Abstract
Context: Elevated urinary albumin excretion has been reported in primary aldosteronism and might partially reflect reversible abnormalities initiated by glomerular hyperfiltration.
Objective: The aim of the study was to examine the outcome of renal function and intrarenal Doppler velocimetric indices in primary aldosteronism.
Design: We conducted a prospective study of patients with primary aldosteronism who were reevaluated 1 yr after either adrenalectomy or treatment with spironolactone.
Setting: The study was conducted at a university referral center.
Patients: Fifty-four patients with tumoral or idiopathic aldosteronism were followed after either surgical (n = 24) or medical (n = 30) treatment. Patients with primary aldosteronism were compared with 100 patients with primary hypertension and comparable severity and duration of disease.
Main Outcome Measures: Changes in renal function and intrarenal echo-Doppler indices were measured.
Results: Patients with primary aldosteronism had greater creatinine clearance and urinary albumin excretion than patients with primary hypertension. Patients with primary aldosteronism and creatinine clearance above the median (105 ml/min per 1.73 m2) had significantly lower resistance and pulsatility index than patients with creatinine clearance below the median, independent of disease subtype. After 1 yr, creatinine clearance and albuminuria declined, and resistance and pulsatility index increased to the same extent in patients with primary aldosteronism treated with either adrenalectomy or spironolactone. Changes in glomerular filtration and albuminuria were inversely related with baseline values of the resistance index. In primary hypertension, echo-Doppler velocimetric indices did not change during follow-up.
Conclusions: In primary aldosteronism, sonographic evidence of decreased intrarenal vascular resistance is associated with glomerular hyperfiltration. Both adrenalectomy and spironolactone revert the intrarenal hemodynamic pattern and decrease urinary protein losses.
In primary aldosteronism, decreased intrarenal vascular resistance is associated with glomerular hyperfiltration, and both adrenalectomy and spironolactone revert the intrarenal hemodynamic pattern and decrease albuminuria.
Although primary aldosteronism has long been considered a relatively benign form of hypertension (1,2), recent studies suggest that chronic exposure to elevated aldosterone might lead to cardiovascular (3) and renal (4) damage out of proportion to blood pressure levels. With respect to the kidney, experimental animal studies clearly demonstrate that aldosterone contributes to intrarenal vascular remodeling and support its role in the progression of renal vascular disease (4,5). However, data showing that aldosterone is detrimental to the kidney are weaker in the clinical setting (6). In fact, the majority of initial reports indicated that primary aldosteronism is not a frequent cause of overt renal damage (1,2,7,8), and recent studies have suggested that the renal dysfunction that is detected in patients with this endocrine disorder could be related to the intrarenal hemodynamic adaptation to the effect of aldosterone excess. For instance, elevated urinary albumin excretion that has been repeatedly reported in these patients might reflect, at least in part, functional and potentially reversible abnormalities initiated by glomerular hyperfiltration (9,10). Increased glomerular filtration rate has been shown consistently in primary aldosteronism patients, even using different assessment methods (9,10,11,12), and renal micropuncture studies have demonstrated glomerular hyperfiltration in animal models of mineralocorticoid-induced hypertension, reflecting increased glomerular blood flow consequent upon vasodilation of both afferent and efferent arterioles (13).
Renal duplex Doppler ultrasound is increasingly used for detection of renal artery stenosis (14) and for assessment of renal artery patency (15) and prediction of clinical outcome (16) after renal revascularization. Moreover, intrarenal velocimetric parameters have been used for assessment of intrarenal hemodynamics, showing significant correlation with intrarenal vascular resistance in hyperfiltering kidneys such as those of patients with early diabetic nephropathy (17,18). In the present study, we have prospectively investigated the changes in intrarenal Doppler sonographic indices and renal function in patients with primary aldosteronism who were treated with adrenalectomy or aldosterone antagonists.
Patients and Methods
Patients
Fifty-four consecutive patients with primary aldosteronism were included in a prospective study. Recruitment of patients, diagnostic criteria, and follow-up have been described in detail in previous publications (19,20). Patients were referred to the hypertension clinic of our university for evaluation of their hypertensive state: 36 patients had persistent hypokalemia, and 27 had hypertension resistant to a triple-drug regimen. Blood pressure was measured by sphygmomanometry, and hypertension was diagnosed according to established guidelines (21). All patients seen at the clinic are screened with extensive testing to define the cause of hypertension. Seven percent of patients with primary aldosteronism were taking no antihypertensive drug, 9% were on monotherapy, and the remaining 84% had multiple-drug treatment with an average of 2.9 drugs per patient. Patients treated with antihypertensive drugs were withdrawn from treatment a minimum of 2 wk before diagnostic assessment. Beta-blockers, diuretics, lipophilic calcium antagonists, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers were withdrawn for a minimum of 3 wk. No patient was taking mineralocorticoid receptor antagonists before the study.
Primary aldosteronism was screened by the demonstration of an increased plasma aldosterone-to-active renin ratio (20 or more) (10,22) in the presence of a plasma aldosterone of more than 150 pg/ml, and the diagnosis was confirmed by the lack of aldosterone suppression (values of 50 pg/ml or more) after an iv saline load (2 liters of 0.9% saline infused over 4 h) (23). This test has been shown to be highly effective in the distinction of both tumoral and idiopathic aldosteronism from low-renin primary hypertension (24). Measurements were performed under a normal sodium diet, and 24-h urinary sodium excretion was checked in all patients. Plasma potassium concentration of 3.5 mmol/liter or less was corrected by oral supplementation before assessment of the plasma aldosterone-to-active renin ratio and saline suppression test. Differentiation between adrenal adenoma and idiopathic aldosteronism was obtained by high-resolution computerized tomography scan followed by selective adrenal vein sampling (successful in 14) and/or adrenal scintigraphy with iodocholesterol (n = 47) that was performed under dexamethasone suppression. In patients who underwent adrenal vein sampling, adrenal vein cannulation was considered successful if the adrenal vein/inferior vena cava cortisol gradient was at least 2 and lateralization was defined by measurement of an aldosterone/cortisol ratio in one adrenal vein that was at least four times the ratio in the other adrenal vein (25). In all patients who underwent adrenalectomy, adenoma was confirmed by histology (solitary nodule of cells originating from the zona glomerulosa with a surrounding capsule) and normalization of plasma aldosterone concentrations. Primary aldosteronism was treated by either unilateral adrenalectomy or spironolactone (range, 50–300 mg/d; average dose, 121 mg/d), and treatment was followed by normalization of blood pressure or significant improvement of hypertension (decrease of 20% or more in mean blood pressure and/or use of fewer antihypertensive drugs) in all patients.
One hundred patients with primary hypertension served as controls for baseline comparisons. These patients were recruited at our hypertension clinic and selected by frequency matching after specification of inclusion criteria to avoid age, gender, body mass index, and estimated duration of hypertension as potential confounding variables. In these patients, secondary causes of hypertension were excluded with extensive laboratory investigations that were performed after appropriate drug washout. All patients who had low active renin values after appropriate drug washout underwent an iv saline load despite an aldosterone-to-renin ratio of less than 20. Among patients with primary hypertension, 11% were taking no antihypertensive drug, 16% were on monotherapy, and 73% had multiple-drug treatment with a mean of 2.3 antihypertensive agents per patient. Written informed consent was obtained from all patients, and the protocol was approved by the local ethics committee.
Renal function studies and renal imaging
Renal function studies included assessment of the glomerular filtration rate and albuminuria and were performed as described previously (10). Briefly, duplicate 24-h urine collections were obtained in the clinical unit for determination of creatinine clearance and urinary protein excretion, and the average of measurements were recorded. Creatinine clearance was normalized for body surface area (ml/min/1.73 m2). Creatinine concentrations in serum and urine were measured by a modification of the Jaffé reaction (26). Urinary albumin excretion was measured by RIA.
Renal ultrasound examination by a duplex Doppler apparatus was performed in patients with primary aldosteronism and patients with primary hypertension after a 12-h fast, as described previously (27). All measurements were obtained by two experienced operators, and tracings were examined by an experienced reader who was blinded to any clinical information of the patients. Doppler scans were performed using a 3.5-MHz convex phased-array probe and color Doppler mapping to identify the renal arteries. Measurements were collected with patients in lateral decubitus position through the flank, using the liver as an acoustic window whenever possible. The Doppler angle was always lower than 60° and as close as possible to 0°; specific care was taken not to compress the kidney. Peak systolic velocity [Vmax (cm/sec)], end diastolic velocity [Vmin (cm/sec)], and mean velocity [Vmean (cm/sec)] were obtained for the calculation of resistance index (Vmax − Vmin/Vmax), pulsatility index (Vmax − Vmin/Vmean), and acceleration [Vmax/t (m/sec2)]. These parameters were calculated from Doppler measurements obtained by placing the sample volume at the level of the color signals visualized on the main tract of the renal arteries and the interlobar arteries, along the edge of medullary pyramids. The average of four to six homogeneous measurements from the upper, middle, and lower third of both kidneys was considered. Kidney measurements were obtained from multiple longitudinal images obtained in sagittal or coronal planes, and kidney volume was calculated (27,28). The kidney volumes were expressed in milliliters, and the values of both kidneys were summed to obtain the total kidney volume. The intraobserver and interobserver coefficient of variability for combined total kidney volume was less than 5%, and for resistance index, pulsatility index, and acceleration coefficients were, respectively, 6.6 and 8.3%, 7.3 and 9.7%, and 10.3 and 12.0%.
Treatment and follow-up
All patients with primary aldosteronism were followed up prospectively (10). Twenty-four of 29 patients with adrenal adenoma underwent adrenalectomy; among the remaining five patients, two had bilateral adenoma and three refused surgery and were treated with spironolactone. Treatment with spironolactone was started with a dose of 100 mg/d that was titrated to reach target blood pressure (average dose during follow-up, 121 mg/d). No patient was treated with amiloride or triamterene. Clinical assessment and laboratory tests were repeated 1, 3, 6, and 12 months after enrollment. At each visit, antihypertensive therapy was adjusted according to the physician’s judgment to reach a target of less than 140/90 mm Hg. Use of all antihypertensive agents was permitted. Renal Doppler sonographic scans were repeated after 1 yr in all patients with primary aldosteronism and primary hypertension.
Statistical analysis
This study size was planned to give a statistical power of more than 95% to detect 10% differences between patients with primary aldosteronism and primary hypertension, with an α value of 0.05. Continuous variables are expressed as means ± sd unless otherwise indicated. Variables with skewed distribution were analyzed after logarithmic transformation. Characteristics of the study subjects were compared among groups by the Student’s t test. The Pearson χ2 test was used to compare categorical variables. The relationship between different variables was examined by linear regression analysis, and the correlation was expressed by the correlation coefficient. Multiple regression analysis was used to ascertain which variables were independently correlated. Changes from baseline of renal function and Doppler ultrasound parameters were assessed by two-way ANOVA. All tests were two-sided with a level of significance of 5%.
Results
Adrenal tumor was demonstrated in 29 (54%) of 54 patients with primary aldosteronism, whereas the remaining 25 (46%) had no evidence of adrenal masses. Baseline clinical and biochemical characteristics of the study patients are summarized in Table 1. After drug washout, patients with primary aldosteronism had blood pressure levels in a range that was similar to that reported in other studies (9,29), and these levels, together with the estimated duration of hypertension, were comparable with those of patients with primary hypertension. As expected, patients with primary aldosteronism had significantly higher plasma aldosterone and significantly lower plasma potassium and active renin levels than patients with primary hypertension. Creatinine clearance was significantly higher in patients with primary aldosteronism than in primary hypertensive subjects. Also, albuminuria was higher in the primary aldosteronism than the primary hypertension group, but this difference was eliminated when urinary albumin excretion was normalized for urinary creatinine concentration, indicating that increase in absolute values could be due to hyperfiltration.
Table 1.
Baseline characteristics of the study population
| Parameter | Essential hypertension (n = 100) | Primary aldosteronism (n = 54) | P value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (yr) | 52 ± 10 | 53 ± 12 | NS |
| Females/males | 31/69 | 16/38 | NS |
| Body mass index (kg/m2) | 28.2 ± 3.2 | 28.6 ± 3.8 | NS |
| Heart rate (beats/min) | 70 ± 3 | 70 ± 4 | NS |
| Systolic blood pressure (mm Hg)a | 165 ± 17 | 167 ± 16 | NS |
| Diastolic blood pressure (mm Hg)a | 102 ± 9 | 103 ± 9 | NS |
| Estimated duration of hypertension (yr) | 10 ± 7 | 10 ± 7 | NS |
| Current smoking, no. (%) | 24 (24) | 15 (28) | NS |
| Alcohol intake (g/d) | 32 ± 11 | 34 ± 9 | NS |
| Laboratory variables | |||
| Plasma glucose (mmol/liter) | 4.8 ± 1.0 | 4.9 ± 0.9 | NS |
| Total cholesterol (mmol/liter) | 5.28 ± 1.05 | 5.13 ± 1.09 | NS |
| HDL cholesterol (mmol/liter) | 1.29 ± 0.32 | 1.26 ± 0.41 | NS |
| Triglycerides (mmol/liter) | 1.36 ± 0.81 | 1.23 ± 0.70 | NS |
| Plasma sodium (mmol/liter) | 140 ± 3 | 141 ± 4 | NS |
| Plasma potassium (mmol/liter) | 4.2 ± 0.3 | 3.2 ± 0.4b | <0.001 |
| Urinary sodium excretion (mmol/24 h) | 107 ± 45 | 101 ± 63 | NS |
| Urinary potassium excretion (mmol/24 h) | 46 ± 21 | 52 ± 19 | NS |
| Plasma aldosterone (pg/ml) | 149 ± 87 | 246 ± 191 | <0.001 |
| Plasma active renin (pg/ml) | 9.1 ± 9.7 | 4.8 ± 6.4 | <0.001 |
| Aldosterone to active renin ratio | 16.4 ± 2.0 | 51.3 ± 3.5 | <0.001 |
| Serum creatinine (μmol/liter) | 100 ± 38 | 91 ± 20 | NS |
| Creatinine clearance (ml/min/1.73 m2) | 87 ± 37 | 105 ± 31 | 0.003 |
| Urinary albumin excretion (mg/24 h)c | 15 [6–26] | 22 [9–38] | 0.016 |
| Urinary albumin/creatinine ratioc | 0.045 [0.019–0.087] | 0.046 [0.021–0.086] | NS |
Values are means ± sd unless otherwise indicated. P values are calculated by the Student’s t test. Normal reference values for plasma measurements: glucose, 4.2–6.4 mmol/liter; total cholesterol, <5.17 mmol/liter; HDL-cholesterol, 1.03–2.19 mmol/liter; triglyceride, 0.57–1.90 mmol/liter; sodium, 132–148 mmol/liter; potassium, 3.5–5.0 mmol/liter, aldosterone, 30–220 pg/ml; active renin, 2.5–18 pg/ml. HDL, High-density lipoprotein; NS, not significant.
Blood pressure was measured after appropriate washout of antihypertensive drugs.
Values are those that were measured before correction with oral supplementation.
Median [interquartile range].
Baseline renal sonographic parameters are summarized in Table 2. Total kidney volume and acceleration were comparable in patients with primary aldosteronism and primary hypertension, whereas both the resistance index and pulsatility index were significantly lower in patients with primary aldosteronism compared with those with primary hypertension. When patients with primary aldosteronism were subdivided according to the median creatinine clearance value for the distribution (105 ml/min/1.73 m2), patients with values above the median had significantly lower resistance index (P = 0.012) and pulsatility index (P = 0.041) than patients with values below the median (Table 3). The same analysis in patients with primary hypertension did not show significant association of creatinine clearance with intrarenal Doppler parameters. Comparison of the resistance index in patients with primary aldosteronism who had plasma renin below the lower limit of detection with our method (2.5 pg/ml; n = 39) to patients who had detectable renin (n = 15) showed a nonsignificant trend to greater values in the latter group (suppressed renin, 0.60 ± 0.06; detectable renin, 0.63 ± 0.07; P = 0.149). Linear regression analysis of renal function, plasma renin, and plasma aldosterone of patients with primary aldosteronism or primary hypertension did not show any significant correlation with intrarenal Doppler parameters.
Table 2.
Baseline renal duplex echo-Doppler parameters of the study population
| Parameter | Essential hypertension (n = 100) | Primary aldosteronism (n = 54) | P value |
|---|---|---|---|
| Total kidney volume (ml) | 414 ± 61 | 411 ± 58 | NS |
| Resistance index | 0.64 ± 0.05 | 0.61 ± 0.07 | 0.003 |
| Pulsatility index | 1.26 ± 0.14 | 1.19 ± 0.15 | 0.005 |
| Acceleration (m/sec2) | 19.2 ± 1.8 | 18.9 ± 1.5 | NS |
Values represent means ± sd. P values are calculated by the Student’s t test for unpaired data. Indicative normal reference values for intrarenal Doppler measurements: resistance index, 0.62 ± 0.07; pulsatility index, 1.20 ± 0.20; acceleration, 18.0 ± 3.0 m/sec2. NS, Not significant.
Table 3.
Renal function and renal duplex echo-Doppler parameters according to baseline creatinine clearance in patients with primary aldosteronism before treatment and after 1 yr follow-up
| Parameter | Creatinine clearance <105 ml/min/1.73 m2
|
Creatinine clearance >105 ml/min/1.73 m2
|
||||
|---|---|---|---|---|---|---|
| Baseline | 1 yr | P value | Baseline | 1 yr | P value | |
| Creatinine clearance (ml/min/1.73 m2) | 96 ± 25 | 87 ± 21 | 0.012 | 113 ± 28 | 90 ± 25 | <0.001 |
| Urinary albumin excretion (mg/24 h)a | 21 [9–36] | 18 [9–29] | NS | 23 [11–38] | 12 [6–20] | <0.001 |
| Total kidney volume (ml) | 409 ± 58 | 404 ± 61 | NS | 413 ± 60 | 402 ± 63 | NS |
| Resistance index | 0.63 ± 0.06 | 0.65 ± 0.07 | NS | 0.59 ± 0.06 | 0.63 ± 0.06 | 0.012 |
| Pulsatility index | 1.23 ± 0.13 | 1.26 ± 0.14 | NS | 1.15 ± 0.15 | 1.23 ± 0.14 | 0.024 |
| Acceleration (m/sec2) | 18.8 ± 1.4 | 19.1 ± 1.3 | NS | 19.0 ± 1.5 | 18.9 ± 1.3 | NS |
Values represent means ± sd unless otherwise indicated. P value in the comparison vs. baseline by the Student’s t test for paired data. NS, Not significant.
Median [interquartile range].
Follow-up
Renal function and Doppler sonographic parameters were reassessed in patients with primary aldosteronism 1 yr after treatment (24 adrenalectomy; 30 spironolactone). Frequency of use of specific types of antihypertensive drugs, including diuretics, was comparable in patients treated with adrenalectomy or spironolactone. Blood pressure declined significantly during follow-up, with average values that were 134/83 and 135/84 mm Hg in patients who were treated with adrenalectomy and spironolactone, respectively. Treatment was followed by normalization of blood pressure in 21 patients with primary aldosteronism (39%; 10 adrenalectomy, 11 spironolactone) and by significant improvement in the remaining 33 (61%; 14 adrenalectomy; 19 spironolactone) (P = 0.71). In the first year, plasma potassium increased significantly from baseline levels (from 3.2 ± 0.4 to 4.2 ± 0.3 mmol/liter; P < 0.001) and remained stable thereafter. No significant changes in plasma sodium levels occurred in patients treated with surgery or mineralocorticoid antagonists. Among 21 male patients treated with spironolactone, four (19%) had clinically relevant breast engorgement that responded well to reduction of dosage.
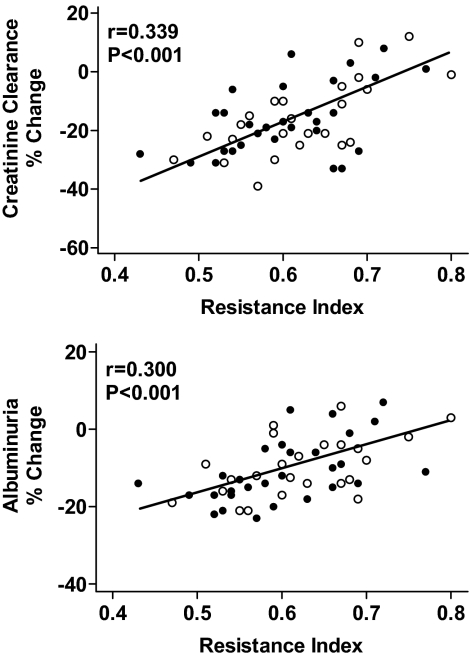
At follow-up, creatinine clearance and urinary albumin excretion declined in patients with primary aldosteronism, and the extent of this decline was significantly greater (both P < 0.01) in patients with baseline creatinine clearance above the median value of 105 ml/min/1.73 m2 (Table 3). Changes in glomerular filtration and urinary albumin excretion were associated with an increase in the average values of the resistance index and pulsatility index that was statistically significant only in primary aldosteronism patients who had baseline creatinine clearance above the median. In primary aldosteronism, linear regression analysis showed a significant inverse relationship between percentage changes in creatinine clearance and albuminuria and the baseline values of the resistance index (Fig. 1). Inclusion of changes in creatinine clearance in a multivariate model eliminated the correlation between baseline resistance index and changes in albuminuria.
Figure 1.
Linear regression analysis of baseline intrarenal resistance index and percentage changes of creatinine clearance (upper panel) and urinary albumin excretion (lower panel) in patients with primary aldosteronism who were treated with adrenalectomy (n = 24; open circles) or spironolactone (n = 30; filled circles). Follow-up measurements were done after 1 yr.
Changes in blood pressure values during follow-up were not significantly different in patients with primary aldosteronism or essential hypertension. The extent of changes in echo-Doppler velocimetric indices did not differ significantly in patients with primary aldosteronism who were treated with adrenalectomy or spironolactone (Table 4). In patients with primary hypertension, intrarenal Doppler parameters did not change significantly during follow-up (Table 4).
Table 4.
Renal duplex echo-Doppler parameters of patients with essential hypertension (n = 100) and patients with primary aldosteronism who were treated with adrenalectomy (n = 24) or spironolactone (n = 30) at baseline and after 1 yr of follow-up
| Parameter | Essential hypertension
|
Adrenalectomy
|
Spironolactone
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 yr | P value | Baseline | 1 yr | P value | Baseline | 1 yr | P value | |
| SBP (mm Hg) | 165 ± 17 | 138 ± 12 | <0.001 | 168 ± 15 | 137 ± 12 | <0.001 | 166 ± 13 | 135 ± 13 | <0.001 |
| DBP (mm Hg) | 102 ± 9 | 82 ± 8 | <0.001 | 103 ± 8 | 81 ± 7 | <0.001 | 102 ± 9 | 82 ± 6 | <0.001 |
| Creatinine clearance (ml/min/1.73 m2) | 87 ± 37 | 85 ± 31 | NS | 104 ± 29 | 90 ± 26 | <0.001 | 106 ± 32 | 94 ± 28 | <0.001 |
| UAE (mg/24 h)a | 15 [6–26] | 11 [4–21] | 0.042 | 21 [8–34] | 14 [5–26] | 0.018 | 22 [10–41] | 16 [5–33] | 0.025 |
| Total kidney volume (ml) | 414 ± 61 | 408 ± 56 | NS | 412 ± 64 | 401 ± 67 | NS | 411 ± 64 | 404 ± 65 | NS |
| Resistance index | 0.64 ± 0.05 | 0.65 ± 0.07 | NS | 0.60 ± 0.06 | 0.63 ± 0.07 | NS | 0.61 ± 0.07 | 0.64 ± 0.06 | NS |
| Pulsatility index | 1.26 ± 0.14 | 1.23 ± 0.16 | NS | 1.18 ± 0.14 | 1.25 ± 0.16 | NS | 1.20 ± 0.15 | 1.24 ± 0.15 | NS |
| Acceleration | 19.2 ± 1.8 | 19.4 ± 1.7 | NS | 18.7 ± 1.4 | 18.8 ± 1.5 | NS | 19.0 ± 1.4 | 18.9 ± 1.7 | NS |
Values represent means ± sd unless otherwise indicated. SBP, Systolic blood pressure; DBP, diastolic blood pressure; UAE, urinary albumin excretion; NS, not significant. P value in the comparison vs. baseline by the Student’s t test for paired data.
Median [interquartile range].
Discussion
The results of the present study demonstrate that glomerular hyperfiltration and increased urinary albumin excretion are associated with decreased intrarenal vascular resistance, as assessed by duplex Doppler sonography, in patients with primary aldosteronism. Both surgical and medical treatment restore a sonographic intrarenal hemodynamic pattern comparable to that of patients with primary hypertension and decrease both glomerular filtration rate and urinary albumin excretion with a change that is inversely related to the pretreatment intrarenal resistance index.
Although preclinical studies conducted in animal models have clearly shown that inappropriate aldosterone levels for sodium status can produce extensive intrarenal vascular damage via proinflammatory and profibrotic effects (reviewed in Refs. 4 and 5), cross-sectional evaluations of renal function in primary aldosteronism have shown high variability in the prevalence of clinically relevant kidney disease (1,7,8,29,30). In a 9-yr prospective study, we have demonstrated that primary aldosteronism is characterized by glomerular hyperfiltration and partially reversible renal dysfunction (10) and, consistently, evidence that albuminuria might be a marker of a hemodynamic rather than structural renal defect has been reported by Ribstein et al. (9) in a short-term, postadrenalectomy evaluation of patients with adrenal adenoma. Therefore, prospective investigations with appropriate control groups indicate that the hallmark of renal dysfunction in primary aldosteronism is reversible glomerular hyperfiltration that contributes to increased urinary albumin losses, and this is in agreement with the findings of studies conducted in more experimental settings (11,12,13). In deoxycorticosterone acetate-salt rats, micropuncture studies have demonstrated increased glomerular filtration, reflecting increased glomerular blood flow consequent upon vasodilatation of both afferent and efferent arterioles, and ascribed to volume expansion (13). Intrarenal vasodilatation may occur, in large part, via a direct mineralocorticoid effect on the vessel wall (31) and is in sharp contrast with the vasoconstrictive response to increased renal perfusion pressure detected in primary hypertension (32). Moreover, in primary aldosteronism, the effects of inappropriate aldosterone-salt status involve changes such as a rightward shift of the pressure-natriuresis curve (12), which is a major contributor to the mechanism of aldosterone “escape” from salt and water retention (11).
The technique of renal duplex Doppler ultrasonography has been used to evaluate renal blood flow in disease states such as main renal artery stenosis and other renal diseases involving the intrarenal vascular compartment, such as diabetic nephropathy and primary hypertension (15,16,17,18,32,33,34). Distal velocimetric indices, obtained by this technique, reliably assess intrarenal hemodynamics (32,35). How much different intrarenal vascular beds contribute to decreased resistance index in primary aldosteronism is not known, although the velocimetric indices better explore the preglomerular hemodynamics. The present study provides evidence of significantly lower intrarenal vascular resistance in patients with primary aldosteronism compared with patients with primary hypertension who were comparable for demographic variables and severity and duration of disease. Detection of significantly lower distal resistance index and pulsatility index in patients with primary aldosteronism indicates that these patients have relative vasodilatation of intrarenal arterial vessels leading to glomerular hyperfiltration and, in turn, to increased urinary albumin excretion. Correction of aldosterone excess by either removal of an adrenal adenoma or treatment with aldosterone antagonists restores intrarenal hemodynamic profiles, corrects glomerular hyperfiltration, and decreases urinary albumin excretion. Changes in glomerular filtration rate and albuminuria occurring after treatment are related with the baseline measurement of the distal renal velocimetric indices, supporting a pathophysiological role of intrarenal hemodynamics in these abnormalities. Whether these intrarenal hemodynamic changes depend upon effects of aldosterone on either vascular smooth muscle or endothelial cells will deserve further investigation. Also, our data cannot rule out an intrarenal vascular effect due to hypokalemia, although in our patients there was no relationship between plasma potassium levels and intrarenal velocimetric indices. Alternative explanations for our present renal ultrasound findings could be related to systemic hemodynamic changes secondary to variations in cardiac performance occurring in patients with primary aldosteronism after treatment, although this seems not to be the case (36), and to resolution of the hypercoagulable state that has been demonstrated in patients with hyperaldosteronism (37).
The extent to which albuminuria reflects a hemodynamic rather than structural response to aldosterone/salt imbalance is not yet clear, but these two separate aspects should be taken into account when considering the effects of aldosteronism on the kidney. On one hand, there are functional adaptations, induced by increased renal sodium reabsorption and leading to volume expansion, hypertension, increased renal perfusion pressure, and suppression of renin with decreased intrarenal vascular resistance. These changes result in glomerular hyperfiltration and increased sodium excretion, with recovery of a steady-state in fluid balance. On the other hand, there is structural damage, involving primarily the intrarenal vessels, that might result from chronic hypertensive insult and/or direct untoward effects of aldosterone (4,5). This might lead to decreased glomerular perfusion, progressive impairment of renal function, and stimulation of renin production that escapes from suppression by excess plasma aldosterone (20,38). Performance of renal biopsy studies in patients with primary aldosteronism and a different stage of renal disease would be important to gain insight into how aldosterone can cause damage to the kidney.
Some limitations of this study need to be underlined. First, similar to most studies that have included patients with idiopathic aldosteronism (30,39,40), we might have underestimated the number of patients with adrenal adenoma. We have performed selective adrenal vein sampling in only 14 of our patients, whereas in the remaining 40, discrimination from idiopathic disease was based on computerized tomography evidence of adrenal masses and adrenal scintigraphy. However, in this respect, it is important to notice that in our study no significant differences in duration of hypertension, blood pressure levels, renal function, and renal Doppler parameters were observed between the two disease subtypes. Also, we have observed no difference in outcomes between patients treated with adrenalectomy or spironolactone. Second, our study might have underestimated the impact of hypertensive burden on renal function because we did not include measurements of the 24-h blood pressure profile, which is superior to clinic blood pressure for its prediction. However, in our hands, as in other studies (41), comparison of ambulatory blood pressure profiles in patients with primary aldosteronism or primary hypertension did not show significant differences (36). Third, the use of certain medications, such as inhibitors of the renin-angiotensin system, might have influenced renal outcomes. In this study, we washed out antihypertensive drugs before both baseline and follow-up evaluations and performed separate analysis of patients who were and were not taking these and/or other types of antihypertensive agents showing no significant differences (data not shown). Finally, the doses of spironolactone that have been used in this study are higher than those that are currently recommended for medical treatment of primary aldosteronism, and therefore extrapolation of the results to a more general context should be done with caution. With regard to this issue, further investigation of the renal effects of the newer aldosterone receptor antagonists, which offer the opportunity to use relatively higher doses without antiandrogenic effects, is warranted.
In conclusion, this Doppler ultrasound study presents first evidence that, in patients with primary aldosteronism, intrarenal vascular resistance is decreased. This intrarenal hemodynamic pattern is associated with glomerular hyperfiltration and albuminuria out of proportion to blood pressure levels that benefit substantially from treatment. In this view, both adrenalectomy and mineralocorticoid receptor blockade appear to be of considerable value, inasmuch as they reverse the intrarenal hemodynamic pattern and decrease glomerular filtration rate and albuminuria. These findings underline the importance of an appropriate timing in the identification of this endocrine disorder to prevent renal complications. Extensive evaluation of renal histology and/or assessment of urinary excretion of markers of renal fibrosis, such as TGFβ, would be important to perform in patients with primary aldosteronism to understand better how aldosterone can damage the kidney.
Footnotes
This work was supported by research grants from the Italian Ministry of the University and Scientific and Technologic Research (to L.A.S. and C.C.) and by a research grant of the Pier Silverio Nassimbeni Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 13, 2009
Abbreviations: Vmax, Peak systolic velocity; Vmean, mean velocity; Vmin, end diastolic velocity.
References
- Conn JW, Knopf RF, Nesbit RM 1964 Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg 107:159–172 [DOI] [PubMed] [Google Scholar]
- Laragh JH 1973 Vasoconstriction-volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med 55:261–274 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Boscaro M, Ronconi V, Funder JW 2005 Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab 16:104–107 [DOI] [PubMed] [Google Scholar]
- Hollenberg NK 2004 Aldosterone in the development and progression of renal injury. Kidney Int 66:1–9 [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier Jr CT 2001 Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab 12:308–314 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW 2008 Primary aldosteronism: cardiovascular, renal, and metabolic implications. Trends Endocrinol Metab 19:88–90 [DOI] [PubMed] [Google Scholar]
- Beevers DG, Brown JJ, Ferriss JB, Fraser R, Lever AF, Robertson JI, Tree M 1976 Renal abnormalities and vascular complications in primary aldosteronism: evidence of tertiary hyperaldosteronism. Q J Med 45:401–410 [PubMed] [Google Scholar]
- Bravo EL, Fouad-Tarazi FM, Tarazi RC, Pohl M, Gifford RW, Vidt DG 1988 Clinical implications of primary aldosteronism with resistant hypertension. Hypertension 11:207–211 [DOI] [PubMed] [Google Scholar]
- Ribstein J, Du Cailar G, Fesler P, Mimran A 2005 Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol 16:1320–1325 [DOI] [PubMed] [Google Scholar]
- Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C 2006 Long-term renal outcomes in patients with primary aldosteronism. JAMA 295:2638–2645 [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP, Smith Jr MJ, Premen AJ 1984 Role of hemodynamics and arterial pressure in aldosterone “escape.” Hypertension 6:I183–I192 [DOI] [PubMed] [Google Scholar]
- Kimura G, Saito F, Kojima S, Yoshimi H, Abe H, Kawano Y, Yoshida K, Ashida T, Kawamura M, Kuramochi M, Ito K, Omae T 1987 Renal function curve in patients with secondary forms of hypertension. Hypertension 10:11–15 [DOI] [PubMed] [Google Scholar]
- Dworkin LD, Hostetter TH, Rennke HG, Brenner BM 1984 Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest 73:1448–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli M, Veglio F, Arosio E, Cataliotti A, Valvo E, Morganti A 2006 New intrarenal echo-Doppler velocimetric indices for the diagnosis of renal artery stenosis. Kidney Int 69:580–587 [DOI] [PubMed] [Google Scholar]
- Blum U, Krumme B, Flugel P, Gabelmann A, Lehnert T, Buitrago-Tellez C, Schollmeyer P, Langer M 1997 Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med 336:459–465 [DOI] [PubMed] [Google Scholar]
- Radermacher J, Chavan A, Bleck J, Vitsthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H 2001 Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 344:410–417 [DOI] [PubMed] [Google Scholar]
- Marzano MA, Pompili M, Rapaccini GL, Covino M, Cotroneo P, Manto A, Todaro L, Ghirlanda G, Gasbarrini G 1998 Early renal involvement in diabetes mellitus: comparison of renal Doppler US and radioisotope evaluation of glomerular hyperfiltration. Radiology 209:813–817 [DOI] [PubMed] [Google Scholar]
- Pelliccia P, Savino A, Cecamore C, Primavera A, Schiavone C, Chiarelli F 2008 Early changes in renal hemodynamics in children with diabetes: Doppler sonographic findings. J Clin Ultrasound 36:335–340 [DOI] [PubMed] [Google Scholar]
- Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Novello M, Favret G, Melis A, Cavarape A, Sechi LA 2006 Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 91:3457–3463 [DOI] [PubMed] [Google Scholar]
- Catena C, Colussi GL, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA 2007 Relationships of plasma renin levels with renal function in patients with primary aldosteronism. Clin J Am Soc Nephrol 2:722–731 [DOI] [PubMed] [Google Scholar]
- 1993 The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 153:154–183 [PubMed] [Google Scholar]
- Ferrari P, Shaw SG, Nicod J, Saner E, Nussberger J 2004 Active renin versus plasma renin activity to define aldosterone-to-renin ratio for primary aldosteronism. J Hypertens 22:377–381 [DOI] [PubMed] [Google Scholar]
- Kem DC, Weinberger MH, Mayes DM, Nugent CA 1971 Saline suppression of plasma aldosterone in hypertension. Arch Intern Med 128:380–386 [PubMed] [Google Scholar]
- Grim CE, Weinberger MH, Higgins JT, Kramer NJ 1977 Diagnosis of secondary forms of hypertension. A comprehensive protocol. JAMA 237:1331–1335 [PubMed] [Google Scholar]
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young Jr WF 2004 Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 89:1045–1050 [DOI] [PubMed] [Google Scholar]
- Seelig HP 1969 The Jaffe reaction with creatinine: reaction product and general reaction conditions. Z Klin Chem Klin Biochem 7:581–585 [PubMed] [Google Scholar]
- Novello M, Catena C, Nadalini E, Colussi GL, Baroselli S, Chiuch A, Lapenna R, Bazzocchi M, Sechi LA 2007 Renal cysts and hypokalemia in primary aldosteronism: results of long-term follow-up after treatment. J Hypertens 24:1443–1450 [DOI] [PubMed] [Google Scholar]
- Al-Said J, O'Neill WC 2003 Reduced kidney size in patients with simple renal cysts. Kidney Int 4:1059–1064 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Bernini G, Desideri G,. Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palombo G, Tizzoni D, Rossi E, Pessina AC, Mantero F 2006 Renal damage in primary aldosteronism. Results of the PAPY study. Hypertension 48:232–238 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Uzu T, Fujii T, Kuroda S, Nakamura S, Inenaga T, Kimura G 1999 Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis 33:261–266 [DOI] [PubMed] [Google Scholar]
- Uhrenholt TR, Schjerning J, Hansen PB, Norregaard R, Jensen BL, Sorensen GL, Skott O 2003 Rapid inhibition of vasoconstriction in renal afferent arterioles by aldosterone. Circ Res 93:1258–1266 [DOI] [PubMed] [Google Scholar]
- Radermacher J, Ellis S, Haller H 2002 Renal resistance index and progression of renal disease. Hypertension 39:699–703 [DOI] [PubMed] [Google Scholar]
- Platt JF, Ellis JH, Rubin JM, Dipietro MA, Sedman AB 1990 Intrerenal renal Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. Am J Roentgenol 154:1223–1227 [DOI] [PubMed] [Google Scholar]
- Jensen G, Bardelli M, Volkmann R, Caidahl K, Rose G, Aurell M 1994 Renovascular resistance in primary hypertension: experimental variations detected by means of Doppler ultrasound. J Hypertens 12:959–964 [PubMed] [Google Scholar]
- Palatresi S, Longari V, Airoldi F, Benti R, Nador B, Bencini C, Lovaria A, Del Vecchio C, Nicolini A, Voltini F, Gerundini P, Morganti A 2001 Usefulness and limits of distal echo-doppler velocimetric indices for assessing renal hemodynamics in stenotic and non-stenotic kidneys. J Hypertens 19:1489–1496 [DOI] [PubMed] [Google Scholar]
- Catena C, Colussi GL, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA 2007 Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 50:911–918 [DOI] [PubMed] [Google Scholar]
- Udvardy M, Harsfalvi J, Rak K 1992 Altered primary haemostasis in Conn’s syndrome. Thromb Res 67:625–628 [DOI] [PubMed] [Google Scholar]
- Oelkers W, Diederich S, Bahr V 2000 Primary hyperaldosteronism without suppressed renin due to secondary hypertensive kidney damage. J Clin Endocrinol Metab 85:3266–3270 [DOI] [PubMed] [Google Scholar]
- Blumenfeld JD, Sealey JE, Schlussel Y, Vaughan D, Sos TA, Atlas SA, Muller FB, Acevedo R, Ulick S, Laragh JH 1994 Diagnosis and treatment of primary aldosteronism. Ann Intern Med 121:877–885 [DOI] [PubMed] [Google Scholar]
- Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P 2006 Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 91:454–459 [DOI] [PubMed] [Google Scholar]
- Matsumura K, Fujii K, Oniki H, Oka M, Iida M 2006 Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens 19:13–18 [DOI] [PubMed] [Google Scholar]