Abstract
Context: Thyroid hormone use is common in older populations, but the frequency of over- or under-replacement is debated.
Objective: We sought to describe the frequency of and factors associated with thyroid hormone over- or under-replacement in a population of older men and women.
Design: Participants were 3678 U.S. community dwelling individuals aged 65 yr or older enrolled in the Cardiovascular Health Study who had thyroid function tests in 1989–1990. Thyroid hormone users (n = 339) were identified and classified into low TSH (<0.45 mU/liter), euthyroid (0.45–4.5 mU/liter), and high TSH (>4.5 mU/liter).
Results: Of the 339 thyroid hormone users, 41% had a low TSH, 16% had a high TSH, and 43% were in the euthyroid range. In multivariable analyses, lower weight (P < 0.001) was independently associated with low TSH status. For every 10 kg lower weight, the likelihood of having low TSH increased by 65% [odd ratio (OR) 1.65; 95% confidence interval (CI) 1.31–2.07]. Those with renal insufficiency were less likely to have low TSH levels (P = 0.02). The presence of diabetes was independently associated with having low (OR 3.35; 95% CI 1.46–7.65) and high TSH levels (OR 2.66; 95% CI 1.14–6.21).
Conclusions: There is a very high prevalence of thyroid function testing abnormalities in older people taking thyroid hormone preparations, particularly in those of low weight or with diabetes. Because of potential adverse cardiovascular and skeletal effects from over-replacement, older people represent a key population for increased TSH monitoring on therapy.
Older individuals taking thyroid hormone preparations, especially those of lower weight or with diabetes, should be monitored for thyroid function abnormalities.
The prevalence of thyroid function testing abnormalities in those taking thyroid hormone preparations may be high, with levels in the euthyroid range present in only 40–60% (1,2). Factors that contribute to the increased risk of over- or under-replacement have not been defined, despite the clinical importance of identifying at-risk patients. We conducted an analysis of individuals taking thyroid hormone preparations who were enrolled in a population-based, longitudinal study of community dwelling individuals aged 65 yr and older. Our goals were to define the prevalence of thyroid function testing abnormalities, and to determine the relationship between sociodemographical factors, comorbidities, pharmacotherapy, and over- or under-replacement in this population.
Subjects and Methods
These analyses are based on data from the Cardiovascular Health Study (3). Enrollment of an original cohort of 5201 adults occurred from 1989–1990. The baseline visit included a medical history, physical examination, assessment of health status, and phlebotomy. The institutional review boards of all study sites and the coordinating center at the University of Washington in Seattle approved the study.
Serum TSH concentration was measured using a chemiluminescent immunometric assay (LumaTag hTSH; Nichols Institute, San Juan Capistrano, CA) with a functional sensitivity of 0.008 mU/liter on a subsample of baseline samples from 3678 individuals from the original cohort, as described previously (4). Free T4 concentrations were measured with a direct, monoclonal antibody assay (Amerlex-MAB; Amersham International, Buckinghamshire, UK) in individuals with serum TSH levels less than 0.10 or more than 4.50 mU/liter, for the 95% of samples with sufficient serum for this additional test.
Within this subgroup of 3678 individuals with thyroid function tests, there were 339 who were taking thyroid hormone preparations. None of these individuals had a history of thyroid cancer. For data analysis, these 339 individuals were classified into one of three groups: low TSH (TSH ≤ 0.44 mU/liter), euthyroidism (TSH 0.45–4.49 mU/liter), and high TSH (TSH ≥4.5 mU/liter). We performed an additional classification in 321 individuals into five categories: overt hyperthyroidism (TSH ≤0.10 mU/liter plus high freeT4); subclinical hyperthyroidism (TSH 0.11–0.44 mU/liter or ≤0.10 U/liter plus normal free T4); euthyroidism (TSH 0.45–4.50 mU/liter); subclinical hypothyroidism (TSH 4.51–19.9 mU/liter plus normal free T4 level); or overt hypothyroidism (TSH ≥20 mU/liter or >4.50 mU/liter plus low free T4). There were 18 individuals that could not be placed into one of these categories and were excluded: two whose testing suggested recent nonadherence (low TSH and low free T4), two whose testing suggested a recent dose increase (high TSH and high free T4), and 14 with either TSH less than or equal to 0.10 mU/liter or elevated TSH without free T4 level.
Statistical analysis
Baseline characteristics were summarized according to thyroid status and compared against those in the euthyroid group using a t test or χ2 test. Multivariable logistical regression models comparing each abnormal TSH group (low or high) with the euthyroid group were performed in the 333 individuals who could be identified as taking either T4 alone or a T4 plus T3 preparation. We included age and sex in all models, and added other covariates, if significant, in stages [stage 1, sociodemographical factors: body mass index, weight, smoking, race, education, and income; stage 2, comorbidities: diabetes, cancer, coronary heart disease, congestive heart failure, renal insufficiency, and lung disease; and stage 3, medications: oral steroids, β-blockers, lithium, and thyroid hormone preparation type], retaining factors that were statistically significant (P < 0.05) in the final model. All models were repeated in those using T4 alone preparations to reflect current prescribing practices. Analyses were done using STATA version 9 (StataCorp LP, College Station, TX).
Results
Of the 3678 individuals with thyroid function testing, 339 (9.2%) were taking a thyroid hormone preparation. In these 339 thyroid hormone users, 41.0% had a low TSH, 16.2% had a high TSH, and 42.8% were in the euthyroid range. The use of preparations containing both T4 and T3 was common, at 26%, though their use did not differ by thyroid category. Ages were similar across all thyroid status categories, with a mean age of 72.9 yr. There were more females in the low TSH group (87.8%) than the euthyroid group (78.6%; P = 0.03). Those with a low TSH had a lower weight and body mass index (65.3 vs. 72.2 kg, P < 0.001; 25.5 vs. 27.1 kg/m2, P = 0.006), had fewer medical diagnoses (P = 0.05), and were taking fewer prescription medications (P = 0.02) than those in the euthyroid group.
In multivariable-adjusted models, lower weight, diabetes, and lack of renal insufficiency were independently associated with low TSH status (Table 1). For every 10 kg lower weight, the likelihood of having low TSH increased by 65%. Those with diabetes were more likely and those with renal insufficiency were less likely to have low TSH levels. When we included only T4 users in our models, results were quantitatively similar. Estrogen use was not an independent predictor of low TSH in a model in women only. Diabetes was the only statistically significant predictor of high TSH status. This association decreased in magnitude and lost statistical significance in the smaller group of those taking only T4 preparations.
Table 1.
Risk factor associations of low TSH status and high TSH status, each compared with euthyroid group, in thyroid hormone preparation users
| All preparations
|
T4 only preparations
|
|||||
|---|---|---|---|---|---|---|
| OR | CI | P value | OR | CI | P value | |
| Low TSH status | ||||||
| Characteristics | ||||||
| Age (yr) | 0.98 | 0.93–1.03 | 0.37 | 0.99 | 0.94–1.05 | 0.78 |
| Male sex | 1.05 | 0.49–2.24 | 0.90 | 1.00 | 0.40–2.48 | 0.99 |
| Weight (per 10 kg decrease) | 1.65 | 1.31–2.07 | <0.001 | 1.53 | 1.19–1.98 | 0.001 |
| Diabetes | 3.35 | 1.46–7.65 | 0.004 | 2.94 | 1.26–6.82 | 0.01 |
| Renal insufficiency | 0.21 | 0.06–0.74 | 0.02 | 0.26 | 0.07–0.94 | 0.04 |
| High TSH status | ||||||
| Characteristics | ||||||
| Age (yr) | 1.03 | 0.97–1.09 | 0.35 | 1.05 | 0.98–1.12 | 0.19 |
| Male sex | 0.56 | 0.23–1.35 | 0.20 | 0.62 | 0.24–1.59 | 0.32 |
| Diabetes | 2.66 | 1.14–6.21 | 0.02 | 1.55 | 0.60–4.04 | 0.37 |
CI, Confidence interval; OR, odds ratio.
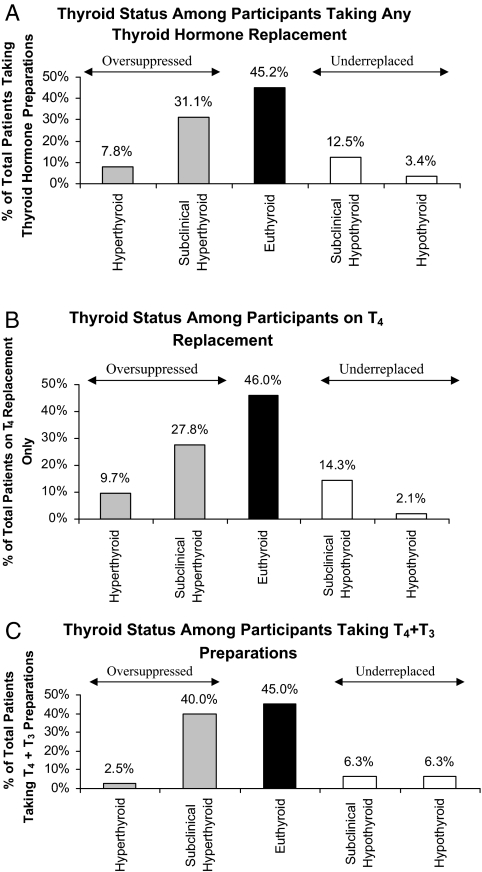
We also sought to classify further the thyroid hormone users into categories that accounted for free T4 levels, in the 321 individuals in whom we could perform this classification (Fig. 1A). When we examined specific preparations (Fig. 1, B and C), the prevalence of euthyroidism was similar to the overall group.
Figure 1.
Thyroid function tests among participants taking all types of thyroid hormone preparations (A), only T4 preparations (B), and only T4 plus T3 preparations (C).
Discussion
In a cohort study of community dwelling individuals aged 65 yr and older, we report a low prevalence of thyroid function tests in the euthyroid range in those taking thyroid hormone preparations, at only 43%. The high prevalence of over-replacement (41%) observed in our study is similar to another population-based sample of elderly enrolled in the Framingham Heart Study during the same time frame, in which 48% of individuals taking thyroid hormone preparations had a low TSH (1), and higher than the 22% prevalence reported in a community health fair and the 5% prevalence in a study of an endocrine clinic population (2,5). In our study, participants were unaware that thyroid function tests were being performed, unlike the latter two studies. In the study of the endocrine clinic population, inadequately controlled patients demonstrated a higher age at diagnosis, consistent with the higher prevalence of TSH abnormalities in our population (5). One important consideration is that our thyroid function test data are from a study visit conducted in 1989–1990. It is possible that providers were less concerned about adverse sequelae of over-replacement at that time, though the concern over exacerbating underlying cardiac conditions in older people has been present in the field for many years, as has the “start low, go slow” recommendation for thyroid hormone replacement (6). We also do not have data on the duration of treatment, and it is likely that a proportion of those who are over-replaced and under-replaced, though not all, were in a titration phase of therapy that would ultimately stabilize.
It has been reported that older hypothyroid patients need approximately 25% less T4 than younger patients (7,8). Not only is there a decrease in the secretion of thyroid hormone with increasing age (9,10), but the half-life of T4 also increases with age, with a mean half-life of 6.7 d for adults aged 18–27 yr of age, and approximately 9 d for those aged 65 yr and older (9). Together, these data suggest that older individuals should be initiated on lower doses of T4 replacement and that a longer duration may be required between dose titrations. Furthermore, continued monitoring is necessary to assess for decreasing thyroid hormone requirements over time. In one British study, physicians failed to adjust the T4 dose in 89% of those with low TSH levels (11).
We sought to identify characteristics of individuals whose TSH levels were not at goal, by examining a series of demographical and health factors that could be related to poor adherence or altered thyroid hormone requirements. A disproportionate number of women was in the low TSH group compared with the euthyroid group. Our data suggest that this difference is due to the lower weight of women because sex was no longer associated with low TSH after adjusting for weight. In this cross-sectional analysis, we cannot determine whether the low weight is a contributor to low TSH levels or a result of over-replacement with thyroid hormone (accidental or intentional), though there are data to support weight-based dosing of thyroid hormone replacement (7,12,13).
There was no association of altered TSH levels with polypharmacy or increased medical comorbidities that could alter thyroid hormone metabolism. In fact, the low TSH group had fewer medical diseases and less prescription medication use, though this effect disappeared in multivariable analyses. When we examined the association between individual diseases and low TSH, those with renal insufficiency were less likely to be in the low TSH group. These data suggest that there is equal or better monitoring of thyroid hormone replacement in those with chronic diseases and no effect of polypharmacy on the ability of older individuals to be able to adhere to thyroid medication daily dosing. The one exception was diabetes. In our study, both low and high TSH levels in older individuals taking thyroid hormone preparations were associated with an increased prevalence of diabetes. This suggests difficulty in managing thyroid hormone replacement in the setting of diabetes, rather than both decreased and increased dose requirements in the diabetic population. Alternatively, in the setting of multiple comparisons, this could be a spurious finding that should be verified in other populations.
A major strength of this study is the use of a large, population-based cohort of older men and women with a wealth of data related to their health status. Our focus on the elderly targets the population most likely to experience deleterious effects from inadequate or overtreatment. In addition, we have TSH and free T4 in a large subset of people who were not presenting for evaluation of thyroid disorders. However, our analyses are cross-sectional in nature and limit causal inference. We do not have information on the reason for thyroid hormone prescription or duration of therapy, and our subcategorization of overt and subclinical hyperthyroidism is limited by a lack of T3 levels.
Clinical implications
Thyroid function testing abnormalities are common in elderly men and women taking thyroid hormone replacement medication. Our data support the recommendation that older individuals taking thyroid hormone preparations, especially those of lower weight or with diabetes, be monitored for thyroid function abnormalities. There are data to support acting on low TSH levels, given cardiac and skeletal effects from thyroid hormone excess that could be particularly deleterious in the elderly (1,4,14,15). The prevalence of older people taking thyroid hormone replacement who have TSH levels above 4.5 mU/liter is lower, and may be of lesser consequence, given the large proportion of older individuals without thyroid disease who have TSH levels in the 6–8 mU/liter range (16) and data to suggest no adverse sequelae from mild TSH elevations in older people (4,17).
Footnotes
This work was supported by National Institute on Aging Grant K23 AG19161 (to A.R.C.), and contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01 HC-15103 from the National Heart, Lung, and Blood Institute. A full list of participating Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 6, 2009
References
- Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB 1994 Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC 2000 The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A 1991 The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW 2006 Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez JJ 2002 Hypothyroidism in patients older than 55 years: an analysis of the etiology and assessment of the effectiveness of therapy. J Gerontol A Biol Sci Med Sci 57:M315–M320 [DOI] [PubMed] [Google Scholar]
- Cappola AR, Ladenson PW 2003 Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab 88:2438–2444 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RL, Barzel US 1982 Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann Intern Med 96:53–55 [DOI] [PubMed] [Google Scholar]
- Sawin CT, Herman T, Molitch ME, London MH, Kramer SM 1983 Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 75:206–209 [DOI] [PubMed] [Google Scholar]
- Gregerman R, Gaffney G, Shock N, Crowder S 1962 Thyroxine turnover in euthyroid man with special reference to changes with age. J Clin Invest 41:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddie TH, Meade Jr JH, Fisher DA 1966 An analysis of published data on thyroxine turnover in human subjects. J Clin Endocrinol Metab 26:425–436 [DOI] [PubMed] [Google Scholar]
- De Whalley P 1995 Do abnormal thyroid stimulating hormone level values result in treatment changes? A study of patients on thyroxine in one general practice. Br J Gen Pract 45:93–95 [PMC free article] [PubMed] [Google Scholar]
- Roberts CG, Ladenson PW 2004 Hypothyroidism. Lancet 363:793–803 [DOI] [PubMed] [Google Scholar]
- Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH 1987 Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 316:764–770 [DOI] [PubMed] [Google Scholar]
- Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FD, Wilson S, Sheppard MC, Franklyn JA 2007 Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 167:928–934 [DOI] [PubMed] [Google Scholar]
- Bauer DC, Ettinger B, Nevitt MC, Stone KL 2001 Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 134:561–568 [DOI] [PubMed] [Google Scholar]
- Surks MI, Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG 2004 Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]