Abstract
At least 50 species of birds are represented in 241 bird bones from five late Pleistocene and Holocene archaeological sites on New Ireland (Bismarck Archipelago, Papua New Guinea). The bones include only two of seabirds and none of migrant shorebirds or introduced species. Of the 50 species, at least 12 (petrel, hawk, megapode, quail, four rails, cockatoo, two owls, and crow) are not part of the current avifauna and have not been recorded previously from New Ireland. Larger samples of bones undoubtedly would indicate more extirpated species and refine the chronology of extinction. Humans have lived on New Ireland for ca. 35,000 years, whereas most of the identified bones are 15,000 to 6,000 years old. It is suspected that most or all of New Ireland’s avian extinction was anthropogenic, but this suspicion remains undetermined. Our data show that significant prehistoric losses of birds, which are well documented on Pacific islands more remote than New Ireland, occurred also on large, high, mostly forested islands close to New Guinea.
Oceanic islands are renowned as locations of extinction. Remote islands in the tropical Pacific, for example, have lost most of their endemic species of birds since humans arrived in the past several millennia (1–3). These losses are documented by studying bones from prehistoric cultural and noncultural sites. By contrast, the large island of New Guinea, standing at the western margin of Oceania, has an avifauna that shows very little evidence of anthropogenic extinction (4–6). Here, we present evidence that major losses of birds occurred prehistorically on New Ireland, a very large island close to New Guinea, and with a modern avifauna that is rich relative to the avifaunas on remote Pacific islands. This fact indicates that even large, mostly forested islands close to New Guinea, and very likely even New Guinea itself, have not been spared in the global extinction crisis that has followed the spread of humans around the world.
New Ireland is the second largest island in Papua New Guinea’s Bismarck Archipelago. It lies north and east of New Britain, which in turn lies just off New Guinea’s Huon Peninsula (Fig. 1). The modern resident landbird fauna of New Ireland consists of about 106 species, of which 69 are non-passerines (including 18 pigeons, 11 parrots, and 7 kingfishers) and 37 are passerines (4, 5, 7, 8). The avifauna of New Ireland is most similar to, and largely a subset of, that of New Britain, which is larger and less isolated from New Guinea and supports 125 species of landbirds (9–11).
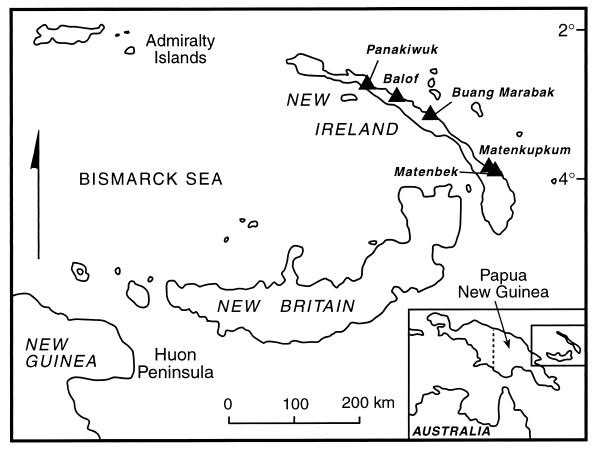
Figure 1.
This map of New Ireland shows the location of the five archaeological sites discussed in this paper.
Many aspects of avian biology in the Bismarcks are understood poorly. For example, new subspecies continue to be described (12, 13), and little is known about avian community ecology (e.g., relative abundance, habitat preference, foraging ecology, and species associations). In this paper, we report how the avifauna of New Ireland has changed in species composition since the first arrival of humans.
MATERIALS AND METHODS
The prehistoric bones (summarized in Table 1) belong to the Papua New Guinea National Museum and Art Gallery (Port Moresby, Papua New Guinea). We believe that most of the bird bones were accumulated by prehistoric peoples rather than non-human predators because of the clearly cultural context in which they were found and because the incidence of burning and breakage are typical of that in other cultural sites in Oceania. Identifications are based on comparisons (by D.W.S.) with modern skeletons from the Florida Museum of Natural History, New York State Museum (Albany, NY), the Smithsonian Institution (Washington, DC), and the University of Washington’s Burke Museum (Seattle, WA). For some taxa in Table 1, identification was limited by a lack of modern skeletons of appropriate species—a large problem with birds throughout Melanesia.
Table 1.
Birds from archaeological sites, New Ireland, Bismark Archipelago
| Species | NISP by site
|
|||||
|---|---|---|---|---|---|---|
| PAN | BAL | BUA | MKK | MBE | Total | |
| Pterodroma sp. (petrel)* | — | — | — | — | 2 | 2 |
| Egretta sacra (Pacific reef heron) | — | — | 1 | — | — | 1 |
| Nycticorax caledonicus (rufous night heron) | — | — | 1 | — | — | 1 |
| Haliastur indus (brahminy kite) | — | 1 | — | — | — | 1 |
| Accipiter novaehollandiae (variable goshawk) | — | 1 | — | — | — | 1 |
| Accipiter sp. 2 (medium goshawk)*†‡ | — | 3 | — | — | — | 3 |
| Accipiter sp. 3 (large goshawk)*†‡ | — | — | — | 2 | 2 | 4 |
| Megapodius eremita (Melanesian scrubfowl) | — | 7 | — | — | — | 7 |
| Megapodius new sp. (large megapode)† | 1 | 1 | — | — | — | 2 |
| Coturnix chinensis (king quail) | — | 1 | — | — | — | 1 |
| Coturnix cf. ypsilophorus (brown quail)* | — | — | — | 4 | — | 4 |
| Rallus/Gallirallus sp. (medium rail)‡ | 1 | — | — | — | — | 1 |
| Gallirallus philippensis (banded rail) | 2 | 1 | — | 7 | — | 10 |
| Gallirallus new sp. (New Ireland rail)† | 1 | 10 | — | 1 | 3 | 15 |
| Porzana cf. tabuensis (sooty crake)* | — | — | — | 2 | — | 2 |
| Poliolimnas cinereus (white-browed crake) | 1 | — | — | — | — | 1 |
| cf. Rallina tricolor (red-necked rail) | — | 1 | — | — | — | 1 |
| Amaurornis olivaceus (bush hen) | 1 | 1 | — | — | — | 2 |
| Porphyrio porphyrio (purple swamphen)* | — | — | — | 1 | — | 1 |
| Porphyrio new sp. (giant swamphen)† | 1 | 16 | — | 1 | — | 18 |
| Rallidae sp. (medium rail)§ | 1 | 7 | — | 1 | — | 9 |
| Ptilinopus sp. 1 (small fruit dove)‡ | 6 | 1 | — | 4 | — | 11 |
| Ptilinopus rivoli (white-bibbed fruit dove) | 2 | — | — | 2 | — | 4 |
| Ducula sp. (imperial pigeon)‡ | — | 1 | — | 1 | — | 2 |
| Columba sp. (pigeon)‡ | — | 2 | — | — | — | 2 |
| Macropygia amboinensis (brown cuckoo dove) | 2 | 1 | — | — | — | 3 |
| Macropygia nigrirostris (bar-tailed cuckoo dove) | — | 5 | — | 1 | — | 6 |
| Chalcophaps stephani (Stephan’s emerald dove) | 1 | — | — | — | — | 1 |
| Gallicolumba beccarii (Beccari’s ground dove) | — | 3 | — | — | — | 3 |
| Gallicolumba jobiensis (white-breasted ground dove) | 2 | — | — | — | — | 2 |
| Caloenas nicobarica (Nicobar pigeon) | — | 6 | — | 1 | — | 7 |
| Columbidae sp. (small dove)§ | 8 | — | — | 14 | — | 22 |
| Columbidae sp. (medium/large dove)§ | 2 | — | — | — | 1 | 3 |
| Charmosyna sp. (lorikeet)‡ | — | 1 | — | — | — | 1 |
| Lorius sp. (large lory)‡ | 3 | — | — | — | — | 3 |
| Loriinae sp. (medium lory)‡ | 3 | — | — | — | — | 3 |
| cf. Cacatua sp. (cockatoo)*† | — | 5 | — | — | — | 5 |
| Cacomantis variolosus (brush cuckoo) | 1 | — | — | — | — | 1 |
| Centropus sp. (coucal)‡ | — | 1 | — | — | — | 1 |
| Cuculidae sp. (another large cuckoo)‡ | — | 1 | — | — | — | 1 |
| Tyto sp. 1 (small tytonid owl)*† | — | — | — | 4 | — | 4 |
| Tyto sp. 2 (large tytonid owl)*† | — | 3 | — | 6 | — | 9 |
| Ninox cf. solomonis (Bismarck boobook owl) | — | 1 | — | — | 1 | 2 |
| Caprimulgus sp. (nightjar)‡ | — | — | — | 1 | — | 1 |
| Collocalia esculenta (glossy swiftlet) | 1 | 6 | — | 1 | — | 8 |
| Alcedo atthis (common kingfisher) | — | 1 | — | — | — | 1 |
| Aceros plicatus (Blyth’s hornbill) | — | 21 | 1 | — | — | 22 |
| Mino dumontii (yellow-faced myna) | — | 3 | — | — | — | 3 |
| Corvus sp. (large crow)*† | — | 2 | — | — | — | 2 |
| Passeriformes sp. 3 (large songbird)‡ | 1 | 1 | — | 1 | — | 3 |
| Passeriformes sp. 4 (medium/large songbird)‡ | 1 | — | — | 2 | — | 3 |
| Passeriformes sp. 5 (medium songbird)‡ | 9 | 4 | — | — | 1 | 14 |
| Passeriformes sp. 6 (small/medium songbird)‡ | 1 | — | — | — | — | 1 |
| Total bones | 52 | 119 | 3 | 57 | 10 | 241 |
| Total*† bones | 3 | 37 | 0 | 19 | 5 | 64 |
| Percentage of *† bones | 6 | 31 | 0 | 33 | 50 | 26 |
| Total species | 20 | 31 | 3 | 18 | 5 | 50 |
| Total *† species | 3 | 6 | 0 | 7 | 2 | 12 |
| Percentage of *† species | 15 | 19 | 0 | 39 | 40 | 24 |
BAL = Balof 1 (17 bones) + Balof 2 (102 bones); BUA = Buang Marabak; MBE = Matenbek; MKK = Matenkupkum; PAN = Panakiwuk. NISP = number of identified specimens. No species of Accipiter are counted for in “Total *† bones” or “Percentage of *† bones.”
Extant species, not recorded previously from New Ireland.
Extinct species.
Identification not precise enough to determine current status on New Ireland; assumed to be extant.
Not necessarily different from a taxon identified more precisely.
The Archaeological Sites.
The five sites yielding bird bones (Fig. 1) are caves or rock shelters developed in reef limestone. Our brief descriptions of the sites follow those given in refs. 14–23.
Panakiwuk is a rock shelter in a large eroded doline in northern New Ireland, ≈150 m above sea level and ≈4 km from either coast. No gardens are maintained near this forested site today, and, given the rugged terrain, it is likely that no gardens were maintained there in the past. Archaeological evidence suggests intermittent use of the shelter by humans moving between coasts and hunting in the forest. Cultural deposits reach an average depth of 1.45 m in the major excavation (3 m2), and two other test pits (1 × 1 m) bottomed out at 60–80 cm. Panakiwuk was first occupied at 15,000–14,000 B.P. [approximate age, based on calibrated radiocarbon (14C) dates reported in detail in refs. 14–23], but occupation seems to have been sporadic. The major archaeological deposits date from 10,000 B.P. to 8,000 B.P. The site was abandoned for most of the Holocene epoch, with minor reoccupation after 1,600 B.P.
Balof 1 and 2 are shelters formed beneath the edges of a sinkhole some 2.7 km from the east coast. The deposits at Balof 1 are ≈1 m deep and substantially reworked by oven pits dug in recent centuries. Deposits at Balof 2 are ≈2 m deep and relatively undisturbed, as corroborated by a sequence of 14C dates. Both sites are as old as 14,000 B.P. but were occupied only intermittently. Large numbers of bones of the culturally important hornbill (Aceros plicatus; confined to Pleistocene levels) suggest that activities at Balof were aimed at particular resources. The differences in avifaunas between Balof and other sites (Table 1) also may relate to Balof’s location at the edge of rugged limestone country that probably has been mostly forested throughout occupation of the site.
Buang Merabak is a limestone cave in central New Ireland, ≈200 m from the east coast and perhaps 50 m above sea level. An excavation (1 × 1 m) reached bedrock at a ≈1.6-m depth. Sparsely scattered Holocene material seems to be disturbed, judging from the 14C dates. The underlying Pleistocene deposits are largely intact. Although further excavation and dating are required, initial studies of the site indicate early, sparse occupation after 32,000 B.P., periodic abandonments, and increased occupation in the terminal Pleistocene epoch.
Matenkupkum is a large limestone cave in southern New Ireland, only ≈30 m inland and ≈15 m above sea level. Although sea level would have been ≈120 m lower at the last glacial maximum (18,000 B.P.), the cave’s distance from the sea would not have been much greater because of very steep submarine contours along this coast. Immediately behind Matenkupkum are extensive grasslands, and further inland are limestone karst and volcanic mountains covered with rainforest. The grassland vegetation is probably a disclimax vegetation, as the soils here are well suited for tree crops and gardens, as well as rainforest. Thus, when first occupied, Matenkupkum may have been surrounded by rainforest. Cultural deposits indicate intermittent occupation from 35,000 to 20,000 B.P., when the site was abandoned until 16,000 B.P. The most intensive occupation was from 14,000 to 10,000 B.P.
Matenbek is another large limestone cave in the same uplifted coral terrace as Matenkupkum, and ≈70 m south of it. The original cave entrance has collapsed and may well overlie deeper and older deposits than those excavated, because the oldest levels in Matenkupkum were at the entrance. The Matenbek faunal sample came from two test pits (80 × 80 cm) in an area that may have been 7–8 m in from the original drip line. There, 90 cm of archaeological deposits are sandwiched beneath 45 cm of in-washed soils and above 30 cm of sterile sands that cover the cave floor. The earlier of two phases of occupation at Matenbek range from 20,000 to 18,000 B.P., after which the site was abandoned and then reoccupied from 9,000 to 6,000 B.P. Dates on the in-washed soils suggest that the entrance collapsed at 2,000 B.P.
At all five sites, the stone artifact assemblage consists mainly of simple unretouched flakes, which, on average, are much smaller at Panakiwuk and Balof than at Matenkupkum and Matenbek, where flaked river cobbles predominate. Shell tools also occur throughout the sequence. Obsidian imported from New Britain first appears in the sites as early as 19,000 B.P. (Matenbek) and as late as 7,000 B.P. (Balof 2). Pottery shards occur in small numbers only in the uppermost deposits (<3,000 B.P.).
Although most sites had some noncultural deposits at their bases, these strata produced only bones of indigenous rodents. Bones in the earliest human layers are mostly of indigenous rats, bats, reptiles, and occasionally birds. Species of mammals brought to New Ireland by humans appear at the sites only after 19,000 B.P. These are phalangers (Phalanger orientalis, Spilocuscus maculatus), wallabies (Thylogale brunii), rats (Rattus praetor, Rattus exulans), pigs (Sus scrofa), and dogs (Canis familiaris), of which only P. orientalis has been recorded from the Pleistocene strata (17, 24). Shell midden and fish bone occur throughout the sequence and in the three coastal sites as well.
Remarks on Extinct/Extirpated or Noteworthy Species of Birds.
Represented by a coracoid and carpometacarpus, a petrel (Pterodroma sp.) belongs to the subgenus Pseudobulweria, consisting of two poorly known species (Pterodroma rostrata and Pterodroma becki) with very localized modern ranges in Oceania. Based on their relatively large size, the two bones from Matenbek may represent the Tahiti petrel (P. rostrata), known today as a breeding species in East Polynesia, with offshore records in the Bismarcks.
Pending availability of skeletons of more Melanesian species of Accipiter, all that can be concluded about Accipiter sp. 2 (ulna, carpometacarpus, tibiotarsus) and Accipiter sp. 3 (coracoid, scapula, humerus, carpometacarpus) is that at least one of these species, both of which are larger than A. novaehollandiae and too disparate in size to represent the same species, no longer occurs on New Ireland. The only species of Accipiter on New Ireland today are the widespread A. novaehollandiae and the smaller Accipiter brachyurus. A recent sight record suggests that the much larger Accipiter meyerianus also may reside on New Ireland (B. M. Beehler, personal communication).
Megapodius new sp. (scapula, tarsometatarsus) is a very large species in the size range of Megapodius molistructor of New Caledonia and Tonga (25–27).
Coturnix ypsilophorus (coracoid, two humeri, ulna) occurs on mainland New Guinea but not in the Bismarcks. Much larger than C. chinensis, this quail prefers grasslands and thus may have never been abundant in New Ireland.
An undescribed flightless rail (Gallirallus new sp.) is well represented (15 bones from four sites). It probably was endemic to New Ireland, where the widespread, volant, more gracile G. philippensis lives today. The undescribed rail is referred to as Gallirallus rather than as other genera of rails in Oceania, following characters in ref. 28. A vast radiation of flightless species of Gallirallus once occupied tropical Pacific islands of the Ryukyus, Marianas, and Bismarcks eastward at least to the Marquesas. Only seven species of estimated hundreds still survive [on Okinawa, Guam (captivity only), New Britain (generic status unconfirmed), New Georgia Group (Solomon Islands), New Caledonia, Lord Howe, and New Zealand; refs. 3 and 29].
Another undescribed rail, Porphyrio new sp., is known from 19 bones (14 skeletal elements). This huge flightless swamphen is much larger than the extinct Porphyrio paepae of the Marquesas (30) and taller but less stout than Porphyrio mantelli of New Zealand. Porphyrio new sp. even exceeds in size the extinct Porphyrio kukwiedei of New Caledonia (25).
Two other rails, Porzana tabuensis (humerus, femur) and Porphyrio porphyrio (tarsometatarsus), are extant and widespread in the Papuan region but had not been recorded previously on New Ireland. The fact that these rails have not been recorded on New Ireland may be only a sampling artifact of modern surveys.
The two coracoids, scapula, femur, and tibiotarsus of cf. Cacatua sp. from Balof are much larger than in the parrots known on New Ireland, the largest of which is Eclectus roratus. These bones differ from those of E. roratus in many qualitative features. In size and proportion, they resemble the bones of several species of Cacatua, of which the nearest population to New Ireland is that of Cacatua [galerita] ophthalmica on New Britain (4, 31). Much larger than the bones of Cacatua ducorpsi of the Solomon Islands, the Balof specimens probably represent an undescribed species or subspecies in the C. galerita species group, the description of which awaits the availability of more comparative skeletons.
No species of Tyto have been recorded from New Ireland, although five species of Tyto are known from Papua New Guinea (4, 6). Bones of the small Tyto sp. 1 (ulna, carpometacarpus, two tarsometatarsi) are about the size of those in Tyto alba (New Guinea and some offshore islands), Tyto aurantia (New Britain), and Tyto capensis (local in New Guinea and Australia). The femur, two tibiotarsi, and six tarsometatarsi of Tyto sp. 2 from Balof and Matenkupkum are much larger than those of T. alba, T. aurantia, and T. capensis. Tyto sp. 2 would seem to be the size of Tyto novaehollandiae (specimens not available); among congeners, T. novaehollandiae is exceeded in size perhaps by only Tyto tenebricosa of New Guinea, Japen, and Australia (32). In Papua New Guinea today, T. novaehollandiae occurs only in lowland forests and savannas of the southern Fly River region and on Manus Island. A large species of Tyto also has been recorded from an archaeological site on Mussau (33). At one time, T. novaehollandiae may have inhabited much of the Bismarck Archipelago.
The humerus and tibiotarsus of Corvus sp. are larger than in Corvus orru, the only corvid on New Ireland. Specimens of Corvus meeki (Bougainville, Buka, Shortlands; see refs. 34 and 35) are not available. The New Ireland bones also are larger than in Corvus woodfordi (Solomon Islands), Corvus bennetti (Australia), Corvus coronoides (Australia), and Corvus macrorhynchos (Philippines). They may represent Corvus tristis, confined today to mainland New Guinea and nearby satellite islands, or a closely related form.
Passeriformes sp. 3–6 represent four different sizes of songbirds that are smaller than Mino and Corvus but larger than, for example, Nectarinia, Dicaeum, and Zosterops. These 21 bones probably represent more than four species.
Taxonomic Biases in the Bone Record.
Dominated by rails and pigeons (Table 2), New Ireland’s prehistoric landbird assemblage resembles those of Holocene (<10,000 B.P.) cultural sites as far away as the Marquesas Islands (3, 36, 37). The prehistoric preference for hunting and eating rails and pigeons has been especially devastating to the former, with nearly all endemic, flightless species now extinct (3). Of seven other families with ≥4% of the total bird bones, only the megapodes, parrots, and passerines occur regularly and in good numbers in prehistoric sites north and east of the Bismarcks.
Table 2.
Family-level summary of bird bones from New Ireland
| Family | Percentage of NISP by site
|
|||||
|---|---|---|---|---|---|---|
| PAN | BAL | BUA | MKK | MBE | Total | |
| Procellariidae (petrels) | — | — | — | — | 20 | 1 |
| Ardeidae (herons) | — | — | 67 | — | — | 1 |
| Accipitridae (hawks) | — | 3 | — | 4 | 20 | 4 |
| Megapodiidae (megapodes) | 2 | 7 | — | — | — | 4 |
| Phasianidae (quail) | — | 1 | — | 7 | — | 2 |
| Rallidae (rails, crakes, swamphens) | 15 | 30 | — | 23 | 30 | 25 |
| Columbidae (pigeons, doves) | 44 | 16 | — | 40 | 10 | 28 |
| Psittacidae (parrots, lories, cockatoos) | 12 | 5 | — | — | — | 5 |
| Cuculidae (coucals, cuckoos) | 2 | 2 | — | — | — | 1 |
| Tytonidae (barn owls) | — | 3 | — | 18 | — | 6 |
| Strigidae (typical owls) | — | 1 | — | — | 10 | 1 |
| Caprimulgidae (nightjars) | — | — | — | 2 | — | <1 |
| Apodidae (swifts) | 2 | 5 | — | 2 | — | 4 |
| Alcedinidae (kingfishers) | — | 1 | — | — | — | <1 |
| Bucerotidae (hornbills) | — | 18 | 33 | — | — | 9 |
| Passeriformes (songbirds) | 24 | 8 | — | 5 | 10 | 11 |
| Total NISP | 52 | 119 | 3 | 57 | 10 | 241 |
Abbreviations are defined in Table 1. All passerine families are combined.
Chronology and Extent of Extinction.
Prehistoric bone assemblages from Remote Oceania typically indicate the loss of 50–90% of the species of native landbirds (1–3). The lower proportion (24%) of extinct/extirpated species from New Ireland may be related to the presence of indigenous rodents (Melomys rufescens and Rattus mordax sanila). In Remote Oceania, birds evolved without native mammalian predators, leading to naïveté and vulnerability to predation when humans and associated nonnative mammals arrived (38). Evolving alongside native rodents exposed New Ireland’s birds to potential predation from terrestrial animals in prehuman times. Nevertheless, the arrival of humans to New Ireland led to the prehistoric introduction of macropods, pigs, dogs, two species of cuscuses, and two more species of rats (17), any of which could have had a negative impact on birds.
Deposits dated to the Pleistocene epoch (>10,000 B.P.) account for 56% of the bird bones from New Ireland. Unlike sites in Remote Oceania with continuous and rich deposition of bird bones (39, 40), the New Ireland record is too spotty to determine precisely when various species were lost. Only 2% of the bird bones identified from the five sites are more than 15,000 years old (Table 3), at which time humans already had occupied New Ireland for 20,000 years. Which species may have been lost during those first 20 millennia remain unknown.
Table 3.
Stratigraphic analysis of bird bones from prehistoric sites on New Ireland
| Site age, B.P. | NISP
|
Percentage of NISP
|
||
|---|---|---|---|---|
| Extant species | Extinct species | Extant species | Extinct species | |
| Panakiwuk | ||||
| <1,600 | 15 | 1 | 94 | 6 |
| 8,000–10,000 | 21 | 0 | 100 | 0 |
| 13,000 | 5 | 0 | 100 | 0 |
| 15,000 | 8 | 2 | 80 | 20 |
| Balof | ||||
| <1,000 | 26 | 1 | 96 | 4 |
| 1,000–5,000 | 7 | 7 | 50 | 50 |
| 5,000–10,000 | 11 | 8 | 58 | 42 |
| 10,000–14,000 | 38 | 21 | 64 | 36 |
| Buang Marabak | ||||
| 20,000 | 2 | 0 | 100 | 0 |
| 32,000 | 1 | 0 | 100 | 0 |
| Matenkupkum | ||||
| >10,000 | 38 | 19 | 67 | 33 |
| Matenbek | ||||
| <2,000 | 1 | 1 | 50 | 50 |
| 6,000 | 3 | 1 | 75 | 25 |
| 6,000–9,000 | 0 | 3 | 0 | 100 |
| 18,000–20,000 | 1 | 0 | 100 | 0 |
| Total | ||||
| <10,000 | 84 | 22 | 79 | 21 |
| >10,000 | 93 | 42 | 69 | 31 |
The analysis is based on data presented in Table 1. NISP = number of identified specimens.
Extinct/extirpated species make up 31% of Pleistocene bones and 21% of Holocene bones (Table 3). Among the three sites with >50 bird bones, Panakiwuk has the fewest bones of extinct species, but these date primarily to 10,000–8,000 B.P., suggesting that much extinction already had taken place by that time. For the two flightless rails (Gallirallus new sp., Porphyrio new sp.), 32 of 34 bones are from Pleistocene strata, suggesting that the two isolated bones may be out of context. However, three other extinct/extirpated species have records at ≤6,000 B.P. (Table 4), suggesting that climate and vegetation changes during the Pleistocene/Holocene transition were not important factors in their extinction. On the other hand, if Lapita peoples in Remote Oceania are any indication, late Holocene horticulturalists may have been just as destructive to bird life as the late Pleistocene hunter/gatherers.
Table 4.
Latest records of extinct or extirpated species of birds in prehistoric sites on New Ireland
| Species | Latest record, B.P. | Number of sites/bones |
|---|---|---|
| Pterodroma sp. | 6,000 | 1/2 |
| Megapodius new sp. | 10,000–14,000 | 2/2 |
| Coturnix cf. ypsilophorus | >10,000 | 1/4 |
| Gallirallus new sp. | <2,000 | 4/15 |
| Porzana cf. tabuensis | >10,000 | 1/2 |
| Porphyrio porphyrio | >10,000 | 1/1 |
| Porphyrio new sp. | <1,600 | 3/18 |
| cf. Cacatua sp. | 1,000–5,000 | 1/5 |
| Tyto sp. 1 | >10,000 | 1/4 |
| Tyto sp. 2 | 5,000–10,000 | 2/9 |
| Corvus sp. | 1,000–5,000 | 1/2 |
Note that the latest records do not necessarily indicate the time of extinction.
Species Richness.
The bird bone samples are too small to estimate New Ireland’s species richness at any one time with much certainty. We have made the very conservative assumption that all taxa marked with a double dagger (‡) in Table 1 represent extant, resident species on New Ireland. If this assumption holds true, then bones of 38 of New Ireland’s 106 current resident species of landbirds were recovered from the archaeological sites, as well as the bones of 12 species no longer occurring on the island. An archaeological record fully representative of New Ireland’s late Pleistocene/early Holocene landbirds probably would include at least 30 species that no longer live on the island. Thus, in the absence of human impact, the landbirds of New Ireland probably would number about 140 species today. A similar number probably existed there in the late Pleistocene.
We believe that few if any of New Ireland’s current landbirds colonized the island within the past several millennia. According to equilibrium theory, the avifauna of New Ireland should have undergone postglacial “relaxation” (41, 42). Because most of the extinct/extirpated taxa belong to families known to be especially vulnerable to human activities, we favor human impact over relaxation as the primary cause of faunal depletion on New Ireland.
CONCLUSIONS
Humans colonized the Bismarck Archipelago and Solomon Islands by 35,000–30,000 years ago (43–45). Thus, unlike in Remote Oceania, where human arrival in the late Holocene was clearly devastating to indigenous birds (3), the Lapita peoples who moved across the Bismarcks and Solomons about 3,500 years ago found a flora and fauna that already had withstood tens of millennia of human activity (33, 46). To provide perspective on how the rich avifaunas of this region have changed over the long course of human occupation, more islands in the Bismarcks and Solomons should be surveyed for bone deposits of prehistoric birds. The limited evidence from New Ireland hints that even New Guinea, occupied by humans at least as long as Island Melanesia (47) and prized since its “discovery” by Europeans for being so wild and forested, has suffered as yet undetected losses of birds since the arrival of humans.
Acknowledgments
We thank G. Petri and M. I. Williams for their help with the manuscript preparation and B. M. Beehler, J. M. Diamond, H. B. Freifeld, P. V. Kirch, A. W. Kratter, and M. Spriggs for helpful comments. Field research was sponsored by Australian Research Council grants to J.P.W. (Balof) and to C. Gosden and J.A. (Matenbek). Panakiwuk, Matenkupkum, and Buang Merabak were excavated as part of the Lapita Homeland Project, with major funding from National Geographic Society Grant 3000-84 and from the Research School of Pacific Studies, Australian National University. D.W.S.’s laboratory research was funded by National Science Foundation Grants BSR-8607535 and EAR-9714819 and University of Florida Division of Sponsored Research Grant RDA 1-23 95-96).
References
- 1.Olson S L, James H F. Ornithol Monogr. 1991;45:1–88. [Google Scholar]
- 2.James H F, Olson S L. Ornithol Monogr. 1991;46:1–88. [Google Scholar]
- 3.Steadman D W. Science. 1995;267:1123–1131. doi: 10.1126/science.267.5201.1123. [DOI] [PubMed] [Google Scholar]
- 4.Coates B J. The Birds of Papua New Guinea. Vol. 1. Alderley, Australia: Dove; 1985. [Google Scholar]
- 5.Coates B J. The Birds of Papua New Guinea. Vol. 2. Alderley, Australia: Dove; 1985. [Google Scholar]
- 6.Beehler B M, Pratt T K, Zimmerman D A. Birds of New Guinea. Princeton: Princeton Univ. Press; 1986. [Google Scholar]
- 7.Hartert E. Novit Zool. 1925;32:115–136. [Google Scholar]
- 8.Beehler B M. Emu. 1978;78:65–70. [Google Scholar]
- 9.Diamond J M. Proc Natl Acad Sci USA. 1970;67:529–536. doi: 10.1073/pnas.67.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond J M. Proc Natl Acad Sci USA. 1970;67:1715–1721. doi: 10.1073/pnas.67.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond J M. Condor. 1971;73:481–483. [Google Scholar]
- 12.Salomonsen F. Steenstrupia (Zool Mus Univ Copenhagen) 1972;2:183–189. [Google Scholar]
- 13.Diamond J M. Emu. 1989;89:58–60. [Google Scholar]
- 14.Downie J E, White J P. Rec Aust Mus. 1978;31:762–802. [Google Scholar]
- 15.Allen J, Gosden C, Jones R, White J P. Nature (London) 1988;331:707–709. doi: 10.1038/331707a0. [DOI] [PubMed] [Google Scholar]
- 16.Allen J, Gosden C, White J P. Antiquity. 1989;63:548–561. [Google Scholar]
- 17.Flannery T F, White J P. Natl Geogr Res Explor. 1991;7:96–113. [Google Scholar]
- 18.Gosden C, Robertson N. Occas Pap Prehist Aust Natl Univ. 1991;20:20–45. [Google Scholar]
- 19.Marshall B, Allen J. Occas Pap Prehist Aust Natl Univ. 1991;20:59–91. [Google Scholar]
- 20.White J P, Flannery T F, O’Brien R, Hancock R V, Pavlish L. Occas Pap Prehist Aust Natl Univ. 1991;20:46–58. [Google Scholar]
- 21.Barton H, White J P. Asian Perspect. 1993;32:169–181. [Google Scholar]
- 22.Rosenfeld A. Bull Pac Prehist Assoc. 1997;16:213–224. [Google Scholar]
- 23.Leavesley M, Allen J. Archaeol Oceania. 1998;33:63–82. [Google Scholar]
- 24.Flannery T F. Mammals of the South-West Pacific & Moluccan Islands. Ithaca, NY: Cornell Univ. Press; 1995. [Google Scholar]
- 25.Balouet J C, Olson S L. Smithson Contrib Zool. 1989;469:1–38. [Google Scholar]
- 26.Steadman D W. Proc Biol Soc Washington. 1989;102:537–552. [Google Scholar]
- 27.Steadman, D. W. (1999) Zool. Verh., in press.
- 28.Steadman D W. Pac Sci. 1987;40:27–43. [Google Scholar]
- 29.Diamond J M. Auk. 1991;108:461–470. [Google Scholar]
- 30.Steadman D W. Proc Biol Soc Washington. 1988;101:162–170. [Google Scholar]
- 31.Hartert E. Novit Zool. 1926;33:122–145. [Google Scholar]
- 32.Rich P V, McEvey A R, Walkley R. Emu. 1978;78:88–90. [Google Scholar]
- 33.Steadman D W, Kirch P V. Emu. 1998;98:13–21. [Google Scholar]
- 34.Schodde R. C S I R O (Aust Div Wildl Res) Tech Pap. 1977;34:1–103. [Google Scholar]
- 35.Hadden D. Wau Ecol Inst Handb. 1981;8:1–107. [Google Scholar]
- 36.Steadman D W. Contrib Sci Nat Hist Mus Los Angeles Cty. 1992;36:329–348. [Google Scholar]
- 37.Steadman D W. Proc Nat Acad Sci USA. 1993;90:818–822. doi: 10.1073/pnas.90.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkinson I A E. Int Counc Bird Preserv Tech Publ. 1985;3:35–81. [Google Scholar]
- 39.Kirch P V, Steadman D W, Butler V L, Hather J, Weisler M I. Archaeol Oceania. 1995;30:47–65. [Google Scholar]
- 40.Steadman D W, Rolett B. J Archaeol Sci. 1996;23:81–94. [Google Scholar]
- 41.Diamond J M. Proc Natl Acad Sci USA. 1972;69:3199–3203. doi: 10.1073/pnas.69.11.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diamond J M. In: Proceedings of the Sixteenth International Ornithological Congress. Frith H J, Calaby J H, editors. Carberra, Australia: Aust. Acad. Sci.; 1976. pp. 616–628. [Google Scholar]
- 43.Wickler S, Spriggs M. Antiquity. 1988;62:703–706. [Google Scholar]
- 44.Pavlides C, Gosden C. Antiquity. 1994;68:604–610. [Google Scholar]
- 45.Spriggs M. The Island Melanesians. Oxford: Blackwell; 1997. [Google Scholar]
- 46.Kirch P V. The Lapita Peoples. Oxford: Blackwell; 1996. [Google Scholar]
- 47.Groube L, Chappell J, Muke J, Price D. Nature (London) 1986;324:453–455. doi: 10.1038/324453a0. [DOI] [PubMed] [Google Scholar]