98:67S–75S [DOI] [PubMed] [Google Scholar]
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA 1992 Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest 90:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL 1990 The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab 70:421–430 [DOI] [PubMed] [Google Scholar]
- Shemesh M 2001 Actions of gonadotrophins on the uterus. Reproduction 121:835–842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.