Abstract
Plants are known to produce NO via the reduction of nitrite. Oxidative NO production in plants has been considered only with respect to a nitric oxide synthase (NOS). Here it is shown that tobacco cell suspensions emitted NO when hydroxylamine (HA) or salicylhydroxamate (SHAM), a frequently used AOX inhibitor, was added. NG-hydroxy-L-arginine, a putative intermediate in the NOS-reaction, gave no NO emission. Only a minor fraction (≤1%) of the added HA or SHAM was emitted as NO. Production of NO was decreased by anoxia or by the addition of catalase, but was increased by conditions inducing reactive oxygen (ROS) or by the addition of hydrogen peroxide. Cell-free enzyme solutions generating superoxide or hydrogen peroxide also led to the formation of NO from HA or (with lower rates) from SHAM, and nitrite was also an oxidation product. Unexpectedly, the addition of superoxide dismutase (SOD) to cell suspensions stimulated NO formation from hydroxylamines, and SOD alone (without cells) also catalysed the production of NO from HA or SHAM. NO production by SOD plus HA was higher in nitrogen than in air, but from SOD plus SHAM it was lower in nitrogen. Thus, SOD-catalysed NO formation from SHAM and from HA may involve different mechanisms. While our data open a new possibility for oxidative NO formation in plants, the existence and role of these reactions under physiological conditions is not yet clear.
Keywords: Hydroxylamine, nitric oxide, oxidative NO formation, reactive oxygen, salicyl hydroxamate, superoxide dismutase
Introduction
Nitric oxide is considered to be an almost universal signalling molecule in plants, involved in the control of growth, development, and stress responses as well as plant defense reactions against pathogens (Lamattina et al., 2003; Besson-Bard et al., 2008). Still much under debate are the pathways of NO synthesis and their regulation, although such knowledge is indispensable for a complete understanding of the multiple roles of NO in plants. Without any doubt, plants are able to produce NO by reducing nitrite to NO. Reduction can be catalysed by assimilatory (cytosolic) nitrate reductase (NR), but also by mitochondrial electron transport (Planchet et al., 2005). The rate of nitrite to NO production depends largely on the nitrite concentration. Nitrite accumulates specifically under conditions where production is high and consumption is low. Such conditions are hypoxia or anoxia, where NO emission from leaves, roots or cell suspensions can be 100 to 1000-fold higher than in air (Planchet et al., 2005). Plants are able to grow and complete their life cycle in the absence of nitrate, with ammonium as the sole N-source. Thus, if NO is to play the proposed roles in signalling, nitrite-independent sources for NO are required that work in the presence of oxygen. So far, a NOS-like reaction appeared to be the only oxidative pathway for NO synthesis that would fulfill these requirements, but the existence of a NOS-like enzyme in plants is still uncertain (Crawford et al., 2006). We are therefore searching for other possibilities for an oxidative NO formation in plants. In animals (Markert et al., 1994; Kouichi et al., 1997) and bacteria (Hooper and Terry, 1979), NO can be synthesized via the oxidation of hydroxylamines and, in animals, hydroxylamine has been shown to posses vasodilatory properties just like NO donors (DeMaster et al., 1989). Here, for the first time, it has been examined whether plant cells could also oxidize R-NHOH-compounds to NO.
Materials and methods
Cell cultures
Tobacco WT (Nicotiana tabacum cv. Xanthi) non-green suspension cells were cultured in 300 ml Erlenmeyer flasks containing 100 ml LS-medium pH 5.8 (Linsmaier and Skoog, 1965) at a constant temperature of 24 °C and a continuous illumination (100 μE m−2 s−1 PAR) with fluorescent tubes, on a rotary shaker (New Brunswick Scientific, NJ, USA). Subcultures were made by transferring 20 ml of the cell suspension into 80 ml of fresh LS-medium. The cells were used for the experiments 3–4 d after subculturing. Cells of the nia30 mutant, which has less than 2% of the WT NR activity (Müller, 1983) were grown on a nitrate-free LS-Medium with casein hydrolysate (Bacto™ Casamino acids, Becton, Dickinson and Company, Sparks, MD, USA, 3 g l−1) as the N-source, and MES at 10 mM in order to maintain the pH close to 6.2.
Prior to the measurements, cells were filtered on paper filters, washed three times and resuspended in a medium containing 20 mM KNO3, 20 mM KCl, 3 mM CaCl2, 1.5 mM MgSO4, 1.25 mM KH2PO4, trace elements, 88 mM sucrose, 5 mM MES, and 20 mM MOPS, pH 7.2. Cells of the nia30 mutant were washed in a nitrate-free medium and without casein hydrolysate. By suitable dilution, cell density was adjusted to 1 g fresh weight in a total volume of 15 ml and used for chemiluminescence measurements.
Cell fresh weight (FW) was determined by filtering 15 ml batches of the cell suspension through 50 mm paper filters by applying a slight vacuum for about 10 s. Subsequently, the filter was weighed and the wet filter weight without cells was subtracted.
Root segments
Roots were harvested from 2-week-old hydroponically cultured barley seedlings. Whole roots were washed carefully with 0.5 mM CaSO4 solution, blotted on filter paper, and cut into segments (1 cm long). One gram (fresh weight) of roots segments were suspended in 15 ml of a solution containing 0.5 mM CaSO4 and 5 mM KNO3, and 5 mM MES–KOH pH 6.8, and used for the chemiluminescence measurements as described above.
Cell-free system
Commercially available enzyme preparations were used (see ‘Chemicals’). The enzyme activities (U) indicated in the legends were those given by the manufacturer. The reaction medium was identical with the ‘wash-medium’ used for the suspensions cells (pH 7.2). The reaction was usually started by substrate addition (HA or SHAM).
NO chemiluminescence measurements
Experiments with cells or cell-free systems were carried out with a chemiluminescence detector (CLD 770 AL ppt, Eco-Physics, Dürnten, Switzerland), as previously described by Planchet and Kaiser (2006a, b). Usually 10 or 15 ml of liquid (cell suspension, enzyme solution) were placed in a glass dish in a transparent glass cuvette (1.0 l gas volume) mounted on a rotary shaker (150 rpm). A constant flow of measuring gas (pressurized air or nitrogen), adjusted by mass flow controllers (FC-260, Tylan General, Eching, Germany), was pulled through the cuvette and subsequently through the detector by a precision vacuum pump connected to an ozone destroyer. The ozone generator of the detector was supplied with dry oxygen (99%). The measuring gas (nitrogen or air) was made free of NO by conducting it through two custom-made charcoal columns (1 m long, 3 cm internal diameter, particle size 2 mm). Data points were obtained three times per minute by automatically sampling the gas for 20 s. Data were transferred to a computer equipped with an AD-converter and analysed with a custom-made program using Visual Designer™ (Intelligent Instrumentation Inc., Tucson, USA). Calibration of the apparatus was carried out with NO-free air and various concentrations of NO (1–35 ppb) adjusted by mixing the calibration gas (500 ppb NO in nitrogen, Messer Griesheim, Darmstadt, Germany) with NO-free air. Rates of NO emission were calculated based on the measured NO concentration (ppb) in the gas stream (1.0 l min−1) and the cell fresh weight (1 g) in the cuvette. Air temperature in the cuvette was usually about 24 °C. Aliquots of various solutions were added to the cell suspensions or enzyme solutions by injecting the appropriate volume (5–100 μl) through the rubber diaphragm of the cuvette directly into the cell suspension using microlitre syringes, without interrupting the NO-measurement.
DAF-2 fluorescence
The time-course of DAF-2 fluorescence was followed in 1 ml of the cell-free system (enzyme mixture) containing 5 μM DAF-2, in fluorescence glass cuvettes closed with a Teflon stopper. The fluorimeter (Jasco Labor- und Datentechnik, Groß-Umstadt, Germany) was adjusted to 495 nm excitation and 515 nm emission wavelength at a band width of 3 nm each. The reaction was usually started by the addition of HA (40 μM).
Nitrite determination
Nitrite contents of the cell media or of the cell-free enzyme solutions were determined usually before and at the end of the reaction (30–40 min). One ml aliquots were mixed with 150 μl zinc acetate (0.5 M), 700 μl N-(1)-(naphthyl)ethylenediamine dihydrochloride (0.02%), and 700 μl sulphanilamide (1%). After 25 min at 24 °C, the mixture was cleared by centrifugation (16 000 g, 5 min), and the nitrite content was determined photometrically.
Chemicals
Hydroxylamine was prepared as 100 mM stock solution by dissolving solid hydroxylammonium-chloride (Sigma-Aldrich, Taufkirchen, Germany) in 1 mM hydrochloric acid. HCl was flushed for 15 min with helium to eliminate oxygen. Solutions were freshly prepared prior to the experiments and kept on ice. Myxothiazol, xanthine, SHAM, XOD, GOD, catalase, and SOD were from Sigma-Aldrich (Taufkirchen, Germany). NG-hydroxy-L-arginine was from Calbiochem (Schwalbach, Germany), DAF-2 from Axxora (Lörrach, Germany).
Results
Tobacco suspension cells oxidize low concentrations of hydroxylamine or SHAM to NO
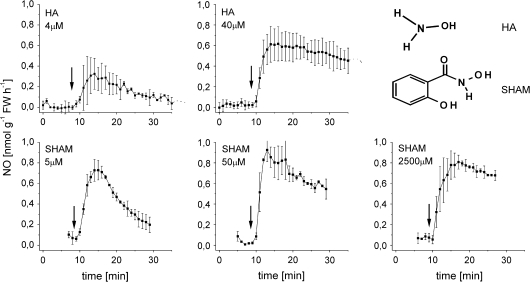
Nitrate-grown tobacco cell suspensions usually emit NO only under anoxia, not in air (Planchet et al. 2005). However, when hydroxylamine (as hydrochloride solution) was added to tobacco suspension cells (15 ml, 1 g FW) on a rotary shaker (154 rpm) in a head space cuvette, NO was emitted from the solution. Transient emission could be observed at HA concentrations as low as 4 μM. At the higher HA concentration (40 μM), rates were higher and more constant (Fig. 1). It has to be noted that chemiluminescence curves as in Fig. 1 are rate curves, i.e. they give the rates of NO emission as a function of time.
Fig. 1.
Rates of NO emission from tobacco suspension cells fed with different concentrations of HA or SHAM. Note that the curves are rate curves, i.e. they give NO emission rates as a function of time (for further details see the ‘Materials and methods’). For drawing curves and SD bars, only every third measuring point was used. For SHAM, emission rates were also followed at the high SHAM concentration (2.5 mM) which is used for the inhibition of AOX. In all cases, NO-emission was negligible when cells were boiled (10 min at 100 °C) (not shown). Bars on the curves give standard deviation (n=3–12).
These aerobic NO emission rates were low. In Fig. 1, the total amount of NO emitted from 4 μM HA until the reaction came to an end (after about 30 min) was 0.075 nmol g−1 FW−1, from the total amount added of 60 nmol HA (in 15 ml). Thus, it appears that only about 0.125% of the HA added was emitted as NO. Immediately after the reaction (20 min), 1 ml samples were also used for the determination of nitrite concentration. However, in all experiments with cell suspensions, nitrite concentrations remained very low (data not shown). This is clearly different from the situation with cell-free enzyme solutions, where at least some nitrite accumulation took place (see below).
NO emission was also observed with another hydroxylamine-like compound, the frequently used AOX inhibitor SHAM. Figure 1 shows that the maximum emission rates were very similar at extremely different SHAM concentrations ranging from 5 μM to 2500 μM. As for HA, at the lowest SHAM concentration, the emission rates were transient, already decreasing just a few minutes after the maximum rate was reached (Fig. 1). At the higher concentrations rates were more constant. As an AOX-inhibitor, SHAM is usually applied in millimolar concentrations.
As another N-hydroxy compound of potential physiological relevance, hydroxy-L-arginine, a putative intermediate of the NOS reaction which was reported to be oxidized to NO (Modolell et al., 1997) was tested. However, up to concentrations of 0.5 mM, hydroxy-L-arginine gave no measurable NO emission, quite in contrast to animal systems (not shown).
Roots also produce NO from HA
It was also examined whether plant tissues would produce NO from HA. Root segments from hydroponically grown barley seedlings (1 cm long, 1 g FW) suspended in 10 ml nutrient solution emitted NO at a mean rate of 0.6 nmol g−1 FW h−1 upon the addition of 10 μM HA, which is within the range of NO emission found with tobacco suspension cells (result not shown separately). With SHAM, root segments were not examined.
Is oxygen required for HA-dependent NO formation?
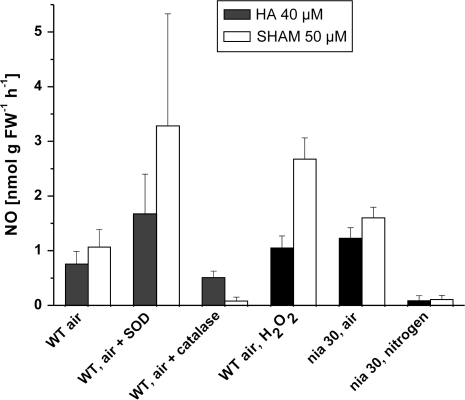
In order to check for an oxygen requirement of NO production from HA or SHAM by tobacco suspension cells, experiments were carried out with the nia30 double mutant, which has almost no detectable NR activity and does not produce nitrite (Müller, 1983). The mutant was used because nitrate-grown WT cells accumulate nitrite under anoxia and then emit nitrite-dependent NO at high rates (Planchet et al. 2005), which would mask the low NO formation from hydroxylamines. In air, rates of NO emission by cells of the nia30 mutant were within the range emitted by WT, both from HA or SHAM. In nitrogen, NO emission from HA and even more from SHAM was much lower than in air (Fig. 2).
Fig. 2.
NO-emission from HA and SHAM by tobacco cell suspensions, as affected by oxygen, by superoxide dismutase (to scavenge superoxide radicals), by catalase (to scavenge hydrogen peroxide, and by the addition of an excess hydrogen peroxide (50 μM). Each column gives the maximum rate obtained during a 20 min time-course. NO emission in air (control) and in nitrogen is shown here for cell cultures from a nia30 double mutant, which has less than 2% of the NR activity of the WT (see the Materials and methods), in order to ensure the absence of NO production by nitrite reduction. All other data have been obtained with WT-cells. Bars at the columns give SD (n=3–6).
Are reactive oxygen species (ROS) involved in hydroxylamine oxidation to NO?
Based on previous literature reports and because of the above-described oxygen requirement of HA and SHAM oxidation to NO, it was suspected that the actual oxidant for HA to NO conversion might be superoxide radicals or hydrogen peroxide produced by the cells. It has to be kept in mind that both ROS are also reacting rapidly with NO, which may complicate interpretation. To check for ROS participation, three different approaches were used.
First, HA-dependent NO emission of cells was measured in the presence of a superoxide-scavenging system (superoxide dismutase, SOD, 10 U per 15 ml), or of excess catalase (3300 U per 15 ml) which decomposes H2O2, or hydrogen peroxide (50 μM) was added directly to the cell suspension. Second, cells were exposed to conditions potentially triggering ROS production. Third, cell-free solutions of the enzyme XOD were used to produce superoxide radicals continuously, and of glucose oxidase (GOD) to produce H2O2 to measure the NO emission from HA or SHAM.
Unexpectedly, the addition of SOD to cells did not decrease NO emission from HA or SHAM, but increased it (Fig. 2). However, as shown later, SOD itself caused an NO emission in the absence of cells (Table 1). After subtraction of this SOD-dependent NO emission from the rate obtained with cells+SOD, cellular NO emission appeared not to be much affected by SOD.
Table 1.
NO emission and nitrite production by solutions of partially purified enzymes
| Reaction mixture | NO-emission (ppb) | Nitrite formation (nmol per 10 ml) | Percentage oxidized to nitrite (%) |
| +HA (400 nmol per 10 ml) | |||
| No addition | 0.08±0.02 | 1.57±0.25 | – |
| +XOD+xanthine | 1.16±0.20 | 24.89±0.59 | 6.22±0.15 |
| +XOD+xanthine+SOD | 2.67±0.52 | Not measured | – |
| +SOD, air | 0.78±0.23 | 2.82±0.49 | 0.71±0.12 |
| +SOD, nitrogen | 2.05±0.05 | Not measured | |
| +GOD+glucose | 0.19±0.07 | 7.70±5.20 | 1.93±1.3 |
| +H2O2 | 0.14±0,02 | Not measured | |
| +SHAM (500 nmol per 10 ml) | |||
| No addition | 0.00±0.02 | 1.86±1.08 | – |
| +XOD+xanthine | 0.19±0.08 | 29.46±0.29 | 5.89±0.06 |
| +XOD+xanthine+SOD | 0.33±0.07 | Not measured | |
| +SOD, air | 0.68±0.10 | 3.12±1.24 | 0.62±0.25 |
| +SOD, nitrogen | 0.31±0.09 | Not measured | |
| +GOD+glucose | 0.02±0.01 | 7.10±4.08 | 1.42±0.82 |
| +H2O2 | 0.02±0.01 | Not measured | Not measured |
XOD (0.5 U)+xanthine (0.25 mM) were used to produce superoxide radicals continuously, and GOD (0.5 U)+glucose (5 mM) were used to produce H2O2. Also shown is HA-dependent NO production by SOD (7 U) alone, in air and in nitrogen, and by H2O2 (50 μM). The last column gives the percentage of hydroxylamine or SHAM which was oxidized to nitrite. NO emission is given as the maximum NO concentration (ppb) reached in the gas stream during a 30 min period. Nitrite in the reaction medium was measured in an aliquot (1 ml) of the Reaction mixture sampled at the beginning and at the end of the reaction (after 30 min). The composition of buffer was identical with the wash medium used for cell suspensions (see the Materials and methods), liquid sample volume was 10 ml (±SD, n=3–5).
The addition of excess catalase to cell suspensions decreased the rate of NO emission from HA, and more strongly from SHAM (Fig. 2). Consistent with that, the addition of hydrogen peroxide (50 μM) to the cell suspension caused a strong increase of NO emission from HA and also from SHAM (Fig. 2). It should be noted that, in the absence of cells, i.e. in a buffer solution supplied with HA or SHAM, hydrogen peroxide caused no or very little NO emission (Table 1).
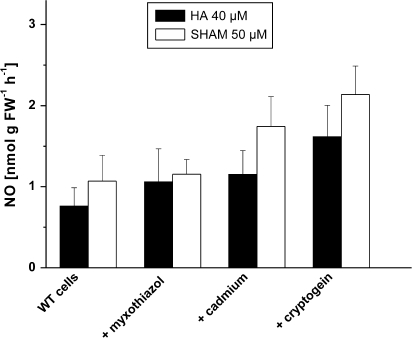
Next, it was examined whether cells would produce more NO from HA and SHAM when they were triggered to produce more ROS. The inhibitor of mitochondrial complex III, Myxothiazol, is known to result in increased ROS production through mitochondrial electron transport. Other well-known conditions causing ‘oxidative stress’ include heavy metal treatment (Schützendübel and Polle, 2002). A 1 h treatment with Myxothiazol produced a slight increase of NO emission, which was non-significant. A 1 h treatment with a CdCl2 (500 μM) increased NO emission somewhat more. But the most significant stimulation of NO emission was obtained by a 1 h pretreatment of cells with the fungal elicitor cryptogein (50 nM, Fig. 3), which is also known to provoke an oxidative burst (Foissner et al., 2000).
Fig. 3.
Attempts to increase ROS production and HA- or SHAM-dependent NO emission from cell suspensions. Cell suspensions were pretreated for 1 h with Myxothiazol (10 μM), with cryptogein (50 nM) or CdCl2 (500 μM). Subsequently, NO emission was followed for 30 min as in Fig. 1, either with 40 μM HA or with 50 μM SHAM. Separate controls were carried with for each treatment. Columns give maximum rates from the time-courses, ±SD, n=3 (treatment), n=9 (controls).
Hydroxylamine oxidation in a cell-free system
Because of the complexity of living cells with respect to simultaneous NO production and metabolism, simple cell-free enzyme solutions producing ROS were also used and checked for NO production from HA or SHAM. XOD+xanthine were used to generate superoxide radicals continuously. Upon the addition of HA, the enzyme solution produced NO and, with a much lower rate, from SHAM as well (Table 1). GOD+glucose, which was used to produce hydrogen peroxide continuously, appeared less effective in oxidizing HA or SHAM to NO. In the cell-free system, NO emission from SHAM was generally lower than from HA, at comparable substrate concentrations.
As shown above (Fig. 2), SOD as a superoxide scavenger increased NO emission by cells from HA or SHAM instead of inhibiting it. Therefore, this unexpected effect of SOD was also examined without cells, just with a SOD solution. Indeed, even without any ROS source added, SOD alone also produced NO from HA or SHAM. Production of NO from HA by SOD was almost three times higher in nitrogen compared to air. By contrast, SOD-dependent NO emission from SHAM was about 50% lower in nitrogen than in air (Table 1).
Cell suspensions fed with HA or SHAM produced no measurable nitrite (not shown), or at least there was no nitrite accumulation above normal levels. In the cell-free system, continuous superoxide or hydrogen peroxide production were probably higher than with cells, eventually leading to a rapid formation of oxidation products. In fact, after a 30 min reaction of XOD+xanthine, 6.2% of the added hydroxylamine or 5.9% of SHAM had been converted to nitrite, whereas production of nitrite via SOD was below 1% (Table 1).
NO emission as measured by DAF-fluorescence
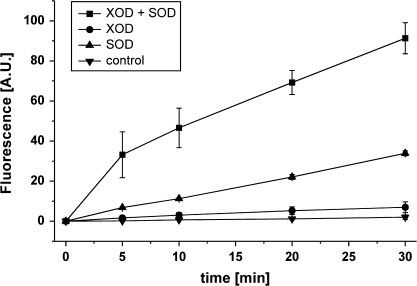
Because NO may react rapidly with oxygen or ROS to produce NO+ or N2O3 and thereby escape chemiluminescence detection, DAF-fluorescence was also used to measure NO. DAF does not react with NO itself, but with the oxidized nitrosonium cation (NO+) or with N2O3 (Planchet and Kaiser, 2006a). Here, data on DAF-fluorescence are only shown for the cell-free system, which has the least interference with DAF-2. To some extent, DAF fluorescence gave similar results as chemiluminescence. Upon HA addition to a solution of SOD alone, or of XOD+SOD, fluorescence increased continuously, indicating a steady production of NO+ and/or N2O3 and accumulation of the highly fluorescing triazole DAF-2 T (Fig. 4). The fluorescence increase with HA was higher with XOD+SOD than with either enzyme alone, consistent with the data from chemiluminescence (Table 1). However, while HA-dependent DAF-fluorescence with XOD alone remained very low, chemiluminescence indicated considerable NO production. To that extent, data from DAF and chemiluminescence were inconsistent.
Fig. 4.
NO emission from a cell-free system as indicated by DAF-2 fluorescence. All reactions were directly carried out in fluorescence cuvettes closed with a Teflon stopper. Conditions were as in Table 1, except that the total sample volume was only 1 ml. HA was 40 μM, XOD 0.05 U, SOD 1 U, and DAF-2 was 5 μM. As a control treatment 40 μl 1 mM HCl instead of HA was applied. Fluorescence at t=0 was set to zero, and the reaction was started by the addition of HA. Fluorescence is given as arbitrary units (A.U.) ±SD, n=3.
Discussion
Is ROS required for HA or SHAM oxidation?
In air, endogenous (nitrite-dependent) NO production by tobacco suspension cells was usually too low to be detectable by chemiluminescence or by DAF-fluorescence (Planchet and Kaiser, 2006b). Only under anoxia, where nitrite reduction is blocked, did nitrite accumulate and NO emission rates were very high (Planchet et al., 2005). Here, after the addition of external N-hydroxylamines, aerobic NO formation took place. Because NO was also produced by simple enzyme systems (see below), it seems probable that the two hydroxylamines were acting as substrates for oxidative NO formation. In order to investigate an oxygen requirement for NO formation further, nia30 mutant cells were used which do not accumulate nitrite and which, therefore, produce no NO under anoxia. Nia cells also produced NO from HA or SHAM with similar rates as WT cells indicating that nitrate reduction was not involved. Contrary to nitrate-dependent NO formation, the generation of NO from HA, with respect to SHAM, by nia cells was low or absent in nitrogen. Thus, NO production by cells from HA, and even more from SHAM somehow required oxygen. In aerated buffer solutions without any cells or enzymes, NO formation from HA or SHAM was very low (compare Table 1). Thus, at air levels of dissolved oxygen and at the applied low substrate concentrations, very little uncatalysed oxidation of hydroxylamines took place.
Because a cell-free system producing superoxide radicals (XOD+xanthine) also emitted NO from HA and, with lower rates, also from SHAM solutions, it was assumed that superoxide might be involved in the oxidation of hydroxylamines to NO. In the example with XOD+xanthine (Table 1), 1.41 nmoles of NO had been formed in 30 min, which equals 0.35% of the added HA. A much larger part of the added HA (6.22%) was oxidized further to nitrite. If all this nitrite had been formed via NO, actual NO production rates would be about 18-fold underestimated due to rapid NO oxidation.
On the other hand, direct addition of hydrogen peroxide (50 μM) to a cell suspension drastically increased NO emission from HA and from SHAM (Fig. 2), but caused no NO emission in a buffer solution without cells (Table 1). Thus, oxidation of HA (or SHAM) by H2O2 requires a catalyst. Continuous hydrogen peroxide production by GOD+glucose produced only a little NO (Table 1), but in this case the hydrogen peroxide concentration in the cell suspension was not measured and might have been lower than upon direct H2O2 addition.
Another hint for a participation of ROS in HA or SHAM oxidation came from the observation that the fungal elicitor cryptogein caused a strong increase in NO emission. Cryptogein is known reliably to produce an ‘oxidative burst’ (Foissner et al., 2000), but also a long-lasting production of ROS in tobacco (van Loon et al., 2008). It should be noted that cryptogein itself caused no or little NO emission from tobacco leaves and suspension cells (Planchet and Kaiser, 2006b). The other attempts to increase ROS formation, such as a 1 h pretreatment of cells with high concentrations (500 μM) of CdCl2, also produced some increase in NO formation from HA or SHAM, whereas treatment with the complex III inhibitor Myxothiazol had only a small effect under the applied conditions. All together, these data suggest that ROS are involved in the oxidation of hydroxylamines to NO, but currently it is not completely clear whether there is any preference for superoxide or hydrogen peroxide as oxidants.
It is a common observation that NO and ROS are generated in response to similar stimuli and with similar kinetics (Vanin et al., 2004). ABA-induced stomatal closure is just one example where this occurs (Neill et al., 2008). ROS and NO may rapidly react with each other, which might explain the rather large nitrite formation.
Peak rates of NO emission from cell suspensions were surprisingly similar over a wide range of substrate concentrations (Fig. 1), and that holds specifically for SHAM, where NO emission was almost the same at 5 μM and 2500 μM SHAM. It appears that the oxidation of hydroxylamines has high substrate (HA or SHAM) affinity and that the rate is eventually limited by oxidant production through cells.
The actual site of cellular NO production from HA or SHAM is not known at this point. Both substrates are able to penetrate membranes, just like NO and ROS. Indeed, ROS are important in cell wall metabolism, and the apoplast compartment could be enriched in ROS, which may be formed inside the cells, but also by PM-NADPH oxidase (for a review see Queval et al., 2008). Thus, oxidation of exogenous HA and/or SHAM may occur outside the cells, as well as inside.
A special case: SOD-catalysed NO formation
SOD addition to cells did not abolish NO formation from HA or SHAM, but rather stimulated it. A possible interpretation was that hydrogen peroxide produced in the SOD reaction could be responsible for the stimulation. However, a solution of SOD alone produced considerable NO emission from HA, without any superoxide-generating system being present (Table 1). In addition, SOD-catalysed NO production from HA was higher in nitrogen than in air (Table 1), whereas cellular NO production from HA or SHAM required oxygen, as shown. Thus, it is speculated that SOD might produce NO from HA through a dismutation-like reaction which would be oxygen independent. Subsequently, SOD might convert the nitroxyl anion to NO, as shown previously (Murphy and Sies, 1991). The higher rate in nitrogen than in air could also be due to partial NO oxidation in air, which would decrease the chemiluminescence signal. Although the actual mechanism of the reaction catalysed by SOD remains unclear, it seems improbable that endogenous SOD was involved in cellular NO production from HA or SHAM, since NO production by cells was oxygen-dependent, as shown.
SHAM as a source for NO
Although SHAM is certainly not a natural intermediate, the fact that it gives rise to NO production is nevertheless of potential importance. Previously, it has been shown that SHAM, together with Myxothiazol, inhibited anoxic NO formation from root segments in solution and from isolated mitochondria (Gupta et al., 2005). Treatment of aerobic cell suspensions with 2.5 mM SHAM caused a strong increase in DAF-fluorescence, which was not well understood at that time (Planchet and Kaiser, 2006a). The data presented here show that, at least in air, SHAM acts as a source for NO, which may explain the previously observed increased DAF-fluorescence of SHAM-treated cells.
Whether NO production from SHAM requires an initial hydrolysis of the hydroxylamine residue to free HA is not clear. In contrast to SOD-dependent NO production from HA, NO production from SHAM was lower in nitrogen than in air. This may indicate that the reaction with SHAM was not exactly the same as the reaction with HA. But whatever the mechanism of NO production from SHAM may be, the use of SHAM as an AOX inhibitor has to be considered with caution because of its partial conversion to NO by plant cells.
Are N-hydroxylamines natural compounds in plants?
While the above results may be interesting, their physiological relevance at this point is not clear. Originally, hydroxylamine was considered to be an intermediate in the reduction of nitrite to ammonia, but this possibility was discarded later (Cresswell et al., 1964). Hydroxylamine is also a putative intermediate in the NOS reaction (DeMaster et al., 1989), and was also considered to be a product in the reaction catalysed by nitrosoglutathione reductase (GSNOR, Jensen et al., 1998) GSNOR is a class III alcohol dehydrogenase which catalyses the NADPH-dependent reduction of GSNO to GSNOH, which may spontaneously form GSSG and hydroxylamine. In that case, a cyclic process might exist whereby GSNO is formed from NO, and hydroxylamine liberated by GSNOR could be converted back to NO and GSNO. However, it is not known whether GSNO may ever reach concentrations high enough to liberate micromolar concentrations of HA, and there are reports that HA is not actually formed at all during the reaction of GSNO reductase (Hedberg et al., 2003). Other potential sources for HA could be ammonia oxidation, for example, catalysed by ammonium mono-oxygenases, as in bacteria (Hooper et al., 1997). Clearly, further experiments are now required to find out whether any natural hydroxylamines can be formed under specific conditions by plants to serve as substrates for an endogenous oxidative NO formation.
Acknowledgments
This work was supported by the DFG, SFB 567. The skilled technical assistance of Maria Lesch is gratefully acknowledged. We also thank R Mendel (University of Braunschweig, Germany) for supplying seeds of the nia30 mutant. Cryptogein was a kind gift of Michel Ponchet (INRA, Unité Mixte de Recherche, Sophia-Antipolis, France).
Glossary
Abbreviations
- GOD
glucose oxidase
- HA
hydroxylamine
- NO
nitric oxide
- NOS
nitric oxide synthase
- SHAM
salicylhydroxamic acid
- SOD
superoxide dismutase
- XOD
xanthine oxidase
- DAF-2
diaminofluorescein
References
- Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signalling in plants. Annual Review of Plant Biology. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Crawford MN, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A. Response to Zemojtel et al.: plant nitric oxide synthase: back to square one. Trends in Plant Science. 2006;11:526–527. [Google Scholar]
- Cresswell CF, Hageman RH, Hewitt EJ, Hucklesby DP. The reduction of nitrate, nitrite and hydroxylamine to ammonia by enzymes from Cucurbita pepo L. in the presence of reduced benzyl viologen as electron donor. Biochemical Journal. 1964;94:40–53. doi: 10.1042/bj0940040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster EG, Raij L, Archer SL, Weir EK. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochemical and Biophysical Research Communications. 1989;163:527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. The Plant Journal. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. Journal of Experimental Botany. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- Hedberg JJ, Griffiths WJ, Nilsson SJF, Höög JO. Reduction of S-nitrosoglutathione by human alcohol dehydrogenase 3 is an irreversible reaction as analysed by electrospray mass spectrometry. European Journal of Biochemistry. 2003;270:1249–1256. doi: 10.1046/j.1432-1033.2003.03486.x. [DOI] [PubMed] [Google Scholar]
- Hooper AB, Terry KR. Hydroxylamine oxidoreductase of Nitrosomonas: production of nitric oxide from hydroxylamine. Biochimica et Biophysical Acta. 1979;571:12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- Hooper AB, Vannelli T, Bergmann DJ, Arciero DM. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie van Leeuwenhoek. 1997;71:59–67. doi: 10.1023/a:1000133919203. [DOI] [PubMed] [Google Scholar]
- Jensen DE, Belka GK, DuBois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochemical Journal. 1998;331:659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouichi O, Rosner G, Graf R. Nitric oxide generation from sodium nitroprusside and hydroxylamine in brain. NeuroReport. 1997;8:2229–2235. doi: 10.1097/00001756-199707070-00028. [DOI] [PubMed] [Google Scholar]
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Linsmaier EF, Skoog F. Organic growth factor requirements of tobacco. Tissue cultures. Physiologia Plantarum. 1965;18:100–127. [Google Scholar]
- Markert M, Carnal B, Mauël J. Nitric oxide production by activated human neutrophils exposed to sodium azide and hydroxylamine: the role of oxygen radicals. Biochemical and Biophysical Research Communications. 1994;189:1245–1249. doi: 10.1006/bbrc.1994.1364. [DOI] [PubMed] [Google Scholar]
- Modolell M, Eichmann K, Soler G. Oxidation of NG-hydroxyl-L-arginine to nitric oxide mediated by respiratory burst: an alternative pathway to NO synthesis. FEBS Letters. 1997;401:123–126. doi: 10.1016/s0014-5793(96)01451-2. [DOI] [PubMed] [Google Scholar]
- Müller AJ. Genetic analysis of nitrate reductase-deficient tobacco plants regenerated from mutant cells. Evidence for duplicate structural genes. Molecular and General Genetics. 1983;192:275–281. [Google Scholar]
- Murphy ME, Sies H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proceedings of the National Academy of Sciences, USA. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- Planchet E, Kaiser WM. Nitric oxide detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. Journal of Experimental Botany. 2006a;67:3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- Planchet E, Kaiser WM. NO as an intermediate in the cryptogein-induced hypersensitive response: a critical re-evaluation. Plant, Cell and Environment. 2006b;29:59–69. doi: 10.1111/j.1365-3040.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Planchet E, Gupta JK, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: rate-limiting factors and evidence for the involvement of mitochondrial electron transport. The Plant Journal. 2005;41:732–743. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- Queval G, Hager J, Gakière B, Noctor G. Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. Journal of Experimental Botany. 2008;59:135–146. doi: 10.1093/jxb/erm193. [DOI] [PubMed] [Google Scholar]
- Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany. 2002;53:1351–1356. [PubMed] [Google Scholar]
- Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. Journal of Biological Chemistry. 2004;279:24100–24107. doi: 10.1074/jbc.M312601200. [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Bakker PAHM, van der Heijdt WHW, Wendehenne D, Pugin A. Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Molecular Plant–Microbe Interactions. 2008;21:1609–1621. doi: 10.1094/MPMI-21-12-1609. [DOI] [PubMed] [Google Scholar]