Abstract
Context: Anorexia nervosa is characterized by hypogonadism and relative hypercortisolemia. We have demonstrated that free testosterone levels are low in women with anorexia nervosa, with the lowest levels in those receiving oral contraceptives (OCPs), and that dehydroepiandrosterone (DHEA) sulfate is reduced only in those receiving OCPs.
Objective: The aim of the study was to determine whether adrenal steroidogenesis dysregulation contributes to decreased androgen levels in anorexia nervosa.
Design and Setting: We conducted a cross-sectional study in a General Clinical Research Center.
Study Participants: We studied 20 women with anorexia nervosa [10 women with anorexia nervosa receiving OCPs (AN+E) and 10 not receiving OCPs (AN−E)] and 20 healthy controls [10 healthy controls receiving OCPs (HC+E) and 10 not receiving OCPs (HC−E)].
Main Outcome Measures: We measured DHEA and cortisol levels in response to 250-μg cosyntropin stimulation after 1-mg overnight dexamethasone suppression.
Results: Mean basal and stimulated, peak stimulated, and area under the curve (AUC) cortisol levels were higher in AN−E than HC−E, but mean basal and stimulated, peak and AUC DHEA were comparable. Mean AUC and peak cortisol were higher and DHEA AUC was lower in AN+E than AN−E. However, after controlling for cortisol binding globulin levels, peak and AUC cortisol were comparable between AN+E and AN–E. After controlling for albumin levels, AUC DHEA was comparable between AN+E and AN−E.
Conclusions: Adrenal glucocorticoid and androgen precursor secretion are dissociated in anorexia nervosa, with relative hypercortisolemia and a preservation of DHEA secretion. Reduced DHEA response to cosyntropin in women receiving OCPs is attributable to decreased albumin levels. In the setting of relative hypercortisolemia, reduced adrenal androgen precursor secretion is not a mechanism underlying low testosterone levels in anorexia nervosa.
Reduced adrenal androgen precursor secretion is not a mechanism underlying low testosterone levels in anorexia nervosa.
We have shown in a large cross-sectional study that total and free testosterone levels are reduced in women with anorexia nervosa, with the lowest levels of free testosterone observed in women with anorexia nervosa receiving oral contraceptives (1). However, it is not known whether the etiology of the hypoandrogenemia in women with anorexia nervosa is adrenal and/or ovarian. In the same study, we reported normal dehydroepiandrosterone sulfate (DHEAS) levels in women with anorexia nervosa, except for those receiving oral contraceptives, in whom mean DHEAS levels were reduced (1). Other groups have described decreased DHEAS levels compared to healthy controls (2,3) or to a normal range (4,5). However, elevated levels of DHEAS have also been reported in this population (6). In addition, an increased prevalence of hypercortisolemia has been demonstrated in women with anorexia nervosa (7,8,9,10). This raises the possibility that shunting of adrenal steroidogenesis toward glucocorticoid production is occurring, resulting in a reduction in the biosynthesis of adrenal androgen precursors. An alternative hypothesis is that up-regulation of adrenal steroidogenesis could affect both glucocorticoid and androgen pathways, as might occur in a CRH-driven state. Finally, it is not clear whether the lower DHEAS levels reported in women with anorexia nervosa receiving oral contraceptives reflect decreased circulating free levels. Alternatively, decreased levels of albumin, DHEAS’s primary binding globulin, may result in reduced total, but not free, DHEAS levels.
Therefore, to determine whether adrenal dysregulation is an underlying pathophysiological mechanism contributing to hypoandrogenemia in anorexia nervosa, we performed cosyntropin stimulation tests after dexamethasone suppression and measured serum cortisol and dehydroepiandrosterone (DHEA) levels in 20 women with anorexia nervosa and 20 healthy controls, with one half of the subjects in each group receiving oral contraceptives. We also measured cortisol binding globulin (CBG) and albumin to investigate whether the elevation of cortisol and reduction in adrenal androgen precursor levels in women with anorexia nervosa receiving oral contraceptives is attributable to effects of oral estrogens on binding globulins.
Subjects and Methods
Protocol
Forty women [10 women with anorexia nervosa not receiving oral contraceptives (AN−E), 10 women with anorexia nervosa receiving oral contraceptives (AN+E), 10 healthy control women not receiving oral contraceptives (HC−E), and 10 healthy control women receiving oral contraceptives (HC+E)] were studied to determine the adrenal contribution to low total and free testosterone levels in anorexia nervosa. Women with anorexia nervosa were all community dwelling, ambulatory women less than 85% of ideal body weight (IBW) and fulfilled all psychiatric diagnostic criteria of the disorder as defined by the Diagnostic and Statistical Manual IV. Nine of 10 AN−E and three of 10 AN+E reported a restrictive subtype; one of 10 AN−E and five of 10 AN+E reported a binge-purge subtype (one subject declined to provide this information). Five of 10 AN−E and four of 10 AN+E were receiving psychotropic medications. Seven of 10 AN−E and four of 10 AN+E reported a history of major depressive disorder (MDD). Seven of 10 AN−E and two of 10 AN+E reported a history of an anxiety disorder. Healthy controls were 90–110% of IBW and without a history of an eating disorder or irregular menses. Other exclusion criteria for healthy controls included pregnancy, history of diabetes mellitus, thyroid, cardiac, liver or renal disease, or medications known to affect cortisol metabolism. None were receiving psychotropic medications or had a history of MDD or an anxiety disorder. Subjects were referred to the study by eating disorder providers in the New England area or recruited through advertisements. The study was approved by Partners Health Care Institutional Review Board, and written informed consent was obtained from all subjects. Study visits took place at the General Clinical Research Center at Massachusetts General Hospital. A history and physical exam were performed at the screening visit, and serum samples for thyroid stimulating hormone, creatinine, and alanine transaminase were obtained. Research dieticians measured metabolic weight and height and calculated body mass index (BMI) (kilograms per square meter). On the night before the cosyntropin stimulation test, each subject took dexamethasone 1 mg by mouth between 2300 h and 2400 h. This approach has been shown to minimize individual variation in hormonal levels at baseline without altering the response of androgen precursors to synthetic ACTH stimulation (11). An iv bolus of 250 μg cosyntropin was administered at 0800 h the following morning. Total testosterone, DHEAS, SHBG, CBG, and albumin were measured at 0 min. DHEA and cortisol were measured at 0, 30, 60, and 90 min.
Biochemical analysis
Cortisol was measured using an RIA by Diasorin Inc. (Stillwater, MN). The intraassay coefficient of variation (CV) was 6.6–7.7%, and the sensitivity was 0.21 μg/dl. DHEA was measured using an RIA by Diagnostic Systems Laboratories, Inc. (Webster, TX). The intraassay CV was 5.6–10.6%, and the sensitivity was 0.02 ng/ml. DHEAS was measured using an RIA from Siemens Medical Solutions Diagnostic (Los Angeles, CA). The intraassay CV was 3.8–5.1%, and the sensitivity was 1.1 μg/dl. The intraassay CV was 2.8–5.3%, and the sensitivity was 0.04 nmol/liter. CBG was measured using an RIA by Immuno-Biological Laboratories, Inc. (Minneapolis, MN) with an intraassay CV of 2.9–3.9% and a sensitivity of 0.25 μg/ml. Albumin was measured on an autoanalyzer, using a calorimetric assay (Roche Diagnostics, Indianapolis, IN) with an intraassay CV of 0.39–0.85% and lower detection limit of 0.2 g/dl.
Data analysis
JMP Statistical Discoveries (version 5.0.1; SAS Institute, Inc., Cary, NC) was used for statistical analyses. After log-transformation, clinical characteristics, binding proteins, and hormone levels were compared using ANOVA, and multivariate regression analyses were performed to assess the relative importance of binding globulins on hormone levels. Statistical significance was defined as a two-tailed P value <0.05. Results are expressed as mean ± sem.
Results
Patient characteristics
Baseline clinical characteristics are presented in Table 1. Women with anorexia nervosa and healthy controls were of similar age. As per study criteria, women with anorexia nervosa had lower weight and percent IBW than healthy controls. There was no difference in mean weight or percent IBW between HC+E and HC−E or AN+E and AN−E. Mean age of menarche was higher in women with anorexia nervosa than in healthy controls (13.9 ± 0.4 vs. 12.6 ± 0.3 yr; P = 0.02), but not significantly different in AN+E than AN−E or HC+E than HC−E.
Table 1.
Clinical characteristics and adrenal hormone data
| AN−E | AN+E | HC−E | HC+E | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (yr) | 27.5 ± 2.4 | 25.8 ± 1.1 | 27.5 ± 2.2 | 23.7 ± 0.9 |
| Weight (kg) | 49.0 ± 1.2a | 48.6 ± 1.5b | 62.4 ± 1.6 | 63.1 ± 2.9 |
| % IBW | 79.8 ± 0.9a | 80.2 ± 1.7b | 101.8 ± 2.3 | 98.5 ± 2.0 |
| BMI (kg/m2) | 18.3 ± 0.3a | 18.0 ± 0.4b | 22.7 ± 0.5 | 22.7 ± 0.6 |
| Duration of OCP use (months) | NA | 38.3 ± 10.7 | NA | 25.7 ± 5.9 |
| Adrenal hormone data | ||||
| Cortisol | ||||
| Baseline (μg/dl) | 6.6 ± 2.3a | 9.7 ± 2.9b | 1.6 ± 0.2 | 4.0 ± 0.6 |
| AUC (μg/dl/90 min) | 2499 ± 240a | 3404 ± 176b,c | 1739 ± 125 | 2673 ± 170 |
| Peak (μg/dl) | 36.9 ± 2.4a | 55.8 ± 2.0b,c | 28.4 ± 1.6 | 43.8 ± 2.7 |
| DHEA | ||||
| Baseline (ng/ml) | 7.1 ± 1.6 | 4.4 ± 0.6 | 4.2 ± 0.6 | 4.6 ± 0.8 |
| AUC (ng/ml/90 min) | 2091 ± 153 | 1571 ± 151c | 1739 ± 296 | 1424 ± 301 |
| Peak (ng/ml) | 29.1 ± 2.0 | 23.8 ± 2.3 | 28.4 ± 4.2 | 20.9 ± 4.4 |
| DHEAS (units) | 94.6 ± 21.3 | 72.3 ± 8.5 | 88.1 ± 15.3 | 115.2 ± 22.7 |
| CBG (μg/ml) | 51.1 ± 2.0a | 102.8 ± 5.1c | 44.4 ± 1.9 | 105.7 ± 5.7 |
| Albumin (g/dl) | 4.7 ± 0.1a | 4.4 ± 0.1c | 4.4 ± 0.1 | 4.3 ± 0.1 |
OCP, Oral contraceptive; NA, not available.
P < 0.05 AN−E vs. HC−E.
P < 0.05 AN+E vs. HC+E.
P < 0.05 AN−E vs. AN+E.
Hormone levels and binding globulins: AN−E vs. HC−E
Mean hormone and binding globulin levels are presented in Table 1. Baseline morning cortisol levels (after 1-mg dexamethasone administration the previous night) were higher in AN−E compared with HC−E. There was no difference in DHEA or DHEAS levels between groups. CBG levels were higher, as were albumin levels, in AN−E compared with HC−E.
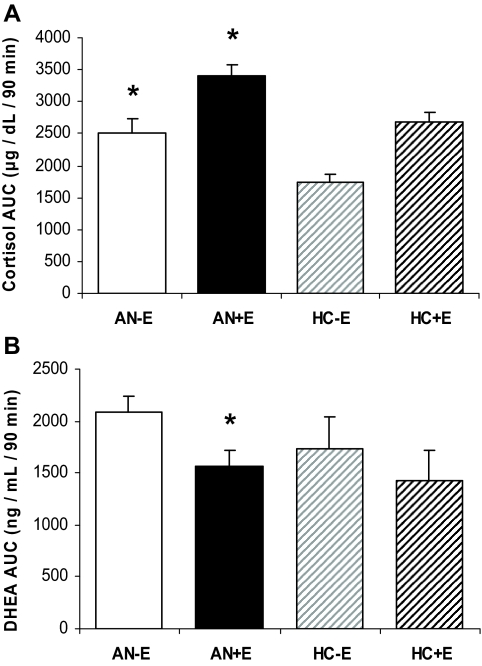
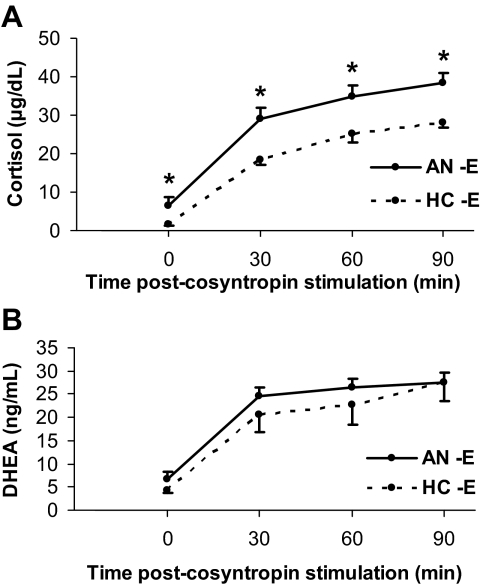
Mean AUC (Fig. 1A) and peak cortisol levels were higher in AN−E compared with HC−E. These differences remained significant after controlling for anorexia nervosa subtype, history of MDD, and history of anxiety disorder. Mean stimulated cortisol levels at all timepoints were higher in AN−E than HC−E (Fig. 2A). After controlling for CBG levels, mean cortisol level at baseline, 30, and 90 min remained significantly higher in AN−E compared with HC−E, and there were trends toward a higher mean AUC and peak cortisol in AN−E than in HC−E.
Figure 1.
A, Cortisol AUC after cortrosyn stimulation was higher in AN−E than HC−E, and in AN+E than both HC+E and AN−E. *, P < 0.05. B, DHEA AUC was lower in AN+E than AN−E. *, P < 0.05.
Figure 2.
A, Mean basal and stimulated cortisol levels were higher in AN−E than HC−E at all time points. *, P < 0.05. B, There were no significant differences in mean basal or stimulated DHEA levels at any time point between the two groups.
After administration of cortrosyn, basal, AUC (Fig. 1B), peak and stimulated (Fig. 2B) DHEA levels at all timepoints were comparable between AN−E and HC−E.
Hormone levels and binding globulins: AN+E vs. HC+E
Mean hormone and binding globulin levels are presented in Table 1. Mean baseline morning cortisol levels (after 1-mg dexamethasone administration the previous night) were higher in AN+E compared with HC+E. There was no difference in DHEA or DHEAS levels between groups. Mean CBG and albumin levels were comparable between AN+E and HC+E.
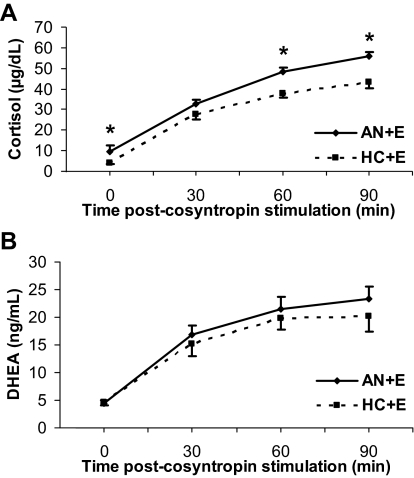
Mean AUC (Fig. 1) and peak cortisol levels were higher in AN+E compared with HC+E. Peak cortisol levels remained significantly different between groups after controlling for anorexia nervosa subtype, MDD, and history of anxiety disorder. Mean AUC cortisol remained significantly different between the groups after controlling for history of anxiety disorder. There was a trend toward a difference in mean AUC cortisol between groups after controlling for anorexia nervosa subtype but not MDD. Mean stimulated cortisol levels at 60 and 90 min were higher in AN+E than HC+E (Fig. 3A). After controlling for CBG, all of these differences between the two groups remained significantly different.
Figure 3.
A, Mean basal and stimulated cortisol levels were higher in AN+E than HC+E at baseline, 60, and 90 min. *, P < 0.05. B, There were no significant differences in mean basal or stimulated DHEA levels at any time point between the two groups.
After administration of cortrosyn, AUC (Fig. 1B), peak and stimulated DHEA levels at all timepoints were comparable between AN+E and HC+E (Fig. 3B).
Hormone levels and binding globulins: AN+E vs. AN−E
Mean hormone and binding globulin levels are presented in Table 1. There was no difference in mean baseline (after 1-mg dexamethasone administration the previous night) cortisol, DHEA, or DHEAS levels between the groups. Mean CBG levels were higher (102.8 ± 5.1 vs. 51.1 ± 2.0 μg/ml; P < 0.0001), and albumin levels were lower (4.4 ± 0.1 vs. 4.7 ± 0.1 g/dl; P = 0.03) in AN+E compared with AN−E.
After cortrosyn administration, mean AUC (Fig. 1A) and peak cortisol levels were higher in AN+E than AN−E. Peak cortisol levels remained significantly different after controlling for history of MDD and history of anxiety disorder but not anorexia nervosa subtype. Mean AUC cortisol levels remained significantly different after controlling for anorexia nervosa subtype, history of MDD, and history of anxiety disorder. Stimulated cortisol levels at 60 and 90 min were higher in AN+E than AN−E. However, controlling for CBG levels, the two were comparable.
After cortrosyn administration, mean AUC DHEA and mean stimulated DHEA at 30 min were lower in AN+E than AN−E. However, after controlling for albumin levels, AUC DHEA was comparable between AN+E and AN−E.
Associations with BMI
In the group as a whole, BMI was inversely associated with baseline morning cortisol (after 1-mg dexamethasone administration the previous night) (R = −0.53; P = 0.0004), cortisol AUC (R = −0.44; P = 0.005), and peak cortisol after cortrosyn stimulation (R = −0.40; P = 0.010). BMI was not significantly associated with any measures of DHEA (baseline after cortrosyn stimulation, AUC or peak), CBG, or albumin levels.
Discussion
We demonstrate that although cortisol levels in women with anorexia nervosa are increased compared with healthy controls, the DHEA response to ACTH stimulation is comparable. This suggests that there is a dissociation between adrenal glucocorticoid and androgen precursor secretion and that hypoandrogenemia in anorexia nervosa is not due to adrenal shunting of androgen precursors to glucocorticoid production. In addition, the higher levels of cortisol and lower levels of DHEA in AN+E compared with AN−E are attributable to estrogen effects on binding globulins alone.
Although hypercortisolemia is well established in women with anorexia nervosa, the effects of undernutrition on adrenal androgen precursors are unclear, as are the effects of oral contraceptives. We previously compared androgen and DHEAS levels in 137 women with anorexia nervosa to those of controls and found normal DHEAS levels despite decreased free testosterone, except in women receiving oral contraceptives (1). Earlier reports in smaller numbers of women had been contradictory, demonstrating low (2,3,4,5), normal (12), and elevated (6) DHEAS levels in women with anorexia nervosa. Three small published studies have investigated the effects of ACTH stimulation on glucocorticoid and adrenal androgen pathways in anorexia nervosa compared with controls. Two studies demonstrated a relative decrease in the ratio of adrenal androgen to glucocorticoid precursors in anorexia nervosa (3,13), and the third reported no difference in cortisol or DHEA in response to synthetic ACTH (14). In contrast to our study, dexamethasone was not administered in the prior studies to decrease the variability of the baseline hormone levels, thereby standardizing the absolute response to ACTH stimulation, and the effects of oral contraceptives on adrenal steroidogenesis in this population have not been studied previously. Our data clarify the dissociation between adrenal glucocorticoid and androgen steroidogenesis in anorexia nervosa and identify the likely mechanism underlying reduced serum DHEAS levels in women with anorexia nervosa receiving oral contraceptives.
To investigate the etiology of hypoandrogenemia in women with HIV wasting syndrome, Grinspoon et al. (15) performed ACTH stimulation testing on 13 patients and 21 matched healthy controls. They found that stimulated cortisol levels were increased at 30 and 60 min and DHEA levels decreased at 90 min in the women with HIV compared with controls, consistent with shunting of adrenal steroidogenesis from androgenic to glucocorticoid pathways. In contrast, our data do not support precursor shunting as an underlying mechanism for hypoandrogenemia in anorexia nervosa. However, our data do not rule out a possible role for hypercortisolemia as a mechanism underlying the suppression of the hypothalamic-pituitary-gonadal axis. Invitti et al. (16) demonstrated that women with anorexia nervosa retain normal sensitivity to glucocorticoids. Therefore, hypercortisolism may exert direct inhibitory effects on the hypothalamic-pituitary-gonadal system, resulting in hypogonadism and thereby contributing to hypoandrogenemia through this mechanism.
To our knowledge, there are no published reports of the effects of oral contraceptives on stimulated DHEA in anorexia nervosa. Oral contraceptives have been shown to decrease DHEAS (17,18,19,20) and DHEA (18) levels in healthy women, possibly due to a binding globulin effect (18). The main binding globulin for DHEA (21) and DHEAS (22) is albumin, which is reduced by oral contraceptive administration (18,20). Twenty-four hour venous blood sampling of 10 healthy women before and 2 to 3 months after starting oral contraceptives showed that oral contraceptives decreased albumin, DHEAS, and DHEA levels (18). Multivariate regression modeling indicated that albumin levels were the most important determinants of DHEAS levels. We have shown previously that DHEAS levels are reduced in women with anorexia nervosa who are receiving oral contraceptives (1). Our current study results are consistent with these data in that they show a decreased response of DHEA to ACTH stimulation. We also show that after controlling for albumin levels, there is no difference in stimulated DHEA. This suggests that despite hypercortisolemia, adrenal androgen precursors are neither reduced nor increased in anorexia nervosa and that free DHEA levels are not affected by oral contraceptives.
In summary, our results are consistent with the hypothesis that there is a dissociation between adrenal glucocorticoid and androgen precursor production, with increased cortisol but not adrenal androgen precursor secretion. Therefore, hypoandrogenemia in anorexia nervosa is not due to a shift of adrenal precursors from androgenic to glucocorticoid pathways. Furthermore, our data indicate that reduced DHEA levels in women with anorexia nervosa on oral contraceptives likely reflect a binding globulin effect rather than reduced bioavailability. Hypercortisolemia may contribute to decreased testosterone levels by other mechanisms, such as suppression of the hypothalamic-pituitary-gonadal axis.
Acknowledgments
We thank the nurses and bionutritionists of the Massachusetts General Hospital GCRC and the patients who participated in the study.
Footnotes
This work was supported by National Institutes of Health Grants NIH-R01-DK52625 and MO1 RR01066.
Disclosure Summary: The authors have no conflicts to declare.
First Published Online January 21, 2009
Abbreviations: AN+E, Women with anorexia nervosa receiving oral contraceptives; AN−E, women with anorexia nervosa not receiving oral contraceptives; AUC, area under the curve; BMI, body mass index; CBG, cortisol binding globulin; CV, coefficient of variation; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; HC+E, healthy controls receiving oral contraceptives; HC−E, healthy controls not receiving oral contraceptives; IBW, ideal body weight; MDD, major depressive disorder.
References
- Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A 2007 Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 92:1334–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinathsinghji DJ, Mills IH 1985 Concentration patterns of plasma dehydroepiandrosterone, Δ5-androstenediol and their sulphates, testosterone and cortisol in normal healthy women and in women with anorexia nervosa. Acta Endocrinol (Copenh) 108:255–260 [DOI] [PubMed] [Google Scholar]
- Devesa J, Perez-Fernandez R, Bokser L, Gaudiero GJ, Lima L, Casanueva FF 1988 Adrenal androgen secretion and dopaminergic activity in anorexia nervosa. Horm Metab Res 20:57–60 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, Gundberg CM, Leboff MS 2002 Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr 141:64–70 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS 2002 Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab 87:4935–4941 [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi M, Colurcio B, Casarosa E, Ioime R, Genazzani AR, Maj M 2001 Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med 63:62–68 [DOI] [PubMed] [Google Scholar]
- Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V 2008 Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology 57:95–115 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A 2004 Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 89:4972–4980 [DOI] [PubMed] [Google Scholar]
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F 2001 Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145:165–171 [DOI] [PubMed] [Google Scholar]
- Walsh BT, Katz JL, Levin J, Kream J, Fukushima DK, Hellman LD, Weiner H, Zumoff B 1978 Adrenal activity in anorexia nervosa. Psychosom Med 40:499–506 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Helke J, Lucky AW 1985 Dexamethasone preparation does not alter corticoid and androgen responses to adrenocorticotropin. J Clin Endocrinol Metab 60:585–589 [DOI] [PubMed] [Google Scholar]
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A 1999 The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 84:4489–4496 [DOI] [PubMed] [Google Scholar]
- Winterer J, Gwirtsman HE, George DT, Kaye WH, Loriaux DL, Cutler Jr GB 1985 Adrenocorticotropin-stimulated adrenal androgen secretion in anorexia nervosa: impaired secretion at low weight with normalization after long-term weight recovery. J Clin Endocrinol Metab 61:693–697 [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Gianotti L, Picu A, Fassino S, Abbate Daga G, Mondelli V, Giordano R, Grottoli S, Ghigo E, Arvat E 2004 The adrenal sensitivity to ACTH stimulation is preserved in anorexia nervosa. J Endocrinol Invest 27:436–441 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Corcoran C, Stanley S, Rabe J, Wilke S 2001 Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab 86:4120–4126 [DOI] [PubMed] [Google Scholar]
- Invitti C, Redaelli G, Baldi G, Cavagnini F 1999 Glucocorticoid receptors in anorexia nervosa and Cushing’s disease. Biol Psychiatry 45:1467–1471 [DOI] [PubMed] [Google Scholar]
- Aden U, Jung-Hoffmann C, Kuhl H 1998 A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception 58:75–81 [DOI] [PubMed] [Google Scholar]
- Carlstrom K, Karlsson R, Von Schoultz B 2002 Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scand J Clin Lab Invest 62:361–368 [DOI] [PubMed] [Google Scholar]
- Moutos D, Smith S, Zacur H 1995 The effect of monophasic combinations of ethinyl estradiol and norethindrone on gonadotropins, androgens and sex hormone binding globulin: a randomized trial. Contraception 52:105–109 [DOI] [PubMed] [Google Scholar]
- Coenen CM, Thomas CM, Borm GF, Hollanders JM, Rolland R 1996 Changes in androgens during treatment with four low-dose contraceptives. Contraception 53:171–176 [DOI] [PubMed] [Google Scholar]
- Dunn JF, Nisula BC, Rodbard D 1981 Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 53:58–68 [DOI] [PubMed] [Google Scholar]
- Plager JE 1965 The binding of androsterone sulfate, ethiocholanolone sulfate, and dehydroisoandrosterone sulfate by human plasma protein. J Clin Invest 44:1234–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]