Abstract
Rice coleoptiles, renowned for anoxia tolerance, were hypoxically pretreated, excised, ‘healed’, and then exposed to a combination of anoxia and pH 3.5. The putative acid load was confirmed by net effluxes of K+ to the medium, with concurrent net decreases of H+ in the medium, presumably mainly due to H+ influx. Yet the coleoptiles survived the combination of anoxia and pH 3.5 for at least 90 h, and even for at least 40 h when the energy crisis, inherent to anoxia, had been aggravated by supplying the coleoptiles with 2.5 mM rather than 50 mM glucose. Even in the case of coleoptiles with 2.5 mM glucose, an accumulation ratio of 6 for Cl– was attained at 4 h after the start of re-aeration, implying plasma membrane integrity was either maintained during anoxia, or rapidly restored after a return to aerated conditions. Cytoplasmic pH and vacuolar pH were measured using in vivo 31P nuclear magnetic resonance spectroscopy with 50 mM glucose in the basal perfusion medium. After 60 h in anoxia, external pH was suddenly decreased from 6.5 to 3.5, but cytoplasmic pH only decreased from 7.35 to 7.2 during the first 2 h and then remained steady for the next 16 h. During the first 3 h at pH 3.5, vacuolar pH decreased from 5.7 to 5.25 and then stabilized. After 18 h at pH 3.5, the initial values of cytoplasmic pH and vacuolar pH were rapidly restored, both upon a return to pH 6.5 while maintaining anoxia and after subsequent return to aerated solution. Summing up, rice coleoptiles exposed to a combination of anoxia and pH 3.5 retained pH regulation and cellular compartmentation, demonstrating tolerance to anoxia even during the acid load imposed by exposure to pH 3.5.
Keywords: Acid load, anoxia, cytoplasmic pH, energy requirements, in vivo NMR, Oryza sativa, pH regulation, proton fluxes, rice coleoptiles, vacuolar pH
Introduction
Few studies have addressed the response of anoxic tissues to acid loads, imposed either by exposure to low pH or by supplying weak organic acids that tend to acidify the cytoplasm due to influx of the undissociated species (Guern et al., 1991). Such acid loads can arise in flooded soils by the accumulation of organic acids and high CO2 (Ponnamperuma, 1984; Greenway et al., 2006). Root tips, of intact maize and wheat plants, were injured earlier when exposed to anoxia at pH 4.0 rather than at pH 5–6 (maize, Xia and Roberts, 1996; wheat, Waters et al., 1991). The maize roots, however, were in 2 mM citrate buffer (Xia and Roberts, 1996), so the uptake of citric acid at low pH would intensify the acid load, while high levels of citrate in the cytoplasm might interfere with metabolism. There were no such problems in the wheat experiments, since no buffers were used but pH was steady owing to a high ratio of solution volume to tissue (Waters et al., 1991). Neither maize nor wheat root tips are particularly tolerant of anoxia; hence in the present study anoxia-tolerant rice coleoptile tips were used.
Cytoplasmic acidosis is one putative cause of anoxia intolerance (Xia and Roberts, 1996; Greenway and Gibbs, 2003; Felle, 2005). Cytoplasmic pH of 20 mm root tips of intact maize, which had been hypoxically pretreated and then exposed to anoxia and pH 4.5, decreased from 7.5 to 7.0 after the first 4.5 h and continued to decline to 6.2 during the next 3 h (Xia and Roberts, 1996). Similarly, even at near neutral external pH, there is often a correlation between cytoplasmic acidosis and severe injury (e.g. in maize root tips, Xia et al., 1995). Such correlations, however, cannot establish whether the cause of injury is the acidosis, or whether the decreases in cytoplasmic pH are a consequence of a slow deterioration, leading eventually to breakdown of trans-membrane gradients caused, among others, by a loss of membrane integrity (Felle, 2005). As persuasively argued by Felle (2005), decreases in cytoplasmic pH demonstrated for several types of plant tissues upon imposition of anoxia are not necessarily due to a lack of pH regulation, but can be viewed as a new set point consistent with an altered metabolism.
The present experiments used 7–10 mm tips of anoxia-tolerant rice coleoptiles supplied with exogenous sugar; these survive anoxia at a pH of 6.5 for at least 120 h (Huang et al., 2005). Survival was tested and net ion fluxes were measured during up to 90 h exposure of rice coleoptile tips to pH 3.5 as compared with pH 6.5. Using in vivo 31P-nuclear magnetic resonance (NMR) spectroscopy, cytoplasmic and vacuolar pH were also measured during anoxia: between 60–90 h at pH 6.5 and between 60–78 h after transfer to pH 3.5 with a subsequent return to pH 6.5 at 78 h and eventually to aerated conditions. Rice coleoptiles tolerated the combination of pH 3.5 and anoxia, even when ethanolic fermentation was reduced by supplying only a low concentration of exogenous glucose; pH regulation and cellular compartmentation were retained during anoxia, and rapid rates of ion net uptake resumed upon re-aeration.
Materials and methods
Preparation of coleoptile tips and incubation solutions
Seeds of an anoxia-tolerant rice cultivar (Oryza sativa L. cv. Amaroo) were dehulled, surface-sterilized in 10% NaClO for 10 min, washed thoroughly in deionized water, and sown on mesh submerged in aerated solution. The solution contained 0.5 mM MES, 0.3 mM CaSO4 and 0.2 mM Ca(OH)2, so that pH was 6.5. At 48 h, the seedlings were given a hypoxic pre-treatment of 0.028 mM O2 (2 kPa). At 66 h, 7–10 mm tips of coleoptiles were excised (Huang et al., 2003, 2005). Excised coleoptile tips were placed in fresh solution of the composition described above, plus 20 mM glucose and 50 mg l−1 ampicillin or 10 mg l−1 carbenicillin. Hypoxia was continued for 5 h to ensure ‘healing’ and then anoxia was imposed. The solution (henceforth called ‘basal medium’) contained Ca2+ 0.5; NH4+ 0.1; NO3− 0.2; MES 0.5 (all in mM), and 50 mg l−1 ampicillin or 10 mg l−1 carbenicillin. SO42− made up the balance between cations and anions. When the experiments included re-aeration, Cl– at 0.3 mM was added to this basal medium, to test for energy-dependent anion uptake, as vigorous uptake would indicate no permanent injury. Glucose and K+ concentrations are given in the captions of the tables and figures, since these solutes varied depending on the experiments and treatments. Important features were (i) glucose was usually at 50 mM and (ii) in experiments that net K+ fluxes were determined, K+ was at 0.25 mM, slightly above the Vmax of the high affinity uptake system, to enhance accuracy of the measurements based on changes in concentrations in the incubation medium.
Treatments
Most experiments on ion fluxes and survival were done in 10 ml Thunberg Tubes, as in Huang et al. (2003). Coleoptile tips were pretreated in anoxia at pH 6.5 prior to perturbations to pH 3.5 with anoxia continued. Recoveries upon transfer back to pH 6.5 with anoxia continued, and subsequently re-aeration, were also assessed. Thus, the main comparisons during anoxia were between coleoptile tips at pH 6.2–6.7 and at pH 3.5–3.7. At pH 6.2–6.7, solutions were buffered with MES at 0.2 mM or 1.0 mM. At pH 3.5–3.7, the solutions contained no MES, but 2.0 mM β-alanine (pKa of 3.55; Dawson et al., 1969). pH was adjusted using H2SO4. The coleoptiles tolerated this exposure to 2.0 mM β-alanine under anoxia for prolonged periods. Whether β-alanine would have any effect on metabolism, or transport, of L-alanine, is unknown.
Analytical methods
Net H+ changes in the medium were measured by titration within 3 h after collecting the samples, using reference solutions taken 5 min after the change from pH 6.5 to pH 3.5. All storage vessels were incubated in the appropriate pH solutions before use. Possible leakage/extrusion of organic solutes to the medium was also assayed; solutions were condensed ∼15 times and then analysed for organic acids (Cawthray, 2003) and amino acids (EZ faast from Phomenex r). No organic or amino acids were detected, supporting that the titrations could be interpreted as net changes in H+. Net uptake or loss of K+ and Cl– was determined from changes in external K+ and Cl–, as described in Huang et al. (2005). Ethanol production rates were also measured as described in Huang et al. (2005).
Measurement of cytoplasmic and vacuolar pH by in vivo 31P-NMR spectroscopy
Perfusion system:
The perfusion system was according to Fan et al. (1986), with the supply flask containing 2.1 l of the incubation solution, purged with high-purity N2. Tygon® PVC tubing was used and the flask and pump system were within a box flushed with argon. Dissolved O2, measured using a Clark-type electrode at the entry point of the NMR tube, did not exceed 0.7 μM, the detection limit of the O2 electrode. To avoid ‘out gassing’ of N2 from the solution, the perfusion medium in the stock flask was maintained at 30 °C and the NMR-probe was at 25 oC. The flow rate was 30 ml min−1.
Plant material and incubation:
Seventy-to-ninety, hypoxically pretreated, excised, coleoptile tips, with a total fresh weight of 500–600 mg, were placed into a 10 mm diameter NMR tube and first exposed for 60 h to anoxia using the perfusion system (at 30 oC), before the tube was inserted into the NMR spectrometer, while perfusion continued. The perfusion medium (composition described above, see ‘basal medium’), was at 0.4 mM K+, the antibiotic was 10 mg l−1 carbenicillin, and was buffered with 1.0 mM MES (pH 6.5). The solution in the flask was continuously flushed with high-purity N2. The solution was replaced at least every 24 h; the new medium was preflushed with high-purity N2. Solutions at pH 3.5 were buffered with 0.2 mM or 2.0 mM β-alanine.
31P-NMR spectroscopy:
Nuclear magnetic resonance spectra were obtained in the sequence: measurements between the final 57–60 h at pH 6.5 (1.0 mM MES), then for 18 h at pH 3.5 with 2.0 mM β-alanine as buffer, followed by 12 h at pH 6.5 with 1.0 mM MES while maintaining anoxia, and subsequently re-aeration for another 12 h at pH 6.5.
31P-NMR spectra were acquired at 202.46 MHz using a Bruker Avance 500 MHz NMR spectrometer (Bruker Biospin GmbH, Germany) equipped with a 10 mm broadband 31P-probe, a 70º pulse, 11 kHz spectral width, and 11 360 data points. For each spectrum, 512 transients were recorded and a repetition time of 2.33 s was used (relaxation delay 1.83 s with an acquisition time of 0.5 s). NMR spectra were processed with software XWIN-NMR (version 3.1, Bruker Analytische Messtechnik GmbH, Germany) and MestreC (version 3.7.9, www.mestrec.com). Chemical shifts are relative to methylene diphosphonic acid (50 mM) in TRIS-buffered D2O at pH 8.9 (16.86 ppm) in a closed capillary inside the NMR tube. A calibration curve for the pH dependence of the chemical shift of the Pi resonance was produced, as described by Lee and Ratcliffe (1983).
NMR experiments with pH perturbations were replicated three times. Chemical shifts of cytoplasmic and vacuolar inorganic phosphate were used to estimate pH in these compartments. New quasi-steady-states in pH, as well as half-times (t1/2) for H+ concentrations to reach the maximal changes, were determined. Half-time analyses were based on the Michaelis–Menten equation, and regression plots of the reciprocals of 1/H+ were typically linear. The extracted data enabled calculation of means ±standard errors for these descriptive parameters.
Results
Assessment of net H+ fluxes
During exposure of anoxic rice coleoptile tips to pH 3.5, net decreases of H+ in the medium were substantial. Expressed as a putative net H+ influx into the coleoptile tips, the rate during the first 12 h was 2.4–3.2 μmol g−1 fresh weight h−1 and it then declined to 0.96 during the last 6 h of exposure to the combination of pH 3.5 and anoxia (Table 1). Such data indicate that pH 3.5 exerted a substantial acid load.
Table 1.
Net H+ uptake (μmol g−1 fresh weight h−1) by excised rice coleoptile tips in anoxia, upon transfer from a medium at pH 6.5 to pH 3.5
| Time after start of anoxia | Transferred to pH 3.5 at 60 h | Continuously at pH 6.5 |
| 60–66 h | 2.4±0.28 | nd |
| 66–72 h | 3.2±0.3 | nd |
| 72–78 h | 0.96±0.14 | nd |
| pH returned to 6.5, with anoxia continued | ||
| 78–86 h | 0.35±0.08 | 0.14±0.1 |
| 86–92 h | 0.07±0.05 | 0.03±0.04 |
Transfer to pH 3.5 was at 60 h after the start of anoxia at pH 6.5. Net H+ uptake was estimated from decreases in H+ in the medium, determined by samples taken at various intervals and titrations back to the reference pH. K+ was 0.25 mM and glucose 50 mM, in the basal medium described in the Materials and methods. Values given are means ±standard errors of three replicates. nd, Not determined.
Survival during 96 h exposure to a combination of anoxia and exposure to pH 3.5
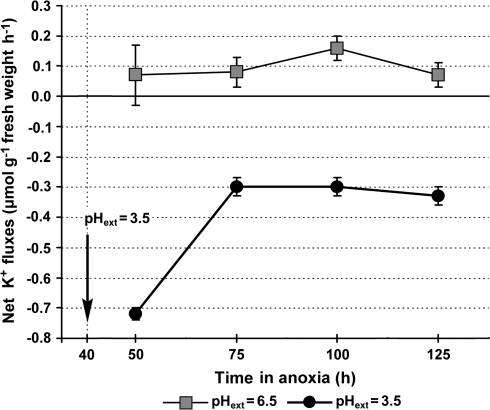
For the first 20 h at pH 3.5, net K+ loss was as high as 0.7 μmol g−1 fresh weight h−1, but the losses subsequently slowed for the next 65 h at pH 3.5 to 0.3 μmol g−1 fresh weight h−1 (Fig. 1). By contrast, at pH 6.5 there was net K+ uptake, albeit at a slow rate (Fig. 1).
Fig. 1.
Net K+ fluxes in rice coleoptile tips during exposure to pH 6.5 (buffered with 1.0 mM MES) or pH 3.5 (buffered with 2.0 mM β-alanine), during anoxia. Excised coleoptile tips were exposed to anoxia at pH 6.5 for 40 h, after which some tips were transferred from pH 6.5 to pH 3.5, which was continued for another 95 h. Rates were plotted at the middle of each interval. Net K+ fluxes after a return to air are reported in the text. K+ was 0.25 mM and glucose 50 mM, in the basal medium described in the Materials and methods. Data are means of five replicates ±standard errors. pHext is an abbreviation for external pH.
Following re-aeration, net K+ uptake over the next 40 h was: 4.5±0.54 and 2.9±0.65 μmol g−1 fresh weight h−1 for coleoptile tips that had been at pH 3.5 or pH 6.5, respectively. These rates were of the same order as the net K+ uptake of ∼4 μmol g−1 fresh weight h−1 by excised coleoptile tips under continuous aeration (Huang et al., 2003). Thus, coleoptile tips exposed to pH 3.5 for as long as 96 h had suffered no irretrievable injury.
That tips survived for at least 96 h anoxia at pH 3.5, was also indicated by the steady total protein concentration during exposure to pH 3.5; furthermore after 44 h of re-aeration, protein had increased by ∼25% in tips that had been under anoxia, regardless of their prior exposure to pH 3.5 or pH 6.5 during the preceding anoxia (data not shown). In another experiment in which the exposure to pH 3.5 lasted 18 h, any losses of organic acids or amino acids from the coleoptile tips were less than 0.08 μmol g−1 fresh weight h−1 (being the detection limit of the methods used), i.e. the K+ losses described above during anoxia at pH 3.5 were not due to a general deterioration of membrane integrity that would increase permeability to low molecular weight solutes as well as to K+.
Changes in cytoplasmic and vacuolar pH
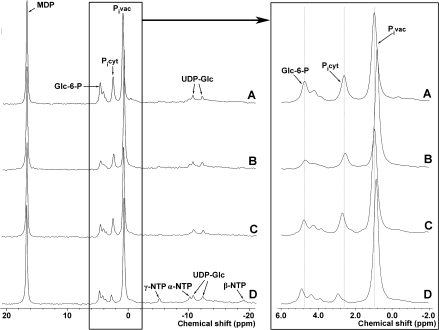
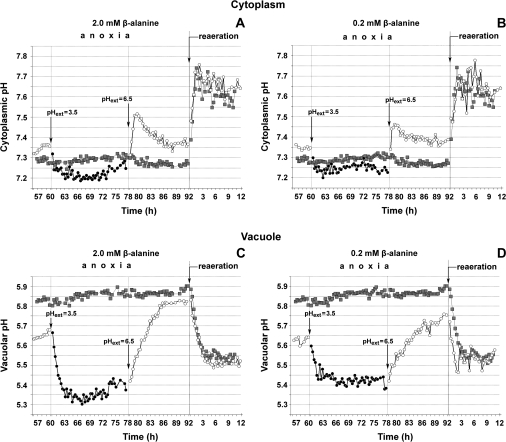
Cytoplasmic and vacuolar pH were measured using in vivo 31P-NMR spectroscopy. NMR spectra for anoxic coleoptile tips exposed to various treatments are shown in Fig. 2. The response to pH 3.5 is shown in Fig. 3, which is a representative from three separate experiments. In Table 2, means and standard errors for the quasi-steady-state values for cytoplasmic and vacuolar pH are given, as well as the time at which the H+ concentrations reached half of the maximum change (i.e. t1/2).
Fig. 2.
Examples of in vivo 31P-NMR spectra, from which cytoplasmic and vacuolar pH were determined, for rice coleoptile tips subjected to anoxia, low pH and recovery with anoxia continued, and eventually re-aeration. Treatment sequence: (A) anoxia at pH 6.5; (B) anoxia at pH 3.5; (C) return from pH 3.5 to pH 6.5 with anoxia continued; (D) return to aerated solution. Glc-6-P, glucose-6-phosphate; MDP, methylene diphosphonic acid; α-, β-, and γ-NTP, signals from α-, β-, and γ-phosphate group of nucleoside triphosphates (NTP), correspondingly; Picyt, cytoplasmic inorganic phosphate; Pivac, vacuolar inorganic phosphate; UDP-Glc, uridine diphosphate glucose.
Fig. 3.
pH of cytoplasmic (A, B) and vacuolar (C, D) compartments in rice coleoptile tips exposed to various treatments, as determined by in vivo 31P-NMR spectroscopy. Symbols: grey squares, tips continuously at pH 6.5; circles show the treatment with pH perturbations—open circles the periods at pH 6.5, closed circles the period at pH 3.5. At pH 3.5, β-alanine was at 2.0 mM (A, C) or 0.2 mM (B, D). (A, C) One example of three experiments is shown, with similar results in the three experiments (see Table 2 for means ±standard errors). Coleoptile tips had been under anoxia for 60 h at pH 6.5, when the NMR tube was perfused with anoxic medium at pH 3.5 for 18 h with β-alanine at 2.0 mM, then for another 12 h anoxia at pH 6.5 (buffered with 1 mM MES), and finally with aerated solution at pH 6.5 for 10 h. (B, D) As for (A) and (C), but at 0.2 mM rather than 2.0 mM β-alanine. K+ was 0.4 mM and glucose 50 mM, in the basal medium as described in the Materials and methods.
Table 2.
pHa of cytoplasmic (pHcyt) and vacuolar (pHvac) compartments in excised tips of coleoptiles of rice at the quasi-steady-state following exposures to anoxia at pH 6.5 and then pH 3.5 for 18 h with anoxia continued, subsequent return to pH 6.5 in anoxia for 14 h, and then to aerated solution at pH 6.5
| Conditions in perfusion solution | pH | pHcyt | t1/2 (min) | pHvac | t1/2 (min) |
| Periods in aeration or anoxia | |||||
| Experiments to establish quasi-steady-states | |||||
| Continuously aerated | 6.5 | 7.65 | na | 5.3 | na |
| Anoxia, 0–92 h | 6.5 | 7.3 | na | 5.8–5.9 | na |
| Re-aerated, 92–104 h | 6.5 | 7.65 | na | 5.5 | na |
| Experiments with pH perturbations | |||||
| Anoxia for 58.5–60 h | 6.5 | 7.35±0.01 | na | 5.7±0.02 | na |
| (i.e. immediately prior to | |||||
| change of external pH to 3.5) | |||||
| Anoxia, 60–78 h | 3.5 | 7.2±0.03 | 28±5 | 5.25±0.05 | 87±17 |
| (i.e. transferred to pH 3.5 | |||||
| after initial 60 h anoxia at pH 6.5) | |||||
| Anoxia, 78–92 h | 6.5 | 7.4±0.01 | 24±1.5b | 5.7±0.04 | 106±10 |
| (i.e. pH 3.5 returned to 6.5 at 78 h) | |||||
| Re-aerated, 92–104 h | 6.5 | 7.65±0.01 | 23.5±0.7 | 5.5±0.03 | 63±7.0 |
Times at which the H+ concentration had reached half the maximum change after a perturbation (t1/2) are also given. Each NMR experiment consisted of 70–90 excised coleoptile tips. K+ at 0.4 mM and glucose 50 mM, in the basal medium as described in the Materials and methods. Values given in the pH perturbation experiments are means ±standard errors of three replicates. na, Not applicable.
Values differ slightly from those in Fig. 3, since values in this table are means of three experiments.
Values for two experiments that did not have ‘overshoots’ following return from pH 3.5 to pH 6.5.
At pH 6.5, cytoplasmic pH in anoxic coleoptile tips was 7.3, compared with 7.65 in aerated tips, while vacuolar pH in the anoxic tips was 5.8–5.9, compared with 5.3 in aerated tips (Table 2). Perturbations after 60 h anoxia at pH 6.5 were in the following order: pH 3.5 for 18 h, return to pH 6.5 for 12 h, followed by a 12 h re-aeration at pH 6.5. In general, half-times for the changes in H+ concentrations after a perturbation were 30 min or less for the cytoplasm and 60–105 min for the vacuole, respectively (Table 2). Following the change in pH from 6.5 to 3.5, cytoplasmic pH decreased only slightly during the first 2 h from 7.35 to 7.2, and then stabilized. After 12 h, cytoplasmic pH had partially recovered to 7.25 (Fig. 3A). Following a return to pH 6.5 after 18 h at pH 3.5, while maintaining anoxia, cytoplasmic pH returned to the quasi-steady-state value of tips continuously exposed to pH 6.5. The substantial transient overshoots during the first 2 h after this perturbation, in the two experiments shown in Fig. 3A and B, were not found in two other experiments, where the rise in cytoplasmic pH was a simple hyperbole (Table 2). Finally, 14 h after the return of the coleoptile tips from pH 3.5 to 6.5 in anoxia, the NMR tube was perfused with aerated solution; cytoplasmic pH rose to 7.65 during the first 1.5 h of re-aeration, the same cytoplasmic pH as in the continuously aerated tips (Fig. 3A; Table 2).
The vacuolar pH in anoxic tips decreased from 5.7 to 5.25 over the first 3 h after transfer from pH 6.5 to 3.5. Subsequently, there was a slight increase to 5.4 (8–18 h in Fig. 3C), which was fairly close to the vacuolar pH of continuously aerated tips at pH 6.5 (Table 2). Following the return of the tips from pH 3.5 to pH 6.5, while maintaining anoxia, vacuolar pH increased over 9 h reaching 5.7 (Fig. 3C; Table 2), a level similar to that of the anoxic tissues continuously kept at pH 6.5 (Table 2). Upon re-aeration, vacuolar pH decreased to 5.5, being 0.2 units higher than the vacuolar pH of continuously aerated tips (Table 2; Fig. 3C).
Similar responses of cytoplasmic pH and vacuolar pH to those described above were also found when β-alanine was at 0.2 rather than 2.0 mM (Fig. 3A, B, C, D). So, there were no indications of side-effects of using 2.0 mM β-alanine.
Tolerance of exposure to pH 3.5 during anoxia, as dependent on exogenous glucose concentration
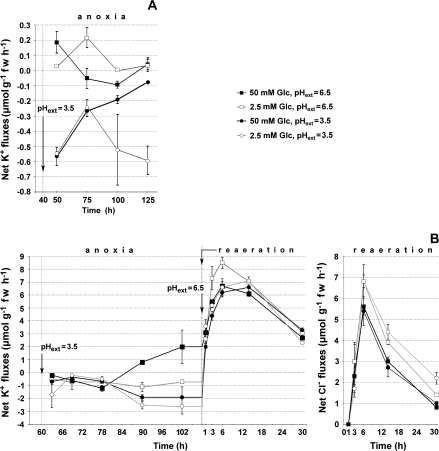
During anoxia, differences in exogenous glucose concentration can be used to manipulate the rate of glycolysis and, hence, the rate of ATP production in this pathway; 2.5 mM glucose was the minimum level required for cell maintenance in rice coleoptile tips (Huang et al., 2005). To test the consequences of reduced rates of glycolysis during exposure to pH 3.5, anoxic coleoptile tips were first exposed to 2.5 mM or 50 mM glucose, for 40 h or 60 h depending on the experiment, before decreasing the pH to 3.5. Two experiments of this type were conducted (Fig. 4). At 50 mM glucose, net K+ fluxes were similar to those shown in Fig. 1, apart from a small net K+ loss alternating with a small net K+ uptake (Fig. 4A, B). At pH 3.5, the net K+ losses differed between the two experiments in degree, although not in kind. In the first experiment (Fig. 4A) net K+ losses at pH 3.5 and 50 mM glucose as usual decreased with time, while at 2.5 mM glucose, net K+ losses accelerated again after the first 48 h at pH 3.5 (Fig. 4A). In the second experiment there was, after 24 h at pH 3.5, not only an increase in net K+ loss at 2.5 mM glucose, but also a smaller increase in net K+ loss at 50 mM glucose (Fig. 4B).
Fig. 4.
Responses of net ion fluxes to different exogenous glucose levels and pH 3.5 or pH 6.5 during anoxia, and recovery following re-aeration at pH 6.5. The coleoptile tips had been exposed to 2.5 mM or 50 mM glucose for 40 h (A) or 60 h (B) since the start of anoxia at pH 6.5. Then for both glucose treatments, three replicates were continued in anoxia at pH 6.5 and three replicates were transferred to pH 3.5, for another 96 h (A) or 50 h (B). Fluxes are shown at the middle of the particular intervals. (A) Net K+ fluxes during anoxia, (B) net K+ fluxes during anoxia, and net K+ and Cl– fluxes after re-aeration, when all treatments were at pH 6.5. Arrows show when some tissues were transferred to pH 3.5, or to aeration. K+ was 0.25 mM during anoxia. After re-aeration, K+ was 0.4 mM, glucose 5 mM, and Cl– 0.3 mM; Cl– was introduced into the medium for the first time at the start of re-aeration. Other constituents in the basal medium are described in the Materials and methods. Tissue concentrations at the end of the experiment in Fig. 4B are shown in Table 3. Data are means of three replicates ±standard errors.
The experiment presented in Fig. 4B included a recovery test by measuring K+ and Cl– net fluxes during re-aeration. The principal results of the re-aeration period are the resumption of large K+ and Cl– net uptakes (Fig. 4B). Net K+ uptake upon re-aeration differed only slightly between the tips that had been in anoxia at pH 3.5 or pH 6.5 combined with either 2.5 mM or 50 mM glucose. There was already substantial net K+ uptake over the first 2 h, while between 2–4 h the rates had reached levels commonly found after introducing continuously aerated coleoptiles for the first time to exogenous K+ (Huang et al., 2003). As usual, rates declined with time and at the end of the experiment the tissues had reached 140–165 mM K+ on a tissue water basis (Table 3).
Table 3.
K+ and Cl– concentrations in rice coleoptile tips after 48 h re-aeration (mM, tissue water basis, calculated from fresh weight:dry weight ratio and assuming 10% water in the free-space), for the experiments in which time trends on net K+ and Cl– uptakes are shown in Fig. 4B
| Tissue ions (mM) | Glucose at 2.5 mM during anoxic phase |
Glucose at 50 mM during anoxic phase |
l.s.d. (P <0.05) | ||
| After anoxic treatments and re-aeration | pH 3.5 60–108 h during anoxia | pH 6.5 continuous | pH 3.5 60–108 h during anoxia | pH 6.5 continuous | |
| K+ | 143±12 | 163±16 | 137±24 | 165±5 | 26 |
| Cl– | 116±18 | 127±3 | 95±1.5 | 106±3 | 14 |
The tips had been at 2.5 mM or 50 mM glucose since transfer to anoxia. At 60 h anoxia, some tips were transferred from pH 6.5 to 3.5. At 108 h anoxia, the tips were transferred to aerated solution and then sampled after 48 h re-aeration. During anoxia, K+ was at 0.20–0.25 mM with no Cl– in the basal medium as described in the Materials and methods. After the start of re-aeration, all treatments contained: K+ 0.4 mM, Cl– 0.3 mM, and glucose 5 mM, in the basal medium. Values are means±standard errors of three replicates.
Net Cl– uptakes, during the first 2 h after the start of re-aeration, were small and variable in all treatments, as also observed for Cl– in the experiments of Huang et al. (2005). Subsequently, net Cl– uptakes reached maximal rates between 4–8 h after the start of re-aeration, but for coleoptile tips at 2.5 mM glucose, the lag was slightly longer if previously in anoxia at pH 3.5, compared with tips at pH 6.5 in anoxia (Fig. 4B). After 48 h re-aeration, accumulation ratios were 315–420 for Cl– and 340–410 for K+ (calculated from Table 3). For tips continuously at pH 6.5 during anoxia, when re-aerated net Cl– uptake was higher in those that had been in anoxia at 2.5 mM, compared with 50 mM glucose (Fig. 4B).
Ethanol production rates
These data are required for the discussion on possible increased requirements for energy in anoxic coleoptile tips at pH 3.5, as compared with those at pH 6.5. At 50 mM exogenous glucose, ethanol production was 5.8 μmol g−1 fresh weight h−1 at both pH 6.5 and 3.5 (Table 4). Consistent results were found in a second experiment. There was also little difference between pH treatments at 2.5 mM glucose, when rates of ethanol production were 3–4-fold lower than at 50 mM glucose (Table 4). Whether the rate of ethanol formation was higher at pH 3.5 than at pH 6.5, when glucose was at 2.5 mM, remains in doubt, because the difference of 0.7 μmol g−1 fresh weight h−1 was not statistically significant owing to the variation between replicates (standard errors were 0.4–0.5; Table 4).
Table 4.
Ethanol production (μmol g−1 fresh weight h−1) in anoxic rice coleoptile tips during 48 h exposure to pH 3.5 or pH 6.5 and 2.5 mM or 50 mM glucose
| Glucose at 2.5 mM |
Glucose at 50 mM |
||
| pH 3.5 | pH 6.5 | pH 3.5 | pH 6.5 |
| 1.9±0.5 | 1.2±0.4 | 5.8±0.9 | 5.8±0.6 |
Coleoptile tips were in anoxia for 60 h with either 2.5 mM or 50 mM glucose at pH 6.5, and then the pH treatments were imposed with continuing anoxia. For each glucose treatment, three replicates were continued in anoxia at pH 6.5 and three replicates were transferred to pH 3.5. The coleoptile tips were also measured in the same experiment for net K+ fluxes (see Fig. 4B). K+ was at 0.25 mM in the basal medium as described in the Materials and methods. Values are means of three replicates ±standard errors.
Discussion
The key objectives of the present experiments were to test whether, or not, anoxic rice coleoptiles could survive the additional adverse condition of an acid load, and once this was established use that system to contribute to the elucidation of pH regulation during an energy crisis. Evidence that the exposure to pH 3.5 was effective as an acid load, consists of the substantial and sustained decreases of H+ in the medium, linked in part with K+ losses from the tissues, presumably due to depolarization of the plasma membrane, triggered by the entry of positive charges (i.e. H+). External pH would also change if organic or amino acids were lost from the tissues to the medium (based on reviews by Gerandas and Schurr, 1999; Greenway and Gibbs, 2003); however, there were no detectable losses of such metabolites (see Results) so the net decrease of H+ in the medium almost certainly represents a net H+ uptake into the cells.
Survival of rice coleoptile tips exposed to a combination of anoxia and pH of 3.5
A high anoxia tolerance of excised coleoptile tips of rice was previously established at the benign pH of 6.5 (Huang et al., 2005). The present experiments demonstrate that this anoxia tolerance persisted, even when the coleoptile tips were also exposed for 96 h to a pH of 3.5, as shown by the vigorous resumption of net K+ uptake by the tips after a return to the aerated solution (Fig. 4 and associated text). After re-aeration, there were also high rates of net Cl– and K+ uptake even after the anoxic coleoptile tips had been exposed for 48 h to a combination of pH 3.5 and 2.5 mM exogeneous glucose (Fig. 4B), which curtailed ethanolic fermentation by 3-fold (Table 4). These tips attained an accumulation ratio of 6 for Cl– at 4 h after the start of re-aeration (calculated from Fig. 4B). Cl– uptake would have been against an electrochemical gradient, so the accumulation ratio of 6 at 4 h re-aeration demonstrated that these tips suffered little injury, or that any injury which might have occurred during anoxia, was rapidly repaired after re-aeration. Thus, the present investigation meets the incisive dictum by Thomas et al. (1973), that recovery upon return to air is crucial to verify that metabolic changes under anoxia are primary effects due to anoxia, rather than the consequences of injury. None of the above negates the likelihood that, after much longer exposures to pH 3.5, cells would deteriorate. So far, the only evidence of an eventual injury was in the experiment in which the combination of 2.5 mM glucose and pH 3.5 was continued for 96 h, when the net K+ loss accelerated again during the last 48 h (Fig. 4A).
pH regulation during an energy deficit
The present responses of intracellular pH in anoxic rice coleoptile tips at pH 6.5 (Table 2; Fig. 3) were broadly similar to the anoxic responses of excised ‘young rice shoots’ and 2 mm excised root tips of rice, although cytoplasmic pH in these two earlier studies stabilized at ∼7.2 rather than ∼7.35 (Menegus et al., 1991; Kulichikhin et al., 2007). In all three cases, vacuolar pH increased to 5.7–6.2 upon imposition of anoxia (Table 2; Menegus et al., 1991; Kulichikhin et al., 2007). These increases of vacuolar pH did not occur in anoxia-intolerant wheat roots (Menegus et al., 1991; Kulichikhin et al., 2007), which is another difference between anoxia-intolerant and -tolerant tissues as reviewed by Greenway and Gibbs (2003). The increase in vacuolar pH might have acclimative value, since at higher vacuolar pH, the driving force for fluxes of H+ to the cytoplasm would be reduced, while the proportion of undissociated acids in the vacuole would decrease and hence their flux to the cytoplasm should also decline (Greenway and Gibbs, 2003).
Our data strongly support the hypothesis by Felle (2005) that decreases in cytoplasmic pH during anoxia are not necessarily injurious, but rather the regulation of cytoplasmic pH at a new set point, consistent with the altered metabolism. Our new contribution to Felle's (2005) notion of a new set point for cytoplasmic pH, which he developed based on responses during the first few hours of anoxia using a presumably anoxia-intolerant tissue, consists of the exposure to anoxia of a tolerant tissue for 4 d, which demonstrated a stable cytoplasmic pH, between 60 h and 90 h after starting anoxia at an external pH of 6.5. Cytoplasmic pH decreased upon transfer of the tips to external pH 3.5, but only from 7.35 to 7.2, while this pH remained stable for the next 18 h (Table 2; Fig. 3A, B). This decrease in cytoplasmic pH could be regarded as small, considering the steep free energy gradient for H+ across the plasma membrane, as well as the increased H+ concentration gradient across the tonoplast (see next paragraph). Regulation of cytoplasmic pH was further demonstrated by the rapid return to the pH level before perturbation, both after transfer from pH 3.5 back to pH 6.5 while maintaining anoxia, and following the return to aerated solution (Fig. 3).
Whether the vacuolar pH is also regulated is not so certain; upon exposure to pH 3.5 the vacuolar pH decreased substantially (from ∼5.7 to ∼5.25; Table 2), in this case, the vacuole can be viewed as a sink for H+ entering the cells. Nevertheless, this decrease in vacuolar pH increased the acid load across the tonoplast, losing the putative advantage of a higher vacuolar pH under anoxia found for rice coleoptiles and roots at the benign pH of 6.5 (discussed above). Nevertheless, upon a return to pH 6.5, the vacuolar pH returned to ∼5.7 (Table 2). The regulation of vacuolar pH implied, at least for the external pH of 6.5, would be consistent with responses by Chara to manipulations of vacuolar pH by perfusion of the large vacuole (Moriyasu et al., 1984).
Possible increase in energy requirements for pH regulation during anoxia at pH 3.5
Exposure to pH 3.5 imposes a steep electrochemical gradient for H+ across the plasma membrane; the concentration gradient and therefore the potential flux increased by 1000-fold (based on an equation in Nobel, 1974). This concentration gradient contributed 21 kJ mol−1 to the free energy gradient and will be further increased by an electrical component of, at most, 12 kJ mol−1, based on membrane potential of ∼–90 mV in rice coleoptiles (Zhang and Greenway, 1993). There are three possible mechanisms contributing to pH regulation in the face of the potentially large H+ influx upon exposure to pH 3.5: a biochemical pHstat to cope with any net entry of H+, H+ excretion across the plasma membrane, and/or a reduction in H+ permeability of the plasma membrane.
Energy requirements would be increased if H+ excretion increased to counteract any H+ influx. Potential for H+ excretion across the plasma membrane was indicated for anoxic maize root tips at pH 6.2, since fusicoccin stimulated net H+ efflux, particularly for hypoxically pretreated roots (Xia and Roberts, 1996); these authors also suggested that the H+-ATPase became engaged at an external pH of 4.5. In a review, Ratcliffe (1999) used the data by Xia and Roberts (1996), and other evidence, to suggest that both the biochemical pHstat and biophysical pHstat (i.e. H+ pumping) are likely to make contributions to cytoplasmic pH regulation, the degree of their engagement depending on other conditions. In the rice coleoptiles, H+ pumping across the tonoplast, at which the concentration gradient for H+ had increased by ∼4–fold (calculated from Fig. 3), would also be expected to consume additional energy. This requirement might be mitigated by a reduced H+ permeability across the plasma membrane. Reduced permeability of membranes during anoxia occurs in tolerant animal cells (Lutz and Nilsson, 2004), with indications of a similar response in anoxic rice coleoptiles by the 17-fold lower permeability for K+ efflux than in aerated tissues (Colmer et al., 2001).
The present data showing increased net K+ losses with time in anoxia by coleoptile tips at 2.5 mM glucose, support the notion that exposure to pH 3.5 increased the requirements for energy, which presumably was diverted from processes essential to ensure long-term survival. One process from which energy seems to have been diverted was the maintenance of membrane integrity (Greenway and Gibbs, 2003), as membrane integrity was compromised when only 2.5 mM glucose was supplied, as indicated by substantial K+ leakage during the longer exposure times to pH 3.5 in anoxia. Yet, this deterioration was not irretrievable even after 48 h exposure to pH 3.5, as shown by the vigorous Cl– and K+ uptakes within 2–4 h after a return to aerated solution at pH 6.5 (Fig. 4B). The long lag before irretrievable injury occurs is surprising, so the previous estimate by Huang et al. (2005) for the energy requirement of 3.5 μmol ATP g−1 fresh weight h−1 for the maintenance of anoxic rice coleoptiles at pH 6.5, as deduced from rates of ethanol production, can be lowered to than less than ∼2 μmol ATP g−1 fresh weight h−1 as based on ethanol production rates in the present study (Table 4). It is still not certain whether in vivo ATP supply via glycolysis is augmented by ATP produced by the haemoglobin–nitrous oxide cycle also during anoxia, as recently discovered in isolated mitochondria of rice roots (Stoimenova et al., 2007).
Conclusions
Anoxic rice coleoptiles, with adequate sugar substrate, maintained cellular compartmentation, despite an additional exposure to an acid load of pH 3.5, as shown by the small decrease in cytoplasmic pH and the rapid recovery of net K+ and Cl– uptake following a return to aerated solution at pH 6.5. Thus, there was no sign of fatal injury, which Felle (2005) postulated occurred due to breakdown of trans-membrane gradients in other, less anoxia-tolerant, plant organs or species. So, rice coleoptiles can tolerate anoxia combined with an acid load, being far superior in tolerance of these conditions than root tips of maize (see Introduction), yet under anoxia both these tissues have a similar energy production on a soluble protein basis (as assessed from rates of ethanol production; Greenway and Gibbs, 2003). These observations reinforce the hypothesis (Fig. 1 in the review by Greenway and Gibbs, 2003) that differences in anoxia tolerance between species are associated with more efficient energy use, rather than merely with rates of energy production via glycolysis linked to ethanol production.
Acknowledgments
Particular thanks for the incisive and comprehensive criticisms by Brian Atwell on a draft close to submission. Hubert Felle and Mark Tester also critically evaluated earlier versions of the manuscript. Shaobai Huang is thanked for introducing KYK to the ‘rice coleoptile tip system’. The DVC Fund at UWA supported KYK to visit UWA for collaborative research. Thanks to an anonymous referee for several incisive suggestions on principles and structure.
References
- Cawthray GR. An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. Journal of Chromatography. 2003;A1011:233–240. doi: 10.1016/s0021-9673(03)01129-4. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Huang S, Greenway H. Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptiles of rice seedlings during prolonged anoxia. Journal of Experimental Botany. 2001;52:1507–1517. doi: 10.1093/jexbot/52.360.1507. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Elliot DC, Elliot WH, Jones KM. Data for biochemical research. 2nd edn. Oxford University: Clarendon Press; 1969. [Google Scholar]
- Fan TW, Higashi RM, Lane AN. Monitoring of hypoxic metabolism in superfused plant tissues by in vivo 1H NMR. Archives of Biochemistry and Biophysics. 1986;251:674–687. doi: 10.1016/0003-9861(86)90377-2. [DOI] [PubMed] [Google Scholar]
- Felle HH. pH regulation in anoxic plants. Annals of Botany. 2005;96:519–532. doi: 10.1093/aob/mci207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendas J, Schurr U. Physicochemical aspects of ion relations and pH regulation in plants: a quantitative approach. Journal Experimental Botany. 1999;50:1101–1114. [Google Scholar]
- Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology. 2003;30:999–1036. doi: 10.1071/PP98096. [DOI] [PubMed] [Google Scholar]
- Greenway H, Armstrong W, Colmer TD. Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Annals of Botany. 2006;98:9–32. doi: 10.1093/aob/mcl076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Felle H, Mathieu Y, Kurkdjian A. Regulation of intracellular pH in plant cells. International Review of Cytology. 1991;127:111–173. [Google Scholar]
- Huang S, Greenway H, Colmer TD. Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-‘intolerant’, but not of a ‘tolerant’, genotype. Journal of Experimental Botany. 2003;54:2363–2373. doi: 10.1093/jxb/erg252. [DOI] [PubMed] [Google Scholar]
- Huang S, Ishizawa K, Greenway H, Colmer TD. Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rates of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia. Journal of Experimental Botany. 2005;56:2453–2463. doi: 10.1093/jxb/eri238. [DOI] [PubMed] [Google Scholar]
- Kulichikhin KY, Aitio O, Chirkova TV, Fagerstedt KV. Effect of oxygen concentration on intracellular pH, glucose-6-phosphate and NTP content in rice (Oryza sativa) and wheat (Triticum aestivum) root tips: in vivo 31P-NMR study. Physiologia Plantarum. 2007;129:507–518. [Google Scholar]
- Lee RB, Ratcliffe RG. Development of an aeration system for use in plant tissue NMR experiments. Journal Experimental Botany. 1983;34:1213–1221. [Google Scholar]
- Lutz PL, Nilsson GE. Vertebrate brains at the pilot light. Respiratory Physiology and Neurobiology. 2004;141:285–296. doi: 10.1016/j.resp.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. Response to anoxia in rice and wheat seedlings. Changes in the pH of intracellular compartments, glucose-6-phosphate level, and metabolic rate. Plant Physiology. 1991;95:760–767. doi: 10.1104/pp.95.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu Y, Shimmen T, Tazawa M. Vacuolar pH regulation in Chara australis. Cell Structure and Function. 1984;9:225–234. [Google Scholar]
- Nobel PS. Introduction to biophysical plant physiology. San Francisco: Freeman and Company; 1974. [Google Scholar]
- Ponnamperuma FN. Effects of flooding on soils. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 9–45. [Google Scholar]
- Ratcliffe RG. Intracellular pH regulation in plants under anoxia. In: Eggington S, Taylor EW, Raven JA, editors. Regulation of tissue pH in plants and animals: a reappraisal of current techniques. Cambridge: Cambridge University Press; 1999. pp. 193–213. [Google Scholar]
- Stoimenova M, Imbargadiev AU, Gupta KJP, Hill RD. Nitrite driven ATP synthesis in barley and rice roots mitochondria. Planta. 2007;226:465–474. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ranson SL, Richardson JA. Plant physiology. 5th edn. London: Longman; 1973. [Google Scholar]
- Waters I, Kuiper PJC, Watkin E, Greenway H. Effects of anoxia on wheat seedlings. I. Interaction between anoxia and other environmental factors. Journal of Experimental Botany. 1991;42:1427–1435. [Google Scholar]
- Xia JH, Roberts JKM. Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology. 1996;111:227–233. doi: 10.1104/pp.111.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Saglio P, Roberts JKM. Nucleotide levels do not critically determine survival of maize root tips acclimated to a low-oxygen environment. Plant Physiology. 1995;108:589–595. doi: 10.1104/pp.108.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Greenway H. Membrane transport in anoxic rice coleoptiles and storage tissue of beetroot. Journal of Experimental Botany. 1995;22:965–975. [Google Scholar]