Abstract
Plant and animal cells release or secrete ATP by various mechanisms, and this activity allows extracellular ATP to serve as a signalling molecule. Recent reports suggest that extracellular ATP induces plant responses ranging from increased cytosolic calcium to changes in auxin transport, xenobiotic resistance, pollen germination, and growth. Although calcium has been identified as a secondary messenger for the extracellular ATP signal, other parts of this signal transduction chain remain unknown. Increasing the extracellular concentration of ATPγS, a poorly-hydrolysable ATP analogue, inhibited both pollen germination and pollen tube elongation, while the addition of AMPS had no effect. Because pollen tube elongation is also sensitive to nitric oxide, this raised the possibility that a connection exists between the two pathways. Four approaches were used to test whether the germination and growth effects of extracellular ATPγS were transduced via nitric oxide. The results showed that increases in extracellular ATPγS induced increases in cellular nitric oxide, chemical agonists of the nitric oxide signalling pathway lowered the threshold of extracellular ATPγS that inhibits pollen germination, an antagonist of guanylate cyclase, which can inhibit some nitric oxide signalling pathways, blocked the ATPγS-induced inhibition of both pollen germination and pollen tube elongation, and the effects of applied ATPγS were blocked in nia1nia2 mutants, which have diminished NO production. The concurrence of these four data sets support the conclusion that the suppression of pollen germination and pollen tube elongation by extracellular nucleotides is mediated in part via the nitric oxide signalling pathway.
Keywords: Calcium signalling, extracellular ATP, guanylate cyclase, nitrate reductase, pollen germination
Introduction
Plant growth and development are regulated by numerous signals, both environmental and hormonal. Communication between cells and structures is critical for the proper development of the plant. While some plant signalling molecules were recognized many decades ago, for example, auxins (Darwin, 1880), their receptors and modes of action have only recently been identified (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). Extracellular ATP (eATP) has been identified for some time as a signalling molecule in animals (Burnstock and Knight, 2004), and the diverse functions of eATP in animal cells are known to be regulated via purinoceptors (Ralevic and Burnstock, 1998).
Evidence that eATP may play a signalling role in plants has accumulated only recently. Thomas et al. (2000) demonstrated the role of eATP in xenobiotic resistance and MDR transport activity, and Tang et al. (2003) showed that higher concentrations of it can inhibit auxin transport and root gravitropism. As summarized by Roux and Steinebrunner (2007), several authors have documented cellular responses to eATP, including changes in membrane potential (Lew and Dearnaley 2000), increases in cytoplasmic calcium (Demidchik et al., 2003; Jeter et al., 2004), and enhanced production of superoxide (Song et al., 2006). Chivasa et al. (2005) showed that depleting eATP induced cell death across several plant species. Some of these studies have also noted the ability of eATP to induce changes in gene expression (Jeter et al., 2004; Chivasa et al., 2005; Song et al., 2006).
Besides inducing increases in cytoplasmic calcium and superoxide, extracellular nucleotides also turn on the production of other signalling agents. Recently, Foresi et al. (2007) showed that eATP can induce nitric oxide (NO) in tomato cell culture. Wu and Wu (2008) found that the eATP-induced production of NO was dependent on its prior induction of increased [Ca2+]cyt in hairy root cultures, and they also detailed the dependence of NO production on eATP dosage. Both Wu and Wu (2008) and Torres et al. (2008) showed that eATP could induce an increase in H2O2 production as well as in NO production, the latter studies carried out in algae, demonstrating the role of eATP in wound signalling early during evolution.
That the signalling changes induced by extracellular nucleotides could have an effect on growth is supported by studies that show chemically or genetically suppressing the activity of ectoapyrases that limit the [eATP] suppresses the growth of hypocotyls, roots, and pollen tubes in Arabidopsis (Wolf et al., 2007; Wu et al., 2007) and tuber growth in potato (Riewe et al., 2008). Especially relevant to the work reported here, Steinebrunner et al. (2003) reported that increasing [eATP] by adding it to the medium or by knocking out AtAPY1 and AtAPY2 could inhibit pollen germination.
While there is evidence for eATP signalling in plants, no receptors have been identified. Complicating their identification is the fact that purinoceptor sequences have not been highly conserved in evolution, as shown by the structural diversity among animal purinoceptors and the sequence difference between algal and mammalian purinoceptors (Fountain et al., 2008). In the absence of receptor identification, one way to investigate the signalling roles of eATP in plant growth and development would be to identify the transduction steps that mediate its effects. The results of Steinebrunner et al. (2003), who showed that applied ATP, but not AMP or phosphate, could inhibit pollen germination, and of Prado et al. (2004), who showed that increases in nitric oxide (NO) inhibited pollen tube growth, raised the question of whether there is a connection between eATP and NO production in the control of pollen germination and growth. A further argument for testing this connection is that eATP induces NO production in cultured tomato cells (Foresi et al., 2007), in Salvia miltiorrhiza hairy roots (Wu and Wu, 2008), and in algae (Torres et al., 2008).
NO signalling and eATP signalling were originally discovered in animals, and both are only recently recognized signalling mechanisms in plants (Crawford, 2006; Roux and Steinebrunner, 2007). NO signalling in plants and animals uses similar mechanisms, although the pathways of NO synthesis are better defined in animals than in plants (Besson-Bard et al., 2008). In both systems, NO plays diverse roles, modifying the structure and activity of multiple different enzymes.
Pharmacological and biochemical studies indicate that, among the enzymes activated in plants by NO is guanylate cyclase, which uses GTP as a substrate to produce cGMP. This cGMP can then serve as a secondary messenger activating other cellular responses. In both plants and animals, some NO-induced responses are diminished by phosphodiesterases that break down the cGMP into GMP. In plants, agonists and antagonists of NO production and/or cGMP accumulation have been used to implicate NO or both of these agents in several major signalling pathways leading to critical growth/physiological responses such as gravitropism (Hu et al., 2005), pathogen defence (Zaninotto et al., 2006), seed germination and seedling de-etiolation (Beligni and Lamattina, 2000), ABA-mediated stomatal closure (Bright et al., 2006), flowering (He et al., 2004), and, especially relevant to this report, UV-B-inhibited germination and tube growth of pollen from Paulownia plants (He et al., 2007), and lily pollen tube elongation (Prado et al., 2004, 2008).
Because eATP does not have to be hydrolysed to induce signalling responses, its effects can be mimicked by the poorly-hydrolysable analogue, ATPγS. It is reported here that eATPγS inhibits Arabidopsis pollen germination and pollen tube growth via increases in NO. An NO-sensitive fluorescent dye (DAF-2D) allowed visualization of NO increases after nucleotide application. NO signalling agonists and antagonists altered the effects of applied nucleotides on pollen germination and pollen tube growth. Applications of eATPγS can induce NO production, and this production is crucial for transducing the inhibitory effects of eATPγS on pollen germination and pollen tube elongation, as shown by the fact that these effects are blocked in mutants suppressed in NO production. To the authors’ knowledge, this is the first report of a key role for the NO signalling pathway in mediating the control of pollen germination and growth by extracellular nucleotides.
Materials and methods
Strains and growth conditions
Arabidopsis thaliana plants were ecotype Wassilewskija (WS), Columbia (Col-0), and nia1nia2 (nia1-1, nia2-5; CS 2356) in a Col-0 background. Medicago truncatula plants were strain A17. All of the plants were grown in Metro-Mix 200 soil at 24 °C with constant light.
In vitro pollen germination
Arabidopsis flowers in stage 12–14 (Smyth et al., 1990) or Medicago truncatula flowers in stage F3 (Firnhaber et al., 2005) were collected and vortexed for 1 min in pollen germination media (PGM) (0.01% boric acid, 1 mM MgSO4, 1 mM CaCl2, 1 mM Ca(NO3)2, 5 mM HEPES, 18% sucrose pH 7.0) (modified from Li et al., 1999). The supernatant containing the pollen was collected by pipette, and up to three rounds of vortexing of the same pollen could be pooled together. The pollen suspended in PGM was then added in 10–12 μl drops to 400 μl PGM+1% agar that had been spread as a thin film across a microscope slide. The slides and pollen were then placed into humidity chambers (Petri dishes with water-saturated kimwipes) and allowed to germinate at 26 °C overnight. Pictures of the germinated pollen were taken with a digital camera attached to a brightfield microscope at ×40. The digital images were scored for the percent of pollen germinated using the AlphaEase software (Alpha Innotech). Pollen with tubes that were shorter than the diameter of the pollen grain were not counted as germinated or non-germinated. The chemicals: ATPγS, AMPS, N2, 2-O-dibutyrylguanosine 3′:5′-cyclic monophospohate (Dib cGMP), 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulphonyl]-4-methylpiperazine citrate (Sildenafil citrate) (Viagra™, Pfizer), and 2-(N,N-diethylamino)-diazenolate 2-oxide (NONOate) were added to the pollen/PGM suspension prior to adding the drops to the PGM+1% agar. The chemicals 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ), N-[4-[1-(3-aminopropyl)-2-hydroxy-2-nitrosohydrazino]butyl]-1,3-propanediamine (SNAP), and 3-isobutyl-1-methylxanthine (IBMX) were diluted in dimethyl sulphoxide (DMSO) and added to the pollen/PGM suspension to a final concentration of 0.1% DMSO.
An alternate method of obtaining Arabidopsis pollen by dabbing flowers onto PGM+1% agar was used in some experiments. To start, 50 μl PGM+1% agar was deposited into concavity slides, and approximately four flowers were dabbed onto the agar to release the pollen. Within 5 min of the pollen being placed onto the agar, 100 μl of PGM or PGM with nucleotides and/or NO pathway agonists was added to the depression so that the pollen/agar was covered in liquid. The concavity slides were placed in a humidity chamber at 26 °C overnight, and photographed as described for the vortexed pollen.
As noted by Boavida and McCormick (2007), optimal conditions for pollen germination can be ecotype-specific. The WS ecotype and germination media used here were different from those reported by Boavida and McCormick (2007), so several temperature and pH values had to be tested before finally selecting the optimal germination conditions for the combination of ecotype and pollen germination media that was employed in our assays. Like Johnson-Brousseau and McCormick (2004), we, too, found that a solid base was necessary for optimal in vitro germination of Arabidopsis pollen. Moreover, for the technique used here, the vortexing and deposition of the pollen/PGM suspension needed to take place in less than 30 min or germination rates dropped considerably.
Measurements of pollen tube elongation
Pollen was germinated as described above except that 10 μl drops of pollen/PGM suspension were added to 60 μl of PGM+1% agar that had been deposited into a concavity slide. The pollen was allowed to germinate for 1–2 h, so that pollen tubes were clearly visible. Then 90 μl of PGM or PGM containing ATPγS, AMPS, DMSO or ODQ was added and a photograph was taken through a brightfield microscope at ×40. The coordinates of each photo were noted, so that the exact pollen tubes could be photographed 50–60 min later. Using Image J (NIH), the pollen tube lengths for individual pollen tubes were measured at the initial and later time points, and a growth rate of μm min−1 was determined.
Measurements of NO in growing pollen tubes treated with ATPγS or AMPS
4,5-Diaminofluorescein diacetate (DAF-2D, Molecular Probes) was dissolved in DMSO to produce a 20 mM stock solution, which was stored at –80 °C in 1 ml aliquots. Pollen was germinated as described for the pollen tube elongation measurements. After 3–4 h, when pollen tubes were clearly visible, 90 μl of PGM containing 5 μM DAF-2D was added with or without ATPγS or AMPS. Using a confocal laser microscope (Leica SP2 AOBS) with emissions at 488 nm and a filter at 522 nm, optical sections were taken of several pollen tubes from 5–30 min after addition of the DAF-2D only and DAF-2D and nucleotides. Using Image J (NIH), the maximum fluorescence readings for the pollen tubes on each slide and each time point were determined. Three or more biological replicates were performed and at least 20 growing pollen tubes with or without nucleotides were compared with each other at similar time points to determine if there was a difference in fluorescence.
Quantitation of NO measurements
The NO generated inside the pollen tubes was calculated by measuring the oxidation of DAF-FM DA. Quantification of fluorescence was achieved by first identifying areas of equal size and cytoplasmic density and measuring the average pixel intensity value for that region with the LSM 5 software (Leica Microsystems).
ATP assay
The luciferin–luciferase assay to determine ATP release was performed using an ATP bioluminescent assay kit (Sigma-Aldrich). After flowers were vortexed, the pollen/PGM solution was filtered through a 0.45 μm filter to remove the pollen, and then the filtered solution was immediately frozen in liquid N2. To measure the released ATP in the solution, 10 μl of non-vortexed PGM or 2 μl of a 1:10 dilution of the vortexed solution was added to 50 μl of the bioluminescent assay solution. The luminescence signal was integrated for 10 s.
Results
Effects of extracellular ATPγS and AMPS on pollen germination
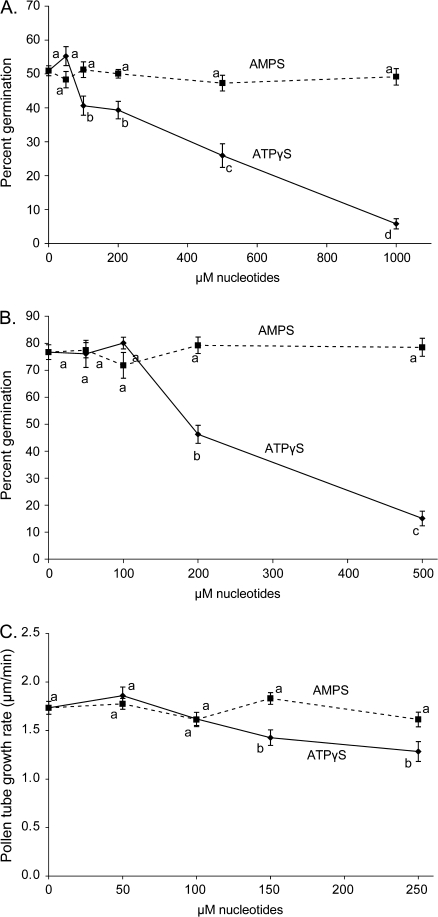
Previous research has shown that eATP can inhibit pollen germination (Steinebrunner et al., 2003), but this was in the mM range, which is higher than would be expected for stable accumulation in the ECM. Poorly hydrolysable versions of adenosine nucleotides could mimic eATP effects at much lower concentrations. Arabidopsis (WS) pollen was germinated in pollen germination media (PGM) containing from zero to 1000 μM ATPγS or AMPS. The addition of AMPS did not significantly change the percentage of germinated pollen. However, beginning at 100 μM ATPγS the pollen germination rate significantly decreased (Fig. 1A).
Fig. 1.
Pollen germination and pollen tube elongation are inhibited by ATPγS but not AMPS. (A) In vitro germination of Arabidopsis WS pollen treated with various concentrations of ATPγS or AMPS. (B) In vitro germination of Medicago truncatula pollen with various concentrations of ATPγS or AMPS. (C) Arabidopsis WS pollen was germinated in vitro, and, after pollen tubes were visible (1–2 h), various concentrations of ATPγS or AMPS were added and the growth rate of the pollen tubes was measured. Different letters above the bars indicate values that are significantly different from each other (P ≤0.05). Error bars are ±SE.
To test whether the effects of ATPγS on pollen germination were specific to Arabidopsis or if this was a more generalized phenomenon, a similar experiment using Medicago truncatula was conducted. Medicago showed decreasing pollen germination rates starting with the application of 200 μM ATPγS (Fig. 1B).
Arabidopsis pollen tube elongation is inhibited by ATPγS
Kim et al. (2006) showed that elongating root hairs release ATP, and Wu et al. (2007) found elongating pollen tubes do the same. One proposed source of eATP is from secretory vesicles as they release their contents to the ECM. Pollen tube elongation involves substantial delivery to the growing tip of secretory vesicles (Cheung and Wu, 2008), which probably include ATP (Wu et al., 2007). Because in pollen the released contents include an acid phosphatase (Ibrahim et al., 2002), any ATP released by the secretory vesicles should be readily hydrolysed. To test whether a nucleotide agonist that could not be hydrolysed by ectophosphatases would affect pollen tube growth, ATPγS and AMPS were used. After Arabidopsis (WS) pollen tubes had begun to grow, from 0 μM to 250 μM ATPγS or AMPS were added and the subsequent growth rate was determined. Up to 250 μM AMPS had no significant effect on pollen tube elongation rates. However, greater than 150 μM ATPγS significantly inhibited pollen tube elongation (Fig. 1C).
Agonists and antagonists of the NO signalling pathway alter the effects of applied nucleotides on pollen
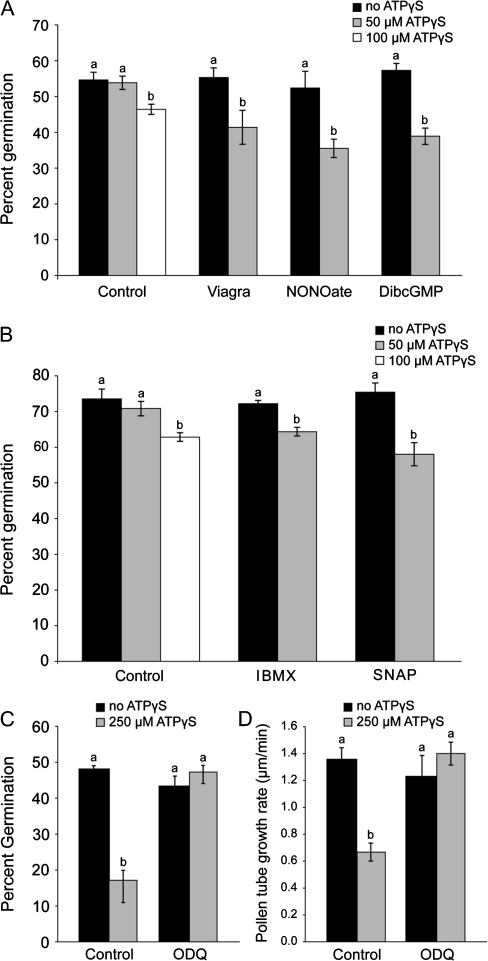
Extracellular nucleotides can induce NO production in tomato cell culture (Foresi et al., 2007), hairy root culures (Wu and Wu, 2008) and in algae (Torres et al., 2008), and NO can inhibit pollen germination and tube growth of Paulownia pollen (He et al., 2007). These results suggested that NO might be involved in the inhibition of Arabidopsis pollen germination by ATPγS, and this was tested using different agonists and antagonists of the known NO signalling pathway. To eliminate non-specific or toxic effects of these chemicals, each agonist or antagonist was used at a concentration that by itself did not change the percentage of pollen germination (Fig. 2). Some of the NO signalling pathway agonists are water-soluble while others are DMSO-soluble.
Fig. 2.
NO signalling agonists promote the effects of ATPγS on pollen germination, and antagonists block the effects of ATPγS on pollen germination and elongation. Different classes of NO signalling agonists and antagonists and various concentrations of ATPγS were added to Arabidopsis WS pollen prior to germination. (A) Water-soluble NO signalling agonists (1 μM Viagra, 20 μM NONOate, 100 μM DibcGMP) shifted the concentration needed for inhibition of germination from 100 μM ATPγS to 50 μM. (B) DMSO-soluble NO signalling agonists (20 μM IBMX, 20 μM SNAP) shifted the concentration needed for inhibition of germination from 100 μM ATPγS to 50 μM. (C) ODQ (100 μM), a DMSO-soluble NO signalling antagonist, reverses pollen germination inhibition by ATPγS. Each agonist or antagonist was used at a concentration that by itself did not affect pollen germination. (D) ODQ also reverses the ATPγS inhibition of WS pollen tube elongation. Treatments were added to growing pollen tubes. Control is PGM+0.5% DMSO, ATPγS concentration is 100 μM+0.5% DMSO and ODQ concentration is 100 μM, which by itself had no effect on pollen tube growth. Different letters above the bars indicate values that are significantly different from each other (P ≤0.05). Error bars are ±SE.
Figure 2A shows the effects of the water-soluble agonists. In controls, 50 μM ATPγS is not inhibitory and 100 μM ATPγS is inhibitory. The active ingredient in Viagra™ (1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulphonyl]-4-methylpiperazine citrate (Sildenafil citrate)) increases cGMP levels by inhibiting the phosphodiesterase that breaks down cGMP (Corbin and Francis, 1999), 2-(N,N-diethylamino)-diazenolate-2-oxide (NONOate) is an NO donor, and N2, 2-O-dibutyrylguanosine 3′:5′-cyclic monophospohate (DibcGMP) is a membrane-permeable cGMP analogue. Adding each of these chemicals activates the NO signalling pathway. Adding any one of these chemicals caused a moderate, but statistically significant, reduction in the [ATPγS] that inhibited pollen germination from 100 μM to 50 μM.
Water-insoluble (DMSO-soluble) NO agonists also reduce the threshold for the inhibitory effects of ATPγS (Fig. 2B). The DMSO-soluble chemicals were all used at concentrations that resulted in a final concentration of 0.1% DMSO. With 0.1% DMSO 50 μM ATPγS does not inhibit pollen germination while 100 μM ATPγS does. Similarly to Viagra™, 3-isobutyl-1-methylxanthine (IBMX) increases cGMP levels by inhibiting the phosphodiesterases that break down cGMP. N-[4-[1-(3-aminopropyl)-2-hydroxy-2-nitrosohydrazino]butyl]-1,3-propanediamine (SNAP) is a NO donor. Treatment with either of these chemicals results in a moderate, but statistically significant, reduction in the [ATPγS] that inhibits pollen germination from 100 μM to 50 μM (Fig. 2B).
Production of cGMP, and therefore downstream effects, can be inhibited by 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ), which inhibits guanylate cyclase. ODQ is DMSO-soluble. The addition of 100 μM ODQ by itself has no effect on pollen germination rates, but it is able to reverse the inhibition of pollen germination by 250 μM ATPγS (Fig. 2C).
Even though inhibitors can have secondary or non-specific effects, five agonists of the NO signalling pathway, with different modes of action, all had the same effect on pollen germination. They all reduced the [ATPγS] that significantly inhibits pollen germination from 100 μM to 50 μM. In agreement with the agonist data, inhibiting cGMP production with ODQ reversed the inhibition of pollen germination by ATPγS. The consistency of these results demonstrates that the NO signalling pathway is activated in response to increased eATPγS.
Due to constraints on the solubility of ODQ in DMSO, pollen tube growth rates were measured in 0.5% DMSO with and without 250 μM ATPγS. This level of DMSO lowers the concentration of ATPγS that inhibits pollen tube elongation (data not shown). Given that DMSO can enhance the permeability of cell membranes (Gurtovenko and Anwar, 2007), one possible explanation for this is that DMSO is affecting the membrane leakiness of the pollen tubes, allowing cytoplasmic ATP, which is typically in the low mM range (Gout et al., 1992; Borisjuk et al., 2003), to leak out of the cell and lower the ATPγS needed to inhibit pollen tube elongation.
The addition of 100 μM ODQ reversed the inhibition of pollen tube elongation by 250 μM ATPγS, while 100 μM ODQ had no effect alone (Fig. 2D). These results indicate that the NO signalling pathway is downstream of the inhibition of pollen tube elongation by ATPγS.
Application of ATPγS increases NO production in Col-0 elongating pollen tubes
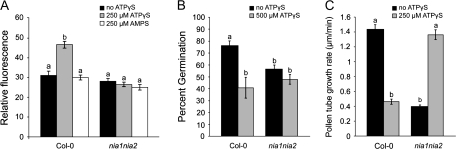
Pollen grains have significant auto-fluorescence, but pollen tubes do not, which allowed direct measurements of NO levels after the application of ATPγS and AMPS in elongating pollen tubes. This was accomplished with DAF-2D, a specific fluorescent marker for the presence of NO (Foissner et al., 2000). For these studies wild-type and mutants with reduced NO were used. The nia1nia2 (nia1-1, nia2-5) plants are double mutants in nitrate reductase, and they have been shown to have only 0.5% of the nitrate reductase activity of wild-type plants (Wilkinson and Crawford, 1993). Because the nia1nia2 mutants are in a Col-0 background, Col-0 pollen was used as a control for these experiments. Elongating wild-type and nia1nia2 mutant pollen tubes were treated with DAF-2D alone or with ATPγS or AMPS. The control DAF-2D fluorescence levels for both wild-type and mutant pollen tubes showed were above background and equal. Adding 250 μM AMPS did not significantly change DAF-2D fluorescence, but the addition of 250 μM ATPγS significantly increased DAF-2D fluorescence in wild-type Col-0 pollen tubes (Fig. 3A). This same induction of DAF-2D fluorescence was also observed in WS pollen tubes (data not shown). However, applying 250 μM ATPγS did not cause increased DAF-2D fluorescence in nia1nia2 mutant pollen tubes. These results indicate that levels of ATPγS that inhibit pollen tube elongation also increase NO levels in elongating wild-type pollen tubes and provides a partial explanation for the altered response to ATPγS in nia1nia2 pollen tubes. Changes in NO levels were first apparent 10 min after treatment with ATPγS, however, the biggest difference in the level of NO between control pollen and ATPγS-treated pollen was observed at 20 min.
Fig. 3.
Effects of ATPγS on intracellular NO levels, pollen germination, and pollen tube growth in Col-0 wild-type and nia1nia2 pollen, (A) DAF-2D and PGM+0.5% DMSO, 250 μM ATPγS, or 250 μM AMPS were added to elongating pollen tubes, and images were taken using confocal laser microscopy. The emission with added ATPγS or AMPS was compared to the control with DAF-2D only at the 20 min time point. Only in wild-type plants did the addition of ATPγS caused a statistically significant increase in fluorescence (n ≥20). (B) Col-0 wild-type pollen germination is inhibited by ATPγS, while nia1nia2 pollen germination is unchanged by ATPγS. Control is PGM+0.5% DMSO, ATPγS concentration is 500 μM+0.5% DMSO. (C) Col-0 wild-type pollen tube elongation is inhibited by ATPγS, while nia1nia2 pollen tube elongation is promoted by ATPγS. Control is PGM+0.5% DMSO, ATPγS concentration is 250 μM+0.5% DMSO. Different letters above the bars indicate values that are significantly different from each other (P ≤0.05). Error bars are ±SE.
Nitrate reductase double mutants show altered pollen germination and elongation
The levels of ATPγS required to inhibit Col-0 pollen germination and elongation were higher than what was required to inhibit WS pollen: inhibition of germination required 500 μM ATPγS and inhibition of tube elongation 250 μM ATPγS (Fig. 3B, C). The control germination rate for nia1nia2 pollen was lower than wild-type pollen, and was unaffected by treatment with ATPγS. Interestingly, the control pollen tube elongation rate for nia1nia2 pollen was also lower than wild-type pollen, but treatment with ATPγS resulted in promoting the nia1nia2 pollen tube elongation rate to the wild-type control rate. Just as in WS pollen, the addition of 100 μM ODQ by itself had no effect on Col-0 pollen germination rates, but it was able to reverse the inhibition of Col-0 pollen germination by 500 μM ATPγS (see Supplementary Fig. 1 at JXB online). Addition of 100 μM ODQ reversed the inhibition of Col-0 pollen tube elongation by 250 μM ATPγS, while 100 μM ODQ had no effect alone (see Supplementary Fig. 1 at JXB online).
Discussion
Before confidently connecting a signal transduction pathway initiated by eATP to the production of NO followed by cGMP, possible non-specific effects of eATP must be considered. Tang et al. (2003) showed that neither calcium chelation nor inorganic phosphate could account for the inhibition of root gravitropism by ATP, and Steinebrunner et al. (2003) also performed controls eliminating chelation or phosphate effects as explanations of the inhibitory effects of eATP on pollen germination. In this study, a poorly-hydrolysable analogue of ATP, ATPγS, was used instead of ATP to prevent the release of inorganic phosphate from the applied nucleotides and also to lower the concentration of nucleotide needed to induce responses. Its addition to the pollen/PGM suspension had no effect on pH. The use of ATPγS enssured that the nucleotide signal would be more stable and thus relatively more potent than ATP. Instead of the 2 mM concentration of ATP needed by Steinebrunner et al. (2003) to inhibit WS pollen germination significantly, 100 μM ATPγS sufficed to have an effect, which lessened the chance of secondary effects being the cause of the inhibition of pollen germination and pollen tube growth. AMPS was tested for its effect on pollen germination and pollen tube elongation at each concentration of ATPγS tested. Even at concentrations several times greater than the effective concentration of ATPγS, no significant effects of AMPS were observed. In addition, Medicago truncatula pollen were tested for their response to ATPγS, and they showed a similar pattern of pollen germination inhibition. These findings and the controls used argue for the specificity of the observed effects of ATPγS on pollen.
The data presented here have emphasized the inhibitory effects of high concentrations of extracellular nucleotides. Are there physiological situations where higher eATP could occur in the pollen ECM to inhibit its germination or growth?
Given evidence in animals and plants that ATP can be released to the exterior of cells by the fusion of ATP-loaded secretory vesicles to the plasma membrane (Lazarowski et al., 2003; Kim et al., 2006; Wu et al., 2007), there are several situations in which pollen could encounter relatively high [eATP]. One example, potentially, would be during pollen development in the lumen of anthers, which is an environment bathed in the secretions of the tapetal layer. If these secretions are high in [ATP], like those of secretions from polarly-growing root hairs and pollen tubes (Kim et al., 2006; Wu et al., 2007), then the documented lack of ectoapyrase expression until late in pollen maturation (Wu et al., 2007) could be a mechanism to maintain high [eATP] in the lumen of anthers and prevent precocious pollen germination, such as occurs in raring-to-go mutants (Johnson and McCormick, 2001). Similarly, if there was a failure of ectoapyrase expression in pollen even at maturity, this could sustain a high [eATP] in pollen walls and contribute to the male sterility phenomena.
The papillar surface of a stigma is another environment of high secretory activity encountered by pollen. To the extent that the environment is high in [eATP], any inhibition of ectoapyrase activity at this site could result in the failure of pollen to germinate; i.e. could contribute to the variety of stigmatic mechanisms that inhibit pollen germination.
Previous findings showed that Arabidopsis pollen lacking two ectoapyrase enzymes, APY1 and 2, fail to germinate (Steinebrunner et al., 2003). Furthermore, when either apyrase inhibitors or anti-apyrase antibodies were added to the pollen germination media, pollen tube growth was significantly slowed, and the decreased pollen tube growth rate was accompanied by an increase in eATP (Wu et al., 2007). These findings predict that to discover whether situations where pollen encounters high [eATP] actually occur, it will be important to document environmental or developmental signals that inhibit ectoapyrase expression in pollen, and to develop tools, like those of Kim et al. (2006), to measure [eATP] in microenvironments in which pollen develops and grows.
The responses of pollen to ATPγS occur at higher concentrations than described for the nucleotide-induced production of reactive oxygen species (ROS) in Arabidopsis leaves (Song et al., 2006) and of NO and ROS in Dasycladus vermicularis (Torres et al., 2008). One explanation for this could be the down-regulation of the ATP receptor at an initial ATP exposure, as is known to occur in animals (Rettinger and Schmalzing, 2004) and protists (Hennessey, 2005). Pollen used for these studies was exposed to from 2 μM to 5 μM ATP that was released into the pollen/PGM suspension during vortexing of the flowers to release pollen (data not shown), a level that in animal cells would trigger a down-regulation of the ATP receptor, thereby decreasing the sensitivity of the cells to eATP. Rettinger and Schmalzing (2004) reported that, in Xenopus, ATP receptor desensitization was responsible for an approximate 1000-fold difference in the ATP signalling threshold.
The inhibition of pollen germination and pollen tube elongation by eATPγS begs the question, what are the possible effectors involved in the transduction of this signal? Given the inhibition of lily pollen tube elongation by NO (Prado et al., 2004), it seemed reasonable to test whether NO and cGMP might be produced in pollen in response to elevated extracellular nucleotides. The results of this test, based on the fluorescent reporter for NO, DAF-2D, indicated there must be NO-producing enzymes in Arabidopsis pollen, and that inhibitory concentrations of eATPγS (but not AMPS) increase their activity. These results also revealed that Arabidopsis pollen, like lily pollen (Prado et al., 2004), has a basal DAF-2D fluorescence above background. The fact that there is a basal level of NO in growing pollen tubes, and that nia1nia2 mutants do not germinate as well as wild-type pollen and have a lower rate of tube growth, point to the possibility that a low level of NO is necessary for pollen tube growth, but higher levels of NO can inhibit that growth.
Agonists and antagonists of the NO signalling pathway were used to test whether inhibition of pollen germination and/or pollen tube growth by increased eATPγS was mediated by NO. The concentration of ATPγS that inhibits pollen germination was lower in the presence of agonists of the NO signalling pathway; in addition, when ODQ, an inhibitor of guanylate cyclase, was applied, the inhibition of pollen germination by ATPγS was reversed (Fig. 2). Using chemical mediators can have secondary or non-specific effects, but it is unlikely that six chemicals that impact the NO signalling pathway at several different points and that have different solubilities would have the same non-specific effects. Also, each chemical was used at a concentration that by itself had no effect on the pollen. Finally, results using ODQ suggested NO is also involved in mediating the ATPγS-induced inhibition of pollen tube elongation. Although this inhibition was only 20% in WS pollen, it was over 3-fold in Col-0 pollen, and it was blocked by ODQ in both WS (Fig. 2D) and Col-0 (see Supplementary Fig. 1 at JXB online). Overall, data from the pharmaceutical experiments indicate that the inhibition of both pollen germination and pollen tube elongation by ATPγS may be transduced via the NO signalling cascade. However these two responses clearly have different sensitivities that change depending on which ecotype is tested, which may reflect differential receptor sensitivities or endogenous levels of eATP.
Both NOS and NR have been identified as being sources of NO in plants (Crawford, 2006), although doubts have been raised about the previously identified AtNOS 1 and 2 (Moreau et al., 2008). Both enzymes are potentially regulated by calcium (Cookson et al., 2005; Crawford, 2006), consistent with the results of Wu and Wu (2008), who documented that eATPγS had to induce an increase [Ca2+]cyt in hairy roots as a required upstream step in order for it to stimulate NO production. If NO is part of a signal transduction chain in pollen, then some NO-producing enzymes must exist in Arabidopsis pollen, as discussed above and previously demonstrated in Paulownia pollen (He et al., 2007).
Microarray data show the expression of potential NO-producing transcripts in pollen. Honys and Twell (2004) document AtNOS1 expression only in uninucleate microspores, but show NR1 and 2 expression in trinucleate pollen. Yet they did not detect AtNOS1 or NR1 or 2 transcripts in mature pollen grains. Conversely, Pina et al. (2005) did detect AtNOS1 and NR1 and 2 in pollen. Both of these studies quantified mRNA levels, which may or may not reflect changes in protein levels.
Initial dose–response tests and NO measurements were carried out in WS pollen so they could be related to the earlier pollen studies of Steinebrunner et al. (2003) and Wu et al. (2007). However, the fact that nia1nia2 mutants were in a Col-0 background required the response of Col-0 wild-type pollen to applied nucleotides to be tested, because different ectotypes of Arabidopsis show significantly different responses to the same stimuli (Maloof et al., 2001). Both for germination and growth responses, the threshold for significant responses to applied ATPγS in Col-0 pollen was about 2-fold greater than in WS pollen. In WS pollen,the applied nucleotides suppressed pollen germination by about 50% but suppressed growth rates by only about 20%, suggesting that, in WS plants, eATP signalling may be more important for pollen germination than for pollen growth. Results in Col-0 pollen, on the other hand, indicated that eATP can strongly influence both germination and tube growth. Applied nucleotides reduced Col-0 pollen germination by about 50%, and they reduced the growth rate of wild-type Col-0 pollen by over 3-fold. However, both these effects were nullified in nia1nia2 mutants, where NO production is suppressed. This result provided the strongest evidence that NO helps mediate the inhibitory effects of ATPγS on pollen germination and tube growth.
Unexpectedly, the low rate of tube growth of nia1nia2 pollen was significantly increased by the same 250 μM ATPγS concentration that inhibited growth of wild-type pollen. This indicates there is some signalling pathway turned on by applied nucleotides that can enhance tube growth in pollen lacking nitrate reductase activity. Given that eATP induces an increase in [Ca2+]cyt in diverse tissues (Demidchik et al., 2003; Jeter et al., 2004; Wu and Wu, 2008), one could plausibly speculate that this growth-enhancing process is turned on by calcium-dependent enzymes. Calcium-dependent signalling pathways play a major role in pollen germination and growth (Malho et al., 2006), and calmodulin and calmodulin-like genes are prominently turned on during pollen germination and growth in Arabidopsis (Wang et al., 2008), so there are diverse ways an eATPγS-induced increase in [Ca2+]cyt could stimulate pathways that promote tube expansion. However, additional studies would be needed to identify what signalling steps other than changes in NO production transduce ATPγS treatment into enhanced growth in nia1nia2 pollen.
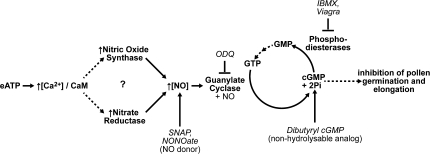
While the literature regarding NO signalling in plants is well established, the idea of eATP signalling is still developing. The results of this paper have further clarified the role of extracellular nucleotides as physiological signals in plants by identifying some of the transduction steps that connect them to growth changes. Although the data do not rule out the involvement of a nitric oxide synthase in the NO production induced by extracellular nucleotides, the results using the nia1nia2 mutant provide support for the participation primarily of nitrate reductase in this response. The ODQ results also imply that the NO effects are mediated through guanylate cyclase and the production of cGMP. Based on these findings a model is proposed for the signal transduction pathway initiated by eATP leading to inhibition of pollen germination and elongation (Fig. 4). Overall, the data presented strengthen the connection between eATP and NO signalling pathways in the regulation of pollen growth.
Fig. 4.
Model for eATP signalling in pollen. eATP causes increased cytoplasmic calcium levels which binds to and activates calmodulin (CaM). NO can be produced via nitric oxide synthase and/or nitrate reductase which can be activated by binding the calcium/calmodulin complex. NO binds to and activates guanylate cyclase which converts GTP into cGMP. The cGMP mediates specific cellular responses such as pollen germination and elongation, and is then broken down by phosphodiesterases. NO signalling agonists and antagonists used in this study are indicated in italics.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. 1. (A) ODQ reverses Col-0 pollen germination inhibition by ATPγS. (B) ODQ also reverses the ATPγS inhibition of Col-0 pollen tube elongation.
Supplementary Material
Acknowledgments
The authors would like to thank the following individuals for their invaluable assistance: Elison Blancaflor for the Medicago truncatula seeds and protocols, and Nigel Crawford for the nia1nia2 seeds and protocols; Angela Bardo and John Mendenhall from the Microscopy and Imaging Facility of the Institute for Cellular and Molecular Biology at The University of Texas at Austin for their help with the confocal microscope; Elizabeth Henaff for ATP measurements; Craig Handley for growing of the nia1nia2 mutant plants; Nicole Smith for help with the nia1nia2 data; and Marianna Grenadier for the artwork on all of the figures. This material is based upon work supported by the National Science Foundation under Grants No. IBN-0344221 and IOS-0718890 to SR.
References
- Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signalling in plants. Annual Review of Plant Biology. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Beligni M, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. The Plant Journal. 2007;52:570–582. doi: 10.1111/j.1365-313X.2007.03248.x. [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Walenta S, Panitz R, Wobus U, Weber H. Energy status and its control on embryogenesis of legumes, ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. The Plant Journal. 2003;36:318–330. doi: 10.1046/j.1365-313x.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock J, Weir I, Neill S. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on HP2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight G. Cellular distribution and functions of P2 receptor subtypes in different systems. International Review of Cytology— A Survey of Cell Biology. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu H. Structural and signalling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- Chivasa S, Ndimba B, Simon W, Lindsey K, Slabas A. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. The Plant Cell. 2005;17:3019–3034. doi: 10.1105/tpc.105.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson S, Williams L, Miller A. Light–dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiology. 2005;138:1097–1105. doi: 10.1104/pp.105.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. Journal of Biological Chemistry. 1999;274:13729–13732. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- Crawford NM. Mechanisms for nitric oxide synthesis in plants. Journal of Experimental Botany. 2006;57:471–478. doi: 10.1093/jxb/erj050. [DOI] [PubMed] [Google Scholar]
- Darwin C. The power of movement of plants. London: John Murray; 1880. [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover B, Davies J. Is ATP a signalling agent in plants? Plant Physiology. 2003;133:456–461. doi: 10.1104/pp.103.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Firnhaber C, Pühler A, Küster H. EST sequencing and time-course microarray hybridizations identify more than 700 Medicago truncatula genes with developmental expression regulation in flowers and pods. Planta. 2005;222:269–283. doi: 10.1007/s00425-005-1543-3. [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. The Plant Journal. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Foresi N, Laxalt A, Tonón C, Casalongué C, Lamattina L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiology. 2007;145:589–592. doi: 10.1104/pp.107.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cao LS, Young MT, North RA. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. Journal of Biological Chemistry. 2008;283:15122–15126. doi: 10.1074/jbc.M801512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Douce R. Regulation of intracellular pH values in higher plant cells. Journal of Biological Chemistry. 1992;267:13903–13909. [PubMed] [Google Scholar]
- Gurtovenko A, Anwar J. Modulating the structure and properties of cell membranes. The molecular mechanism of action of dimethyl sulfoxide. Journal of Physical Chemistry B. 2007;111:10453–10460. doi: 10.1021/jp073113e. [DOI] [PubMed] [Google Scholar]
- He YK, Tang RH, Hao Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- He J-M, Bai X-L, Wang R-B, Cao B, She X-P. The involvement of nitric oxide in ultraviolet-B-inhibited pollen germination and tube growth of Paulownia tomentosa in vitro. Physiologia Plantarum. 2007;131:273–282. doi: 10.1111/j.1399-3054.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- Hennessey T. Responses of the ciliates Tetrahymena and Paramecium to external ATP and GTP. Purinergic Signalling. 2005;1:101–110. doi: 10.1007/s11302-005-6213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Neill S, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H, Pertl H, Pittertschatscher K, Fadl-Allah E, El-Shahed A, Bentrup FW, Obermeyer G. Release of an acid phosphatase activity during lily pollen tube growth involves components of the secretory pathway. Protoplasma. 2002;219:176–183. doi: 10.1007/s007090200019. [DOI] [PubMed] [Google Scholar]
- Jeter C, Tang W, Henaff E, Butterfield T, Roux SJ. Evidence of a novel cell signalling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. The Plant Cell. 2004;16:2652–2664. doi: 10.1105/tpc.104.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, McCormick S. Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiology. 2001;126:685–695. doi: 10.1104/pp.126.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Brousseau SA, McCormick S. A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically expressed genes. The Plant Journal. 2004;39:761–775. doi: 10.1111/j.1365-313X.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Sivaguru M, Stacey G. Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signalling. Plant Physiology. 2006;142:984–992. doi: 10.1104/pp.106.085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Molecular Pharmacology. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lew R, Dearnaley J. Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Science. 2000;153:1–6. [Google Scholar]
- Li H, Lin Y, Heath R, Zhu M, Yang Z. Control of pollen tube tip growth by a Rop GTPase–dependent pathway that leads to tip-localized calcium influx. The Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malho R, Liu Q, Monteiro D, Rato C, Camacho L, Dinis A. Signalling pathways in pollen germination and tube growth. Protoplasma. 2006;228:21–30. doi: 10.1007/s00709-006-0162-6. [DOI] [PubMed] [Google Scholar]
- Maloof JN, Borevitz JO, Dabi T, et al. Natural variation in light sensitivity of Arabidopsis. Nature Genetics. 2001;29:441–446. doi: 10.1038/ng777. [DOI] [PubMed] [Google Scholar]
- Moreau M, Lee G, Wang Y, Crane B, Klessig D. AtNOS/A1 is a functional Arabidopsis thaliana cGTPase and not a nitric oxide synthase. Journal of Biological Chemistry. 2008;283:32957–32967. doi: 10.1074/jbc.M804838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina C, Pinto F, Feijó JA, Becker JD. Gene family analysis of the arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiology. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado AM, Colaco R, Moreno N, Silva AC, Feijo JA. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signalling. Molecular Plant. 2008;1:703–714. doi: 10.1093/mp/ssn034. [DOI] [PubMed] [Google Scholar]
- Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP for the P2X1 receptor. Journal of Biological Chemistry. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Fernie AR, Wucke C, Geigenberger P. The potato-specific apyrase is apoplastically localized and has influence on gene expression, growth, and development. Plant Physiology. 2008;147:1092–1109. doi: 10.1104/pp.108.117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends in Plant Science. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Steinebrunner I, Wang X, Stout S, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiology. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ. Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiology. 2003;131:1638–1647. doi: 10.1104/pp.102.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady S, Sun Y, Muday G, Roux SJ. Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiology. 2003;131:147–154. doi: 10.1104/pp.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. A role for ectophosphatase in xenobiotic resistance. The Plant Cell. 2000;12:519–533. doi: 10.1105/tpc.12.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Rivera A, Clark G, Roux SJ. Participation of extracellular nucleotides in the wound response of Dasycladus vermicularis and Acetabularia acetabulum (Dasycladales, Chlorophyta) Journal of Phycology. 2008;44:1504–1511. doi: 10.1111/j.1529-8817.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiology. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Crawford NM. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes Nia1 and Nia2. Molecular General Genetics. 1993;239:289–297. doi: 10.1007/BF00281630. [DOI] [PubMed] [Google Scholar]
- Wolf C, Hennig M, Romanovicz D, Steinebrunner I. Developmental defects and seedling lethality in apyrase AtAPY1 and AtAPY2 double knockout mutants. Plant Molecular Biology. 2007;64:657–672. doi: 10.1007/s11103-007-9184-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ. Apyrases (nucleoside triphosphate-diphosphohydrolases) play key role in growth control in arabidopsis. Plant Physiology. 2007;144:961–975. doi: 10.1104/pp.107.097568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Wu JY. Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. Journal of Experimental Botany. 2008;59:4007–4016. doi: 10.1093/jxb/ern242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto F, La Camera S, Polverari A, Delledonne M. Cross-talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiology. 2006;141:379–383. doi: 10.1104/pp.106.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.