Abstract
Context: The distribution of serum TSH shifts progressively to higher concentrations with age.
Objective: The aim of the study was to determine whether the population shift in TSH distribution to higher concentrations with aging extends to people of exceptional longevity, namely centenarians, and to assess the relationship between concentrations of TSH and free T4 (FT4).
Design/Setting/Patients: We analyzed TSH, FT4, and TSH frequency distribution curves in thyroid disease-free Ashkenazi Jews with exceptional longevity (centenarians; median age, 98 yr), in younger Ashkenazi controls (median age, 72 yr), and in a population of thyroid disease-free individuals (median age, 68 yr) from the U.S. National Health and Nutrition Examination Survey 1998–2002 (NHANES controls).
Results: Serum TSH was significantly higher in centenarians [1.97 (0.42–7.15) mIU/liter] than in Ashkenazi controls [1.55 (0.46–4.55) mIU/liter] and NHANES controls [1.61 (0.39–6.29) mIU/liter] (median, 2.5 and 97.5 centiles) (P < 0.001). The TSH frequency distribution curve of centenarians was relatively similar in shape to controls but shifted significantly to higher TSH, including TSH concentration at peak frequency. The TSH distribution curve of the NHANES control group was superimposable to and not significantly different from the Ashkenazi controls. FT4 was similar in centenarians and Ashkenazi controls, and there was a significant inverse correlation between FT4 and TSH in both groups.
Conclusions: The TSH population shifts to higher concentrations with age appear to be a continuum that extends even to people with exceptional longevity. The inverse correlation between TSH and FT4 in our populations suggests that changes in negative feedback may contribute to exceptional longevity.
In individuals who achieve extreme longevity, median and upper reference limits of serum TSH are shifted to levels even higher than the oldest groups reported in NHANES surveys, suggesting that routine levothyroxine treatment should not be recommended.
Subclinical hypothyroidism is diagnosed when serum TSH concentration is above the upper reference limit and free T4 (FT4) remains within the reference range. Because the designation of subclinical hypothyroidism is so dependent on raised serum TSH, accurate and population-specific reference ranges are of major clinical importance.
Recent reports indicate that serum TSH concentrations and distribution gradually increase with age (1), suggesting either a decline in thyroid function or a reset in the TSH set point, which may occur with aging. The prevailing recommendation of professional societies (2), which is controversial (3), is to treat such patients routinely with levothyroxine. However, despite extensive study, the clinical implications of higher serum TSH observed with aging remain unclear, and it is uncertain whether the raised serum TSH adversely affects health, has no clinical importance, or is a factor that contributes to healthy aging (3,4).
Because hypothyroidism is associated with extreme longevity in certain animal models (5) and various studies in humans show an association between low thyroid function and longevity (6,7), we hypothesized that a progressive age-related shift in TSH might be a continuum that would extend even to people with exceptional longevity and possibly contribute to their longevity. To explore this hypothesis, we examined the distribution of serum TSH and FT4 in a well-characterized population of Ashkenazi Jews with exceptional longevity (centenarians) and related their findings both to younger Ashkenazi controls and to NHANES controls that are age-matched to the Ashkenazi controls.
Subjects and Methods
Study population
A group of 232 Ashkenazi Jewish centenarians living independently (166 females, median age, 97.8 yr; and 66 males, median age 97.6 yr) were recruited to participate in the study; 188 younger unrelated Ashkenazi Jews served as a control group (Ashkenazi controls) (95 females, median age, 69.7 yr; and 93 males, median age, 72.3 yr). The study population was identified from the Longevity Genes Study at Albert Einstein College of Medicine, Bronx, NY. Recruitment for the Longevity Genes study has been described in detail elsewhere (8). Briefly, subjects were recruited through publicity, and age was verified by birth certificates or U.S. passports. Medical history, demographic characteristics, and clinical data were obtained uniformly using a structured questionnaire. All subjects underwent a physical examination and provided a blood sample. Informed written consent was obtained in accordance with the policy of the Committee on Clinical Investigation of the Albert Einstein College of Medicine.
Exclusion criteria included: acute or debilitating medical condition (not recruited), history of thyroid disease or subjects taking thyroid medications (42 centenarians and 47 controls), or subjects with serum TSH less than 0.4 mIU/liter and/or FT4 outside the reference limits (three centenarians and three controls).
Another control group obtained from NHANES 1998–2002 data included all 605 subjects in the 60- to 79-yr-old group who had serum TSH determinations, did not have thyroid disease, or were taking thyroid medications. We studied TSH distribution in this cohort (NHANES controls), with median age 68 yr, comparable to the Ashkenazi controls, to exclude the possibility of an ethnicity-related bias in the interpretation of the data.
TSH and FT4 analyses
Measurements of TSH were performed at the laboratories of Montefiore Medical Center (Bronx, NY) using a solid-phase, two-site chemiluminescent immunometric assay (Immunolite 2000 Third Generation TSH; Siemens Corp., Tarrytown, NY); manufacturer’s reference limits were 0.4–4.0 mIU/liter. FT4 was measured using ELISA (Alpco Diagnostic, Salem, NH); manufacturer’s reference limits were 0.8–1.8 ng/dl.
Due to logistic and technical restrictions, serum TSH was analyzed in 232 centenarians and 185 controls, and serum FT4 was analyzed in 137 centenarians and 172 controls. However, only 97 centenarians and 150 controls had both serum TSH and FT4 determined. Also, due to insufficient serum available, we could not determine the antithyroid antibody levels in our study population.
Statistical analysis
We used SAS software, version 9.12 (SAS Institute Inc., Cary, NC) for data analysis. Plasma TSH and FT4 distributions were tested for normality using Shapiro-Wilk test and D’Agostino’s K-squared test and were found to violate the normality assumption. Thus, a comparison between nonparametric variables was performed using Kruskal-Wallis one-way ANOVA by ranks and Mann-Whitney rank sum test. Spearman’s rank correlation was used to examine the correlation between TSH and FT4. The frequency distribution curves of TSH concentration were prepared using log transformed values of TSH. To determine whether various drugs taken by participants may affect TSH concentration, we applied a linear regression model where serum TSH levels served as a dependent variable, and various medications taken by our study subjects served as independent variables. Data are presented as median (2.5 and 97.5 centiles). A P value <0.05 was considered statistically significant.
Results
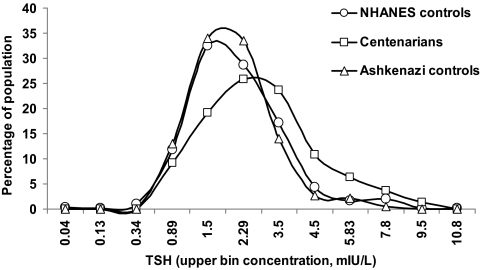
Serum TSH was significantly higher in the centenarians [1.97 (0.42–7.15) mIU/liter] compared with the Ashkenazi controls [1.55 (0.46–4.55) mIU/liter] (median, 2.5 and 97.5 centiles) (P < 0.001). The frequency distribution curves for TSH appeared relatively similar in shape but shifted to higher TSH concentrations in the centenarians, including the TSH concentration at peak frequency (Fig. 1). The distribution curve for centenarians may possibly be minimally skewed to higher values compared with the Ashkenazi controls. The percentage of subjects with TSH greater than 2.5 mIU/liter was 15.4% in the controls and 35.2% in the centenarians.
Figure 1.
TSH Distribution in disease-free centenarians, Ashkenazi controls, and NHANES controls. The frequency distribution curves of TSH concentration were prepared using log-transformed values of TSH concentrations. Bin values were calculated by using the antilog function on the equal interval concentrations. Note that intervals on horizontal axis are not equal.
We further compared the TSH distribution curves of the centenarians and Ashkenazi controls to the thyroid disease-free NHANES control group (Fig. 1). Median TSH concentrations were similar in the Ashkenazi and NHANES control groups [1.55 (0.46–4.55) mIU/liter and 1.61 (0.39–6.29) mIU/liter, respectively (P = 0.18)]. TSH distribution in the Ashkenazi control group did not differ significantly from the NHANES control group (P = 0.17). However, TSH distribution in the centenarians was shifted significantly to higher serum concentrations compared with Ashkenazi and NHANES control groups (P = 0.01 and P = 0.002, respectively).
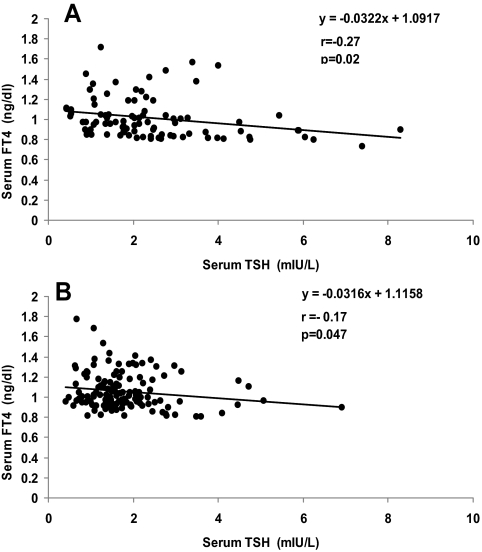
Serum FT4 concentrations were similar in the centenarians and Ashkenazi control group with a median of 1.02 (0.62–2.02) ng/dl in centenarians and 1.02 (0.63–1.67) ng/dl in controls (P = 0.37). There was an inverse correlation between FT4 and TSH in the centenarians (r = −0.27; P = 0.02; Fig. 2A) and in the control group (r = −0.17; P = 0.047; Fig. 2B). FT4 determinations were not available for the NHANES control group.
Figure 2.
Correlation between FT4 and TSH in the centenarians (A), and in Ashkenazi controls (B).
Analysis of the association between various medications taken by the centenarians and serum TSH concentrations showed no significant effect of any medication. Furthermore, applying a statistical stepwise regression of a mixed model where probability to enter was 0.25 and probability to leave was 0.05 demonstrated that none of the medications had any significant effect.
Discussion
Our data demonstrate that centenarians have significantly higher median serum TSH concentrations compared with younger Ashkenazi controls. Furthermore, TSH distribution in centenarians was shifted toward higher TSH levels, underscoring the fact that the majority of the centenarian population had higher serum TSH values compared with the Ashkenazi control group. Because the selection of Jewish subjects was a basic requirement in the Longevity Genes Project (8,9), all the centenarian and Ashkenazi control population was Jewish. The reason we chose NHANES data as an additional control group was to determine whether there might be an ethnicity-related effect on serum TSH concentrations that could affect our results. Indeed, our control group had a median serum TSH concentration and distribution comparable to its age-matched cohort from NHANES, demonstrating that our results were not biased by the ethnicity of the centenarians and Ashkenazi controls. Moreover, serum TSH determinations for the centenarian and Ashkenazi control populations were performed at the same laboratory (Montefiore Medical Center), excluding the possibility of laboratory-related bias.
A previous analysis of TSH distribution in the U.S. population demonstrated a progressive increase in median TSH with aging (1). Recent reports showed that this progressive increase in serum TSH resulted from a population distribution shift to higher TSH, independent of antithyroid antibodies (10,11). Median TSH of centenarians (1.97 mIU/liter) was higher than Ashkenazi or NHANES controls (1.51 and 1.61 mIU/liter, respectively), as well as the older than 80 yr group in NHANES III (1.90 mIU/liter) (1). Taken together, our findings suggest that the progressive population increase in serum TSH with aging extends even to individuals with extreme longevity.
Some of the increase in serum TSH distribution associated with aging could be due to an increase in prevalence of acquired autoimmune thyroid disease. Indeed, antithyroid antibody prevalence does increase with age (1,10), appears to plateau after 60 yr of age at approximately 26%, and may decrease in those with exceptional longevity (12). We believe that autoimmune thyroid disease, while probably present in some individuals, is not primarily responsible for the population shifts observed here and elsewhere (10,11). A progressive increase in the incidence of acquired thyroid disease with age should not result in a shift to higher TSH at peak frequency in TSH distribution curves and should produce a notable skew to higher TSH. Neither of these findings was apparent in our study, or in other reports (10,11).
Our findings support other reports demonstrating elevated serum TSH concentrations in extreme longevity (7,13). Interestingly, other studies show a decrease in serum TSH levels in centenarians (14,15). We suggest that these data should be interpreted cautiously because the reported studies have smaller numbers of subjects, some living in areas of variable iodine deficiency, and have different genetic backgrounds. Our findings should not, therefore, be extrapolated to populations outside of North America.
Among possible explanations for a continuum of progressive increase in TSH with aging, even to centenarians, are effects of medications, age-related alterations in TSH glycosylation, atrophic nonautoimmune changes in the thyroid, negative feedback set points, or hypothyroidism. The inverse correlation of FT4 and TSH observed here and previously (11) provides support either for an altered set point or subtle hypothyroidism. Because the population shifts in TSH with aging do not appear related primarily to autoimmune thyroid disease (10), it is possible that the same FT4 and TSH concentrations in these individuals were present from a young age and contributed to healthy aging. This could account for the progressive increase in prevalence of raised TSH with aging in cross-sectional population studies because the remainder of the population might die at earlier ages. Finally, we cannot completely exclude the possibility that autoimmune thyroid disease, for which prevalence also increases with age, might play a role in our findings. However, the fact that centenarians have higher serum TSH concentration suggests that the increase in serum TSH is related to a favorable role in their healthy aging regardless of the underlying mechanism. In this regard, it is notable that animal studies (5,16,17) suggest an association of hypothyroidism with improved longevity.
Although it remains unclear from numerous clinical studies whether altered negative-feedback between FT4 and TSH or subtle hypothyroidism raises the risk of adverse health outcomes, this, a priori, does not seem likely for individuals who have achieved exceptional longevity. Data concerning adverse health consequences of subclinical hypothyroidism remain conflicting (3,4). Numerous studies of cardiovascular outcomes in subclinical hypothyroidism and even several meta-analyses have come to opposite conclusions (18). However, two recent meta-analyses suggest that increased risk for adverse cardiovascular outcomes occurs in patients younger than 65 yr of age, but not in those more than 65 yr old (19,20). Until these issues are settled by further research, it seems prudent not to treat routinely elderly patients with levothyroxine because they are found to have a minimal increase in serum TSH.
Acknowledgments
The authors thank Ms. Zhao Hu and Mr. Temuri Bodagov for laboratory determinations.
Footnotes
This work was partially supported by Grants AG-027734, AG-18728, RR-12248, DK-20541, and M01-RR12248 from the National Institutes of Health and the Glenn Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 21, 2009
Abbreviation: FT4, free T4.
References
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT 2005 Consensus statement 1: subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. Thyroid 15:24–28; response, 32–33 [DOI] [PubMed] [Google Scholar]
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ 2004 Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228–238 [DOI] [PubMed] [Google Scholar]
- Biondi B, Cooper DS 2008 The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Pinto M 15 July 2008 Endocrine function in naturally long-living small mammals. Mol Cell Endocrinol 299:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG 2004 Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Savarino L, Cucinotta D, Cavalli G 2000 Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab 85:2260–2265 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR 2003 Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA 290:2030–2040 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Gabriely I, Gabriely M, Iankowitz N, Sorkin JD 2001 Offspring of centenarians have a favorable lipid profile. J Am Geriatr Soc 49:76–79 [DOI] [PubMed] [Google Scholar]
- Surks MI, Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- Boucai L, Surks MI 25 August 2008 Reference limits of serum thyrotropin (TSH) and free thyroxine (free T4) are significantly influenced by race and age in an urban outpatient practice of medicine. Clin Endocrinol (Oxf) 10.1111/j. 1365-2265.2008.03390.x [DOI] [PubMed] [Google Scholar]
- Mariotti S, Franceschi C, Cossarizza A, Pinchera A 1995 The aging thyroid. Endocr Rev 16:686–715 [DOI] [PubMed] [Google Scholar]
- Tietz NW, Shuey DF, Wekstein DR 1992 Laboratory values in fit aging individuals—sexagenarians through centenarians. Clin Chem 38:1167–1185 [PubMed] [Google Scholar]
- van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SW 2005 Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab 90:6403–6409 [DOI] [PubMed] [Google Scholar]
- Magri F, Muzzoni B, Cravello L, Fioravanti M, Busconi L, Camozzi D, Vignati G, Ferrari E 2002 Thyroid function in physiological aging and in centenarians: possible relationships with some nutritional markers. Metabolism 51:105–109 [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H 2004 Life extension in the dwarf mouse. Curr Top Dev Biol 63:189–225 [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A 1996 Dwarf mice and the ageing process. Nature 384:33 [DOI] [PubMed] [Google Scholar]
- Ladenson PW 2008 Cardiovascular consequences of subclinical thyroid dysfunction: More smoke but no fire. Ann Intern Med 148:880–881 [DOI] [PubMed] [Google Scholar]
- Ochs N, Aur R, Bauer DC, Nanchen D, Gussekloo J, Cornuz J, Rodondi N 2008 Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 148:8321–8845 [DOI] [PubMed] [Google Scholar]
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SHS 2008 The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 93:2998–3007 [DOI] [PubMed] [Google Scholar]