Abstract
Objective: Our objective was to determine whether excessive adiposity is associated with alteration of the normal hormonal changes of early pubertal girls.
Design and Participants: Healthy 6.4- to 9.5-yr-old, prepubertal (PRE, n = 20) and 9.4- to 13.0-yr-old pubertal premenarcheal volunteers (PUB, n = 20) were divided into excessive-weight (EW) or normal-weight (NW) groups according to the 85th percentile body mass index.
Interventions and Setting: Overnight blood sampling; GnRH agonist (GnRHag), low-dose ACTH, oral glucose tolerance tests, and pelvic ultrasonograms were performed in our Clinical Research Center.
Results: EW girls were similar in age and baseline and ACTH- and GnRHag-stimulated androgen levels to stage-matched NW girls. However, the sleep-related LH rise was blunted in EW-PUB girls compared with NW-PUB girls. The sleep-related rise of mean LH in EW-PUB [0.68 ± 0.35 (sem) U/liter] was insignificant, less than that of NW-PUB (2.1 ± 0.45, P < 0.05) and not significantly different from that of PRE girls (0.08±0.03). EW-PUB had slower LH pulse frequency and a lower rise in LH pulse amplitude during sleep than NW-PUB girls (both P < 0.05). Overnight FSH patterns paralleled LH patterns, whereas estradiol levels were similar in stage-matched NW and EW groups, differing between stages as expected. Early morning and peak LH, FSH, and estradiol responses to GnRHag were similar in EW-PUB and NW-PUB and significantly greater than those of PRE girls.
Conclusions: Healthy EW-PUB girls have significantly blunted sleep-related LH production. These data suggest that excess adiposity, in the absence of sex steroid excess, may subtly suppress hypothalamic-pituitary-gonadal function in premenarcheal pubertal girls.
Healthy excessive-weight pubertal premenarcheal girls have blunted sleep-related gonadotropin production in the absence of hyperandrogenemia.
Excess adiposity is a risk factor for pubertal disorders. Evidence is mounting that excess adiposity advances the age of onset of puberty, making it a risk factor for precocious puberty (1,2). Girls with complete precocious puberty have high LH levels and a high LH-to-FSH ratio for age, albeit normal for stage of puberty (3). Obesity also is a risk factor for polycystic ovary syndrome (PCOS) (4) and has been reported to be associated with increased androgen levels in early pubertal girls, an association also found in PCOS (5). Adolescent girls with PCOS have high LH levels and LH-to-FSH ratio for age (6). Although the phenomena of precocious puberty and PCOS do not seem to be related, obesity appears to influence both disorders, potentially by altering gonadotropin production.
Consequently, we hypothesized that pubertal premenarcheal girls with excessive adiposity, indexed by elevated body mass index (BMI), would have higher levels of LH and androgens than those with normal BMI. To test our hypothesis, in developing normative data, we recruited healthy volunteers with normal and elevated BMI and surveyed their hypothalamic-pituitary-ovarian function by analyzing gonadotropin secretion during a sleep test, by assessing their gonadotropin and sex steroid responses to a GnRH agonist (GnRHag) test, and by testing their adrenal androgenic sensitivity to ACTH. Contrary to our expectations, however, the LH increase with the onset of sleep, the earliest hormonal change of puberty (7), was blunted in healthy girls whose BMI was excessive; furthermore, these girls had no evidence of androgen excess. Our findings suggest that excess adiposity, in the absence of hyperandrogenemia, may subtly blunt hypothalamic-pituitary gonadotrope function during the premenarcheal stages of puberty.
Subjects and Methods
Subjects
We recruited healthy volunteers by advertisement from January 2000 to June 2004 such that excessive weight (EW, BMI >85th percentile) and normal-weight (NW, BMI 8–85th percentile) girls were approximately equally represented in both prepubertal (PRE) and pubertal premenarcheal (PUB) stages. The volunteers had no chronic illnesses or hirsutism. They were normal height for age and were not taking medications. PRE girls (n = 20) were 6.4–9.5 yr old and had no breast or pubic hair development (Table 1). PUB girls (n = 20) were 9.4–13.0 yr old, had Tanner 2–4 breast development that was predominantly stage 3 (Fig. 1) (pubarche was of a variable degree, stage 1–4), and were premenarcheal; none reported menses in a survey 1 month after the studies reported here. Two extreme outliers were excluded from this tally post hoc because of key pubertal data more than 3 sd from the mean of the rest of their group: one 9.0-yr-old NW-PRE girl had a mean sleep LH (1.24 U/liter) indicative of peripuberty and one 12.7-yr-old NW-PUB girl with stage 3 breast development had a plasma free testosterone (T) level (15 pg/ml) and 17-hydroxyprogesterone response to GnRHag (256 ng/dl) indicative of occult PCOS (8). Ethnicity was not evenly distributed among groups (P = 0.035): NW-PRE was 60% African-American and 40% Caucasian; EW-PRE was 46% African-American, 27% Caucasian, and 27% Hispanic; NW-PUB was 58% African-American, 33% Caucasian, and 9% Hispanic; and EW-PUB was 88% African-American and 12% Hispanic. These studies were approved by the University of Chicago Institutional Review Board and were performed after obtaining assent of the girls and consent of the parents.
Table 1.
Study group baseline characteristics
| Group | Age (yr) | BA (yr) | BMI (percentile) | Max Ov (ml) | Max Foll (number) | LH (U/liter) | FSH (U/liter) | E2 (pg/ml) | Free T (pg/ml) | SHBG (nm) | DHEAS (μg/dl) | HOMA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NW-PRE (n = 9) | ||||||||||||
| Mean± sem | 7.7 ± 0.3 | 7.2 ± 0.4 | 56 ± 10 | 2.5 ± 0.8 | 2.7 ± 0.8 | ≤0.15 ± 0.00 | 1.1 ± 0.2 | 6.1 ± 0.4 | ≤2.0 ± 0.0 | 38 ± 3.5 | 21 ± 5.3 | 3.7 ± 0.6 |
| Range | 6.5–9.0 | 5.8–8.8 | 8–85 | 0.2–6.3 | 0–6 | ≤0.15 | 0.5–2.4 | ≤5.0–8.0 | ≤2.0 | 24–58 | <5–42 | 0.7–6.2 |
| EW-PRE (n = 11) | ||||||||||||
| Mean± sem | 8.1 ± 0.4 | 8.4 ± 0.4c | 95 ± 1d | 3.5 ± 0.5 | 5.2 ± 1.5 | ≤0.21 ± 0.06 | 1.3 ± 0.2 | 5.5 ± 0.3 | ≤2.1 ± 0.1 | 26 ± 4.5 | 17 ± 4.1 | 8.0 ± 2.1 |
| Range | 6.4–9.5 | 6.3–11.0 | 88–99.9 | 1.2–5.6 | 0–10 | <0.15–0.85 | ≤0.2–2.1 | ≤5.0–8.0 | ≤2.0–3.0 | 8–60 | <5–50 | 1.3–25.1 |
| NW-PUB (n = 12) | ||||||||||||
| Mean± sem | 11.5 ± 0.3 | 11.7 ± 0.3 | 48 ± 6 | 4.3 ± 0.5 | 7.1 ± 0.6 | 2.1 ± 0.42 | 5.0 ± 0.5 | 30.3 ± 4.5 | 4.4 ± 0.6 | 28 ± 3.7 | 35 ± 5.2 | 6.4 ± 0.9 |
| Range | 9.4–13.0 | 8.8–13.0 | 9–74 | 1.6–7.3 | 4–10 | 0.50–5.6 | 3.0–7.8 | 6.0–50.0 | ≤2.0–8.0 | 3–44 | 8–60 | 2.9–13 |
| EW-PUB (n = 8) | ||||||||||||
| Mean± sem | 11.3 ± 0.3 | 11.5 ± 0.6 | 95 ± 2d | 6.8 ± 1.6 | 6.0 ± 1.4 | 2.1 ± 0.71 | 3.7 ± 0.8 | 24.8 ± 6.5 | 6.6 ± 1.1 | 24 ± 3.2 | 40 ± 7.4 | 10.2 ± 1.4c |
| Range | 10.6–12.8 | 8.8–13.0 | 86–99 | 1.8–12.3 | 0–10 | <0.15–5.8 | 0.5–6.2 | ≤5.0–53.0 | 4–10 | 9–40 | 11–72 | 5.8–17.3 |
Observed range for each group is shown. For conversions to SI units, multiply as follows: T × 0.0347 = nmol/liter; free T × 3.47 = pmol/liter; DHEAS × 0.0271 = μmol/liter. Max Foll, Maximum follicle number in largest plane; Max Ov, largest ovary.
P values comparing EW with NW within pubertal stage:
P < 0.05;
P < 0.01.
Figure 1.
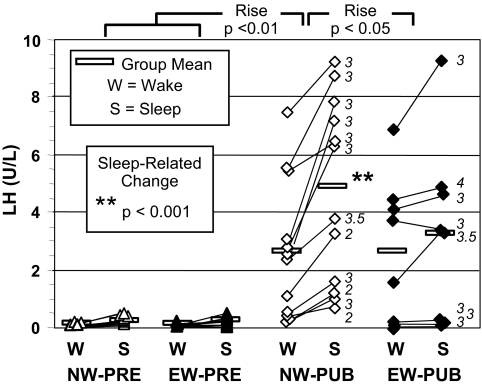
Individual wake and sleep mean LH concentrations. Statistical significance of sleep-related rises is shown. The significant sleep-related LH rise characteristic of NW-PUB was not seen in EW-PUB and was also significantly greater than that of the PRE groups. Breast stage of each pubertal girl is shown in italics.
Study protocol
All subjects were admitted to the University of Chicago General Clinical Research Center. At 1900 h, a hormonal sleep test was begun by inserting an iv line for continuous blood withdrawal, with sampling at 20-min intervals until 2 or more hours after sleep onset, as assessed by observation (9), with the exception of two subjects in each PRE group whose iv access lasted only 1.0–1.7 h. LH was measured at 20-min intervals, FSH and estradiol (E2) in 2-h pools. Thereafter, the study protocol was conducted as previously described for postmenarcheal girls (8). Briefly, at 0800 h the next morning, baseline fasting blood samples were obtained for steroid assays. An oral glucose tolerance test (OGTT) was then performed according to American Diabetes Association guidelines (10): after a fasting sample was obtained, subjects were given glucose in solution (Glucola), 1.75 g/kg body weight up to a maximum of 75 g, with repeat sampling at 2 h for serum glucose and insulin. During the OGTT, blood was sampled at 15-min intervals to determine the baseline gonadotropin mean. At 1200 h, dexamethasone was given orally to attenuate spontaneous adrenocortical secretion during the subsequent ACTH test (11); a low dose (0.25 mg/m2) of dexamethasone was used to provide a relatively short-term effect. Then a pelvic ultrasound examination was performed. At 1600 h, a low-dose ACTH1-24 test (1.0 μg/1.7 m2 iv) (12,13) was performed to test adrenal sensitivity to ACTH by sampling at 0 (basal), 15, and 30 min for steroids. At 1800 h, after collecting blood every 15 min for 1 h for basal gonadotropins, a GnRHag (leuprolide acetate) test dose (10 μg/kg sc) (14) was given to assess responsiveness of LH, FSH, and ovarian steroids and intermediates. Blood was sampled for these hormones 0.5–18 h later. Dexamethasone was then readministered 18 h after GnRHag (1200 h) to attenuate coincidental adrenocortical secretion during the remaining sampling for steroidogenic responses to GnRHag (15), which was performed 20–24 h after GnRHag (i.e. at 1400–1800 h). Blood volumes withdrawn were adjusted so that no more than 5% total blood volume was removed. Subjects were given meals at 0800, 1200, and 1800 h, except for the morning of the OGTT.
Laboratory and procedural methods
Serum LH and FSH were measured by immunofluorometric assays (Delphia; Wallac, Turku, Finland); total T, dehydroepiandrosterone sulfate (DHEAS), and cortisol by Diagnostic Products Corp. (Los Angeles, CA) RIA kits; and E2 by Pantex (Santa Monica, CA) immunoassay kit (14). Free T and SHBG were calculated from a binding assay as previously reported (14,16). RIAs were used for steroid intermediates (17-hydroxyprogesterone, androstenedione, 11-deoxycortisol, 17-hydroxypregnenolone, and DHEA) (17) and serum insulin (18). Each hormone on a study subject was assayed simultaneously. Values below the limits of sensitivity were set to this limit for statistical analysis. Assay characteristics (sensitivity and midrange intra- and interassay precision) were LH, 0.15 U/liter and 2.8 and 7.1%; FSH, 0.2 U/liter and 2.3 and 3.8%; total T, 10 ng/dl and 9 and 11%; DHEAS, 5 μg/dl and 3.7 and 12%; cortisol, 0.2 μg/dl and 3.0 and 12%; E2, 5.0 pg/ml and 10 and, 10%; free T, 2 pg/ml and 2.9 and 9%; SHBG, 3 nm and 7.7 and 12%; steroid intermediates, 25 pg/ml and 8.7 and 10.7%; and insulin, 4 μU/ml and 11.4 and 14.9%. Plasma glucose was measured using a glucose analyzer (YSI Model 2300 STAT; Yellow Springs Instruments, Yellow Springs, OH). Insulin resistance was approximated by homeostatic model assessment (HOMA) according to the equation HOMA = [fasting plasma insulin (milliunits/liter) × fasting plasma glucose (millimoles/liter)]/22.5 (19). Real-time pelvic ultrasound imaging was performed in the Pediatric Radiology Department by the abdominal route using an Acuson Sequoia with a 4-Mhz transducer (Acuson, Mountain View, CA) according to the manufacturer’s specifications using standard algorithms and a phantom probe for periodic calibration (20). There was no significant variation between readings by the two radiologists who performed these studies. Ovarian volume was calculated according to the formula for ellipsoid bodies: volume = 0.523 (longitudinal diameter × anteroposterior diameter × transverse diameter). Bone age was determined by a modification of the Greulich-Pyle method (21). BMI percentiles and Z-scores were determined from a national database (22).
Data analyses
Group data, including hormonal responses log-transformed as necessary for data normalization, were compared by Kruskal-Wallis test (hormone rises during sleep test), one-way ANOVA (comparisons among groups), Fisher’s exact test (for categorical variables), two-way repeated-measures ANOVA (GnRHag test), paired and unpaired Student’s t tests (two-sample comparisons), and linear regression analysis (relationships between variables). Because a rise in sleep-related LH is the earliest hormonal change of puberty, the mean LH rise was the a priori primary outcome variable. Correction for multiple comparisons was carried out for this variable by Dunn’s post hoc test and for secondary outcome variables by Fisher’s protected least significant differences test or Bonferroni correction. Results are expressed as mean ± sem unless otherwise stated. Two-tailed P values <0.05 were considered significant. Post hoc power calculations indicated that our studies have approximately 80% or more power to detect the primary observed differences between NW-PUB and EW-PUB. However, about 40 or more subjects per group would be required to detect differences of the magnitude seen in this study for most other variables of secondary interest that did not reach statistical significance.
Significant pulses of LH in PUB girls were identified with the Chronobiologic Series Analyzer (University of Chicago, Chicago, IL) program of Van Cauter, Hasak, and Leproult. This program uses the ULTRA algorithm to determine and quantify significant LH pulses by eliminating all peaks of concentration for which either the increment or the decrement did not exceed a threshold three times the intraassay coefficient of variation (23). Because of the variable duration of the sleep tests (2.7–8.3 h), LH pulse data for both the NW-PUB and EW-PUB groups were first separated into 3-h segments. Comparison of LH pulse data within these 3-h segments was by one-way ANOVA. After finding no statistically significant differences, the data from the 3-h segments was pooled in each individual before performing between-group comparisons.
Results
Baseline
Baseline characteristics of each study group are shown in Table 1. Within stage, baseline pubertal hormone levels were not significantly different in NW compared with EW girls. Notably, EW girls had no evidence of baseline hyperandrogenism as assessed by total or free T, DHEAS, and androstenedione (Table 1 and supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem. endojournals.org). EW-PRE had significantly higher bone age than NW-PRE, as expected. EW-PUB had the expected higher HOMA than NW-PUB; there was no significant difference in the prevalence of impaired glucose tolerance among groups, which ranged up to 25% in both PUB groups.
Sleep test
NW-PRE and EW-PRE did not have significantly different wake or sleep LH parameters (Table 2), so they were pooled for statistical analysis. LH of the combined PRE group underwent a small but significant rise (from ≤0.15 ± 0.00 awake to 0.23 ± 0.03 asleep; rise 0.08±0.03), reflecting significant rises in seven of the 20 girls.
Table 2.
Summary of wake and sleep gonadotropin and E2 levels of prepubertal and pubertal NW and EW groups
| Group | LH (U/liter)
|
FSH (U/liter)
|
E2 (pg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean wake | Mean sleep | Peak sleep | Mean wake | Mean sleep | Peak sleep | Mean wake | Mean sleep | Peak sleep | |
| NW-PRE (n = 9) | |||||||||
| Mean± sem | 0.15 ± 0.00 | 0.21 ± 0.04 | 0.29 ± 0.08 | 0.90 ± 0.15 | 1.4 ± 0.31 | 1.4 ± 0.31 | 11.3 ± 1.9 | 5.8 ± 0.5 | 5.8 ± 0.5 |
| Range | ≤0.15 | ≤0.15–0.52 | ≤0.15–0.85 | 0.43–1.5 | 0.40–3.0 | 0.40–3.0 | ≤5.0–24.0 | ≤5.0–10.0 | ≤5.0–10.0 |
| EW-PRE (n = 11) | |||||||||
| Mean± sem | 0.15 ± 0.00 | 0.25 ± 0.04 | 0.40 ± 0.09 | 1.0 ± 0.13 | 1.7 ± 0.30 | 1.8 ± 0.31 | 9.2 ± 1.1 | 5.6 ± 0.4 | 5.7 ± 0.5 |
| Range | ≤0.15 | ≤0.15–0.51 | ≤0.15–0.60 | 0.38–1.6 | 0.50–3.8 | 0.60–3.8 | ≤5.0–16.0 | ≤5.0–9.0 | ≤5.0–9.0 |
| NW-PUB (n = 12) | |||||||||
| Mean± sem | 2.7 ± 0.70 | 4.8 ± 0.92b | 7.7 ± 1.2 | 4.0 ± 0.44 | 5.4 ± 0.58c | 6.1 ± 0.60 | 19.5 ± 2.9 | 19.4 ± 3.5 | 25.8 ± 4.8 |
| Range | 0.23–7.5 | 0.77–9.3 | 1.8–12.9 | 1.9–7.0 | 2.8–8.1 | 3.4–9.5 | 5.5–36.0 | 5.0–42.3 | ≤5.0–49.0 |
| EW-PUB (n = 8) | |||||||||
| Mean± sem | 2.6 ± 0.89 | 3.3 ± 1.1 | 5.3 ± 1.7 | 4.0 ± 0.87 | 4.2 ± 0.86 | 4.4 ± 0.88 | 25.5 ± 4.4 | 25.8 ± 6.2 | 28.9 ± 7.2 |
| Range | ≤0.15–6.9 | ≤0.15–9.3 | ≤0.15–13.5 | 0.68–7.2 | 0.54–6.6 | 0.60–6.8 | 10.5–43.7 | 5.3–48.7 | 7.0–63.0 |
P values comparing sleep with wake within group:
P < 0.001;
P < 0.01.
The NW-PUB group had the expected highly significant sleep-related rise in mean LH (2.1 ± 0.45 U/liter) that was greater than in any other group (Fig. 1); a significant rise occurred in every girl. This brought all above the PRE range, as expected (Fig. 1 and Table 2).
In contrast, the EW-PUB group’s rise in mean LH during sleep (0.68 ± 0.35 U/liter) was not significant and was significantly blunted in comparison with that of NW-PUB girls (Fig. 1). Only two of eight EW-PUB girls had a significant rise in mean LH during sleep. Thus, the mean wake and sleep LH levels of EW-PUB girls were intermediate between PRE and NW-PUB: they overlapped with PRE, and they did not differ significantly from either mean wake or sleep LH levels of NW-PUB girls (Fig. 1 and Table 2). The peak sleep LH of EW-PUB girls was likewise intermediate.
As expected, all sleep LH parameters correlated with breast stage and bone age across pubertal stage (P < 0.001). Similarly, they were related to baseline E2 and total and free T across pubertal stage (P < 0.001) as well as to DHEAS (P < 0.05). However, no sleep LH parameters were related to BMI percentile or Z-score by linear (Fig. 2A) or multiple linear regression analysis controlling for pubertal status, glucose tolerance parameters, insulin level, or HOMA.
Figure 2.
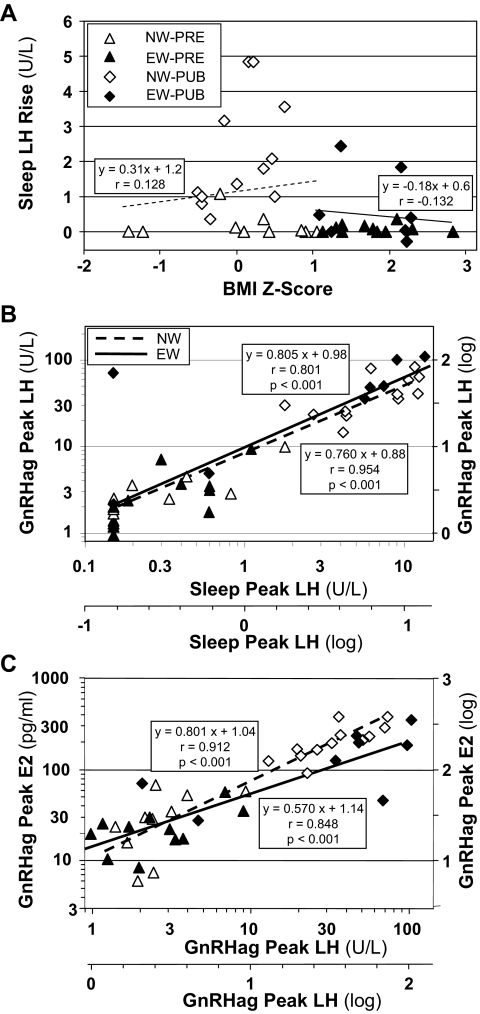
Scatterplots showing relationships among LH peak during sleep, BMI, LH response to GnRHag, and E2 response to GnRHag in individual EW and NW girls. A, Relationship of sleep-related rise in mean LH to BMI Z-score, which affords a broader display of the data than BMI percentile. The correlations were not significant (slopes of NW and EW represented by light trend lines). B, Relationship of peak LH during sleep to that in response to GnRHag. The slopes, determined and shown on a logarithmic scale, are not significantly different in EW than in NW girls. C, Relationship of post-GnRHag peak of E2 to that of LH. The slopes, determined and shown on a logarithmic scale, are steeper in NW than in EW girls (P = 0.05).
FSH patterns paralleled those of LH. Notably, FSH rose significantly during sleep in NW-PUB (1.3 ± 0.40 U/liter) and PRE (0.57 ± 0.17 U/liter) girls but not in EW-PUB girls (0.16 ± 0.25 U/liter) (Table 2). Because FSH levels were assessed as 2-h pools during the sleep test, there were insufficient data to determine the proportion of individuals having a significant sleep-related FSH rise.
E2 levels were similar in NW and EW girls of like stage (Table 2). PRE girls had a small significant decrease in E2 levels from wake to sleep. Although E2 levels were significantly higher overnight in the PUB groups, they showed no significant sleep-related variation.
LH pulse analysis
LH pulse analysis was performed to determine the basis of the blunted sleep-related LH rise in EW pubertal girls. The NW-PUB girls had a greater overnight LH pulse frequency than EW-PUB girls that became significant during sleep (Table 3). NW-PUB girls also had significantly higher LH pulse amplitude asleep than awake, whereas in EW-PUB girls, this difference was not manifest (P < 0.1). Consequently, the sleep-related rise in LH pulse amplitude was significantly greater in NW-PUB than in EW-PUB (Table 3). Too few PRE girls (two NW and three EW) had significant sleep-related pulses of LH to permit meaningful statistical comparison with PUB.
Table 3.
LH pulse analysis
| Group | Wake
|
Sleep
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duration (h) | Freq (pulses/3 h) | Mean Amp (IU/liter) | Peak Amp (IU/liter) | Duration (h) | Freq (pulses/3 h) | Mean Amp (U/liter) | Peak Amp (U/liter) | Mean Amp rise (U/liter) | Peak Amp rise (U/liter) | |
| NW-PUB (n = 11) | 3.0 ± 0.4 (1.3–5.7) | 1.7 ± 0.34 (0.81–4.6) | 3.4 ± 0.85 (0.25–8.3) | 3.6 ± 0.92 (0.30–9.0) | 5.2 ± 0.6a (3.0–8.3) | 1.5 ± 0.15 (0.39–2.0) | 7.3 ± 1.1b (2.8–12.6) | 8.2 ± 1.2b (2.8–12.9) | 4.0 ± 0.55 (1.5–7.2) | 4.6 ± 0.65 (2.4–8.6) |
| EW-PUB (n = 8) | 3.0 ± 0.3 (2.0–4.3) | 1.1 ± 0.39 (0.0–3.0) | 3.2 ± 1.1 (0.0–8.0) | 3.3 ± 1.1 (0.0–8.6) | 6.5 ± 0.6a (3.0–8.0) | 0.88 ± 0.24 (0.00–1.7) | 4.7 ± 1.6 (0.0–13.0) | 5.3 ± 1.7 (0.0–13.5) | 1.5 ± 0.64 (0.0–5.0) | 2.0 ± 0.70 (0.45–4.9) |
| P, EW-PUB vs. NW-PUB: | <0.05 | 0.01 | <0.02 | |||||||
Results are shown as Mean ± sem (observed range). One NW-PUB patient was excluded due to sleep study ending at 2 h at the peak level. Amp, Amplitude; Freq, frequency.
P < 0.01 vs. wake.
P < 0.001 vs. wake.
ACTH test
Adrenal steroid levels of EW and NW were similar within stage during the ACTH test. Androstenedione, DHEA, and other adrenarcheal steroids were higher in PUB than in PRE girls before and/or in response to ACTH, while progesterone responses were lower (supplemental Table 2).
GnRHag test
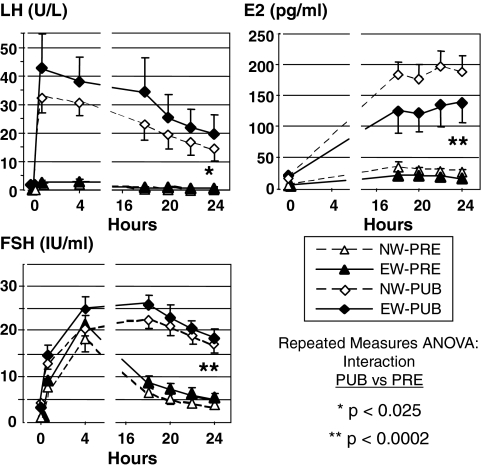
Despite the differences between EW and NW pubertal LH during sleep, no significant differences in pubertal hormone levels were observed within stage during the GnRHag test. Pubertal hormone levels were generally higher in PUB than in PRE girls before and in response to GnRHag (Fig. 3 and supplemental Table 1). All EW-PUB girls, including those with very low sleep LH levels, had an LH, FSH, or E2 response to GnRHag that exceeded the 95th percentile for pooled PRE girls.
Figure 3.
Baseline and peak LH, FSH, and E2 responses to GnRHag testing. NW-PUB and EW-PUB did not differ significantly, nor did NW-PRE and EW-PRE. PUB groups were significantly greater than PRE according to repeated-measures ANOVA for interaction (P < 0.025). Error bars depict sem.
The peak LH response to GnRHag, a simple correlate of area under the curve (P < 0.001), was not related to BMI percentile or Z-score, glucose tolerance parameters, insulin level, or HOMA. However, it was related to breast stage, bone age, E2, total and free T, and DHEAS across pubertal stage (P < 0.001), as expected.
Relationships between sleep LH and responses to GnRHag
Because EW-PUB and NW-PUB girls’ responses to GnRHag testing did not differ significantly although EW-PUB girls had a blunted sleep-related rise in LH, we examined the relationships between sleep LH and responses to GnRHag. As expected, peak LH responses to GnRHag reflected peak LH during sleep, as indicated by their high correlation across pubertal stage (Fig. 2B). This relationship did not differ significantly between NW- and EW-PUB girls, suggesting no significant difference between them in pituitary sensitivity to GnRH.
To in turn examine ovarian responsiveness to LH, the peak LH response to GnRHag was related to the peak E2 response to GnRHag. These relationships were again highly correlated across pubertal stage (Fig. 2C), but in this case, the NW girls had an increasingly higher E2 response to GnRHag as the peak LH response rose than did the EW girls (P = 0.05).
Discussion
We found that healthy EW-PUB girls had insignificant sleep-related rises in mean LH and FSH, which contrasted to NW-PUB girls. These alterations occurred in the absence of significantly different sex steroid levels. The LH changes were attributable to both a significantly lower LH pulse frequency and a significantly lower rise in LH pulse amplitude during sleep. The lesser LH pulse frequency of EW-PUB suggests a less vigorous hypothalamic drive to puberty in EW than NW girls, because LH pulse frequency is a surrogate for GnRH pulse frequency (24). This may in turn indicate a higher central nervous system abnormality in the regulation of the GnRH pulse generator.
This blunting of the sleep-associated LH increase in EW-PUB girls is consistent with the tendency for early morning LH to be low in obese early pubertal girls (5). It seems, at least in part, related to the recent findings that adult obesity is associated with suppression of LH levels in women. Obese eumenorrheic normoandrogenic women have drastically blunted LH levels (25). In addition, LH levels of PCOS women are inversely related to BMI (26,27). The LH suppression is due to blunting of LH pulse amplitude in both settings. This effect of obesity alone appears to counteract that of hyperandrogenism, moderate degrees of which enhance LH secretion (28). This blunting of LH pulsatility in PCOS is in part due to accelerated metabolism of LH (29); this is postulated to result from obesity decreasing the sialylation of LH, which would be expected to shorten its plasma half-life and, depending upon the degree and molecular localization, decrease its in vitro bioactivity (30,31). This may be mediated by insulin, an infusion of which under euglycemic clamp conditions has recently been reported to suppress baseline LH (32).
We could not document the clear-cut suppression of ovarian activation that might be expected to result from an overall decrease in LH output; EW-PUB girls had hormone responses to GnRHag testing similar to NW-PUB girls. There are a number of possible explanations for this, other than use of a maximal GnRHag test dose (14). Notably, EW-PUB girls tended to have lower E2 responses (Fig. 3), reminiscent of the report that the lowered LH of obese eumenorrheic adults had only a subtle effect on estrogen output; overall estrogen production, judged from estrone conjugate excretion, appeared to be normal during the follicular phase of the adult menstrual cycle (the portion that is akin to the early pubertal phase of ovarian activation) but was significantly depressed at the time of the midcycle ovulatory surge, after which corpus luteum function was severely blighted, as judged from progesterone metabolite excretion (25). In addition, the E2 response of EW girls to secreted gonadotropin was lower (P = 0.05) than that of NW girls, judging from the slope of the relationship of the peak E2 to the peak LH level achieved in response to gonadotropin. This may be explicable by desialylation-related decreased LH bioactivity in the EW state, as discussed above.
Alternatively, it is possible that blunting of sleep LH by obesity is compensated by another mechanism. We cannot rule out the possibility of a shift in diurnal LH rhythm, such that 24-h LH secretion is not altered. Reversal of diurnal LH periodicity has been reported in adolescents with PCOS, in whom it has been considered evidence for an intrinsic chronobiological abnormality of neuroendocrine function (33). However, the original and subsequent studies (6,34) have not controlled for the possibility of independent effects of obesity and hyperandrogenism. The possibility exists that the hyperinsulinemic insulin resistance of obesity accounts for this or exerts other stimulatory effects on hypothalamo-gonadotropic function (35,36,37,38,39), analogous to its apparent effect in the ovary (40). However, we did not find significantly different relationships between sleep LH and LH responsiveness to GnRHag challenge in EW compared with NW girls, as might be expected if these conditions pertain.
We could demonstrate no evidence of androgen or estrogen excess in ordinary EW-PUB girls at baseline or after provocative testing with GnRHag or a submaximal dose of ACTH. This contrasts with the report of hyperandrogenemia in frankly obese early pubertal girls (5) and the potential of the excess aromatase activity of adiposity to generate estrogen from androstenedione. The normal gonadotropin responses to GnRHag of the EW-PUB girls militate against excess negative feedback by sex steroids. We could also demonstrate no relationship of the measured indices of adiposity or insulin resistance to LH parameters. Taken together, these considerations suggest a threshold effect of moderate adiposity that is independent of sex steroid excess.
This study can only be considered exploratory. Although our groups were sufficiently large to provide important normative data, only minority subjects comprised the EW-PUB group. Our study had limited statistical power and was not designed to detect interactions with other hormones that link obesity to sleep or gonadotropin secretion (41,42). Because it was designed as a survey of the many aspects of puberty potentially disturbed by adiposity, our sleep data are limited in these young girls. Sleep onset was documented by observation, and we cannot document sleep integrity. However, this is unlikely to affect the validity of the data because there was no difference between EW- and NW-PUB girls in the apparent duration or intactness (data not shown) of sleep; furthermore, acute deviation from the usual sleep pattern does not eliminate increased nocturnal LH secretion during puberty (43). We also do not have 24-h hormonal data to discern the mean concentrations. Furthermore, we cannot be certain that the apparent reduction in LH pulse frequency in EW-PUB girls is real or is an artifact of reduction of LH pulse amplitude to levels below assay sensitivity. Despite these limitations, our data point to new research directions that warrant further study.
A blunted sleep-related LH rise is compatible with a blunted stimulus to pubertal progression in EW girls. This may provide a mechanism for the possibility that the pace of puberty is slowed in obese girls. The available longitudinal data are compatible with excess adiposity after the onset of puberty slowing pubertal tempo, although less so than prepubertal adiposity advances puberty onset (44,45,46).
In summary, the spontaneous sleep-related gonadotropin rise is blunted in healthy EW-PUB girls, but pituitary-ovarian responses to GnRHag testing do not seem to be proportionately affected. The LH disturbance seems to be due to both a lower increase in LH pulse amplitude and slower LH pulse frequency during sleep. Consequently, a suppressive effect of excess adiposity on the regulation of hypothalamic GnRH secretion during puberty is suggested. Thus, these data suggest that excess adiposity, in the absence of sex steroid excess, may subtly suppress hypothalamic-pituitary-gonadal function in premenarcheal pubertal girls.
Supplementary Material
Acknowledgments
We thank Kristen Kasza for biostatistical support, Kate Feinstein and David Yousefzadeh for ultrasonographic support, and Eve Van Cauter and Rachel Leproult for pulse analysis consultation.
Footnotes
Disclosure Summary: B.B., E.L., and R.L.R. have nothing to declare.
This work was supported in part by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54-041859) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and HD-39267 (R.L.R.), 5T32DK064582 and a Lawson Wilkins Pediatric Endocrine Society Research Fellowship (B.B.), and RR-00055 and UL1RR024999 from the National Center For Research Resources.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
A preliminary report was presented at the Pediatric Academic Society Annual Meeting, Toronto, Canada, May 3–8, 2007.
First Published Online February 3, 2009
For editorial see page 1094
Abbreviations: BMI, Body mass index; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; EW, excessive weight; GnRHag, GnRH agonist; HOMA, homeostatic model assessment; NW, normal weight; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; PRE, prepubertal; PUB, pubertal; T, testosterone.
References
- Herman-Giddens ME, Kaplowitz PB, Wasserman R 2004 Navigating the recent articles on girls’ puberty in Pediatrics: what do we know and where do we go from here? Pediatrics 113:911–917 [DOI] [PubMed] [Google Scholar]
- Rosenfeld RL, Lipton RB, Drum ML 2009 Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 123:84–88 [DOI] [PubMed] [Google Scholar]
- Boyar RM, Finkelstein JW, David R, Roffwarg H, Kapen S, Weitzman ED, Hellman L 1973 Twenty-four hour patterns of plasma luteinizing hormone and follicle-stimulating hormone in sexual precocity. N Engl J Med 289:282–286 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL 2007 Identifying children at risk of polycystic ovary syndrome. J Clin Endocrinol Metab 92:787–796 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC 2007 Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 92:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter D, Bützow T, Laughlin G, Yen S 1994 Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 79:119–125 [DOI] [PubMed] [Google Scholar]
- Boyar R, Finkelstein J, Roffwarg H 1972 Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287:582 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Rosenfield RL, Littlejohn E 2006 Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 91:3786–3790 [DOI] [PubMed] [Google Scholar]
- Ghai K, Cara JF, Rosenfield RL 1995 Gonadotropin releasing hormone agonist (nafarelin) test to differentiate gonadotropin deficiency from constitutionally delayed puberty in teen-age boys: a Clinical Research Center study. J Clin Endocrinol Metab 80:2980–2986 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2000 Type 2 diabetes in children and adolescents. Pediatrics 105:671–680 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Helke J, Lucky AW 1985 Dexamethasone preparation does not alter corticoid and androgen responses to adrenocorticotropin. J Clin Endocrinol Metab 60:585–589 [DOI] [PubMed] [Google Scholar]
- Dickstein G, Shechner C, Nicholson W, Rosner I, Shen-Orr Z, Adawi F, Lahav M 1991 Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 72:773–778 [DOI] [PubMed] [Google Scholar]
- Bridges NA, Hindmarsh PC, Pringle PJ, Honour JW, Brook CG 1998 Cortisol, androstenedione (A4), dehydroepiandrosterone sulphate (DHEAS) and 17 hydroxyprogesterone (17OHP) responses to low doses of (1-24)ACTH. J Clin Endocrinol Metab [Erratum (1999) 84:2972, 4177] 83:3750–3753 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Perovic N, Ehrmann DA, Barnes RB 1996 Acute hormonal responses to the gonadotropin releasing hormone agonist leuprolide: dose-response studies and comparison to nafarelin. J Clin Endocrinol Metab 81:3408–3411 [DOI] [PubMed] [Google Scholar]
- Barnes RL, Ehrmann DA, Brigell DF, Rosenfield RL 1993 Ovarian steroidogenic responses to the gonadotropin-releasing hormone agonist nafarelin in hirsute women thought to have 3β-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 76:450–455 [DOI] [PubMed] [Google Scholar]
- Moll Jr G, Rosenfield R 1979 Testosterone binding and free plasma androgen concentrations under physiologic conditions: characterization by flow dialysis technique. J Clin Endocrinol Metab 49:730–736 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA 1994 Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 79:1686–1692 [DOI] [PubMed] [Google Scholar]
- Morgan CR, Lazarow A 1963 Immunoassay of insulin: two antibody system. Plasma insulin levels of normal, subdiabetic, and diabetic rats. Diabetes 12:115–126 [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- de Vries L, Horev G, Schwartz M, Phillip M 2006 Ultrasonographic and clinical parameters for early differentiation between precocious puberty and premature thelarche. Eur J Endocrinol 154:891–898 [DOI] [PubMed] [Google Scholar]
- Roche AF, Eyman SL, Davila GH 1971 Skeletal age prediction. J Pediatr 78:997–1003 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL 2000 CDC growth charts: United States. Adv Data 8:1–27 [PubMed] [Google Scholar]
- Van Cauter E 1988 Estimating false-positive and false-negative errors in analyses of hormonal pulsatility. Am J Physiol 254:E786–E794 [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow TL, Laughlin GA, Yen SS 1993 Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 76:940–949 [DOI] [PubMed] [Google Scholar]
- Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N 2007 Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 92:2468–2473 [DOI] [PubMed] [Google Scholar]
- Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE 1997 Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Laughlin GA, Morales AJ, Yen SS 1997 Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 82:3728–3733 [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Srouji SS, Pagan YL, D’Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE 2007 Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab 92:1347–1352 [DOI] [PubMed] [Google Scholar]
- Burgon PG, Stanton PG, Robertson DM 1996 In vivo bioactivities and clearance patterns of highly purified human luteinizing hormone isoforms. Endocrinology 137:4827–4836 [DOI] [PubMed] [Google Scholar]
- Burgon PG, Stanton PG, Pettersson K, Robertson DM 1997 Effect of desialylation of highly purified isoforms of human luteinizing hormone on their bioactivity in vitro, radioreceptor activity and immunoactivity. Reprod Fertil Dev 9:501–508 [DOI] [PubMed] [Google Scholar]
- Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ 2008 Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab 93:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumoff B, Freeman R, Coupey S, Saenger P, Markowitz M, Kream J 1983 A chronobiologic abnormality in luteinizing hormone secretion in teenage girls with the polycystic ovary syndrome. N Engl J Med 309:1206–1209 [DOI] [PubMed] [Google Scholar]
- Porcu E, Venturoli S, Magrini O, Bolzani R, Gabbi D, Paradisi R, Fabbri R, Flamigni C 1987 Circadian variations of luteinizing hormone can have two different profiles in adolescent anovulation. J Clin Endocrinol Metab 65:488–493 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Unger JW, Lange W 1997 Insulin receptors in the pituitary gland: morphological evidence for influence on opioid peptide-synthesizing cells. Cell Tissue Res 288:471–483 [DOI] [PubMed] [Google Scholar]
- Adashi EY, Hsueh AJ, Yen SS 1981 Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 108:1441–1449 [DOI] [PubMed] [Google Scholar]
- Xia YX, Weiss JM, Polack S, Diedrich K, Ortmann O 2001 Interactions of insulin-like growth factor-I, insulin and estradiol with GnRH-stimulated luteinizing hormone release from female rat gonadotrophs. Eur J Endocrinol 144:73–79 [DOI] [PubMed] [Google Scholar]
- Dorn C, Mouillet JF, Yan X, Ou Q, Sadovsky Y 2004 Insulin enhances the transcription of luteinizing hormone-β gene. Am J Obstet Gynecol 191:132–137 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL 1995 Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16:322–353 [DOI] [PubMed] [Google Scholar]
- Van Cauter EV, Polonsky KS, Blackman JD, Roland D, Sturis J, Byrne MM, Scheen AJ 1994 Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. J Clin Endocrinol Metab 79:1797–1805 [DOI] [PubMed] [Google Scholar]
- Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K 2000 Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest 105:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapen S, Boyar R, Finkelstein J, Hellman L, Weitzman E 1974 Effect of sleep-wake cycle reversal on luteinizing hormone secretory pattern in puberty. J Clin Endocrinol Metab 39:293–299 [DOI] [PubMed] [Google Scholar]
- de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB 1992 Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab 75:442–446 [DOI] [PubMed] [Google Scholar]
- Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, Daniels S 2006 Pubertal correlates in black and white girls. J Pediatr 148:234–240 [DOI] [PubMed] [Google Scholar]
- Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC 2007 Weight status in young girls and the onset of puberty. Pediatrics 119:e624–e630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.