Abstract
In Crocus vernus, a spring bulbous species, prolonged growth at low temperatures results in the development of larger perennial organs and delayed foliar senescence. Because corm growth is known to stop before the first visual sign of leaf senescence, it is clear that factors other than leaf duration alone determine final corm size. The aim of this study was to determine whether reduced growth at higher temperatures was due to decreased carbon import to the corm or to changes in the partitioning of this carbon once it had reached the corm. Plants were grown under two temperature regimes and the amount of carbon fixed, transported, and converted into a storable form in the corm, as well as the partitioning into soluble carbohydrates, starch, and the cell wall, were monitored during the growth cycle. The reduced growth at higher temperature could not be explained by a restriction in carbon supply or by a reduced ability to convert the carbon into starch. However, under the higher temperature regime, the plant allocated more carbon to cell wall material, and the amount of glucose within the corm declined earlier in the season. Hexose to sucrose ratios might control the duration of corm growth in C. vernus by influencing the timing of the cell division, elongation, and maturation phases. It is suggested that it is this shift in carbon partitioning, not limited carbon supply or leaf duration, which is responsible for the smaller final biomass of the corm at higher temperatures.
Keywords: Acclimation, carbon partitioning, carbon translocation, corm growth, leaf senescence, low temperature, photosynthesis, spring ephemerals
Introduction
Although many plants have adapted to grow at low temperatures, their growth rate and final size are usually reduced under low temperatures (Körner and Larcher, 1988). In contrast, spring ephemerals are known to grow better at lower temperatures, with larger underground perennial organs as a result (De Hertogh and Le Nard, 1993; Nault and Gagnon, 1993; Lapointe and Lerat, 2006). The greater biomass accumulation in the perennial organs of spring ephemerals at lower temperatures may be due to thermal effects on leaf longevity, as extended periods of epigeous growth would prolong the duration of photosynthetic carbon assimilation and storage. Studies have shown that under constant low temperatures, senescence is delayed; for example, in Erythronium japonicum, senescence is delayed by 1 month at 10 °C compared with plants grown at 20 °C (Yoshie and Fukuda, 1994). However, longer leaf life spans do not invariably result in increased biomass accumulation. For instance, in Erythronium americanum (Lapointe and Lerat, 2006) and Crocus vernus (Badri et al., 2007), longer leaf life span is correlated with higher corm biomass, while in Floerkea proserpinacoides, another spring ephemeral but with an annual cycle, it results in lower final plant biomass (McKenna and Houle, 2000). Prolonged foliar growth as the key explanation of final corm size in C. vernus and in E. americanum has been questioned due to the fact that the corm reaches its final size well before any visual signs of senescence appear at 18/14 °C, while at lower temperature the corm continues to increase in size even after leaf senescence is well advanced (Gutjahr, 2006; Badri et al., 2007). Thus, the early induction of leaf senescence in spring ephemerals may be more strongly connected to the reduction in sink demand, once the corm or bulb is filled with carbohydrates, rather than to any environmental cues such as temperature (Lapointe, 2001). If the reduction in sink strength occurs before any substantial changes in light or temperature, leaf senescence would be induced even if there is a possibility for continued photosynthetic activity.
In addition to leaf life duration, C translocation rates could influence the growth of perennial organs. However, most studies have reported either transient (Lundmark et al., 2006) or longer term reduction in C translocation rates at lower temperatures (Paul et al., 1990; Leonardos et al., 2003), which would not explain the increased size of perennial organs observed in spring ephemerals. Carbon partitioning has also been shown to be affected by growth temperatures. For example, low temperature exposure during post-harvest induces sweetening in potato tuber (Isherwood, 1973). Sweetening during active growth could influence the rate of cell growth in the perennial organ through changes in osmotic pressure (Zonia and Munnik, 2007). Changes in the partitioning between cell wall and non-structural carbohydrates could also alter the growth of sink organs (Fujita et al., 2004). Finally, more subtle changes, such as modifications of the ratio of hexoses to sucrose, might also influence perennial organ growth. High hexose to sucrose ratios are reported during the early stages of organ development and would stimulate cell initiation and elongation, whereas cell differentiation would be triggered by lower ratios of hexoses to sucrose (Kock, 2004; Gibson, 2005). Thus, growth temperature may induce a number of shifts in C partitioning and thereby cause changes in final corm size in spring ephemerals such as C. vernus.
High biomass accumulation under low growth temperatures is an interesting example of cold adaptation but it also puts such species at risk under future global warming scenarios. Data are already accumulating to show that the leafing out of trees is occurring earlier due to earlier and warmer spring temperatures (Menzel, 2000). Earlier spring and an increase in the rate at which temperatures rise early in the spring may well restrict the growth of spring ephemerals. Furthermore, an earlier closure of the canopy due to increased spring temperatures (Menzel, 2000) could also reduce their growth, by limiting the period during which favourable light conditions occur on the forest floor, in the event that canopy closure is advanced more than the sprouting of the spring ephemerals. In combination, these factors might reduce the size of the underground perennial organ, decreasing the frequency of both clonal growth and sexual reproduction in these species. This could, in turn, lead to changes in the species composition of the forest floor. Spring ephemerals might therefore be amongst the first species in temperate hardwood forests to be negatively affected by global warming because of their sensitivity to higher growth temperatures. Thus, understanding how temperature influences their growth and reproductive success is crucial to predicting their response to earlier spring thaws and to warmer spring temperatures.
In the work reported here, the fate of carbon from the leaf to the corm was followed over time in C. vernus (L.) Hill var. Flower Record to identify changes that could explain the higher biomass accumulation observed at lower growth temperatures. The partitioning of carbon between soluble sugars, starch, and cell wall material was also tracked throughout the growth season. This study increases our understanding of the mechanisms that spring ephemerals have evolved to cope with the low spring temperatures and the way they will respond to global warming.
Material and methods
Growth conditions
Crocus vernus corms (var. Flower Record) were purchased from a local supplier (Bröderna Nelsons Frö, Tingsryd, Sweden). Corms of 11–12 g fresh weight were planted in individual pots (7×7 cm, 18 cm deep), in a potting mixture containing peat, perlite, and sand. The corms were cold stratified from early September to early January in order to allow root and shoot growth. Temperatures during cold stratification were 9 °C for the first 2 months then 5 °C for the following 2 months. The plants were then transferred to controlled-environment growth chambers set at either 12/8 °C or 18/14 °C (day/night) for the duration of the epigeous growth period. The lower temperature regime was chosen to represent early spring conditions and the higher to represent conditions later in spring during senescence. A photoperiod of 14 h was employed and the irradiance was kept at 500 μmol m−2 s−1 at plant height for both treatments. Relative humidity was set to 50% and the plants were fertilized once a week with a complete fertilizer (Blomstra, Cederroth International AB, Väsby, Sweden: N, 0.05 g l−1; P, 0.01 g l−1; K, 0.05 g l−1; Ca, 0.003 g l−1; Mg, 0.004 g l−1; plus S, Fe, Mn B, Z, Cu, and Mo as micronutrients).
Harvesting
In order to assess biomass accumulation in the perennial tissue, plants were harvested throughout the epigeous growth period. At 18/14 °C, plants were harvested every week for a total of 10 harvests over 67 d, while at 12/8 °C they were harvested every week or every 2 weeks for a total of nine harvests over a 90 d period. Leaf area was recorded separately for green and yellowing segments at both temperatures. Both green and yellow leaf areas were measured using an area meter (LI-COR LI-3100; Linolnn, NE, USA). Leaf senescence was then estimated based on the percentage of total leaf area that showed yellowing. Growth rate (g d−1) at each temperature was estimated by linear regression using all data points except the initial dry mass since no change in mass occurred between the first and second harvest (see Fig. 1A).
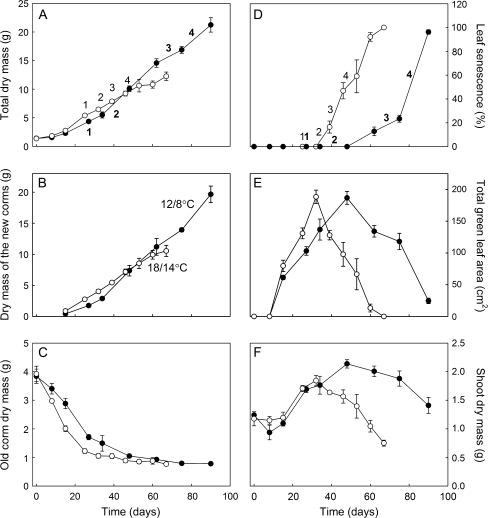
Fig. 1.
Change in total plant biomass (excluding the old corm) (A), and biomass of the new corms (B) and of the old corm (C) in C. vernus throughout the epigeous growth period. (D) Leaf senescence, as a proportion of senescing leaf area of the total leaf area. Total green leaf area (E) and shoot biomass (F) throughout the epigeous growth period. Filled circles represent plants grown at 12/8 °C and open circles plants grown at 18/14 °C. Numbers 1–4 indicate the different 14C labelling dates: (1) at the beginning of corm growth; (2) during the active phase of corm growth; (3) at the beginning of leaf senescence; and (4) at mid leaf senescence. Each value represents the mean (±SE) of six plants.
14C labelling and partitioning
The aerial parts of the plants were labelled with 14C at four different times during the growth period, representing four different phenological stages. At 12/8 °C the labelling was done at day 27, 41, 68, and 80, and at 18/14 °C the labelling occurred at day 25, 33, 39, and 47 (see Fig. 1 for the timing between labelling dates and plant growth). New corm growth had just begun when the first labelling occurred. The second labelling occurred during the fast growth stage of the corm. The third labelling occurred when leaves had just started to senesce and the last labelling occurred when leaf senescence had reached 50%.
Labelling with 14C took place in the growth chamber between 9:00 h and 10:00 h in the morning, 1 h into the light period. At the time of labelling, each plant was hermetically enclosed in a 17.5 l plastic bag. Hydrochloric acid (1 N) was injected in a sodium bicarbonate solution (total of 145 mM NaHCO3 from cold bicarbonate solution and 50 μCi of NaH14CO3) to allow the vaporization of CO2 (final gaseous CO2 concentration: 425 ppm; final specific activity: 115 μCi mmol−1 of CO2). After 30 min of incubation, the bags were removed under a fume hood. Plants were returned to the growth chamber and harvested after six different chase periods: 1, 2, 4, 8, 24, and 48 h. For each chase period, four plants were harvested. Plants were dissected and subsamples of the different organs were immediately frozen in liquid nitrogen, and then stored at –80 °C.
A 50 mg aliquot of leaf material was digested by the addition of, in sequence, 400 μl of 7% perchloric acid, 400 μl of 30% (v/v) hydrogen peroxide, and 200 μl of of 2-methoxyethanol. Between the additions of each chemical the sample was incubated for 24 h. Then the mixture was left for another 24 h before the scintillation cocktail was added. The radioactivity of the sample was measured 1 week later because peroxide gives rise to a lot of chemiluminescence (Farrar, 1993). Only samples from the 1 h chase period were analysed to determine maximum 14C incorporation by the leaf tissue.
The frozen corms were ground to a fine powder using a bead beater and 50 mg of ground tissue was used for extracting the different fractions. The corm material was extracted at 80 °C in 80% ethanol. The ethanol-soluble and -insoluble fractions were prepared as described previously (Nielsen and Veierskov, 1990). The soluble fraction and the lipid fraction were obtained from the ethanol supernatant by the addition of water and chloroform separating the aqueous phase (soluble fraction) from the organic phase (lipid fraction). The cell wall fraction was obtained from the ethanol-insoluble pellet, by first digesting and removing the starch by enzyme degradation. The amount of 14C present in different fractions was then determined by liquid scintillation counting. Total 14C in corm per g fresh weight (FW) was estimated by adding up the 14C detected in the four fractions: digested starch, water-soluble fraction, cell wall, and lipid fraction. The mean value of the four samples was then used to calculate total dpm in corms, based on the biomass of the corms at each phenological stage (data reported in Fig. 1B). Similarly, total 14C in leaves was calculated from the mean 14C in leaf per g FW and the total green leaf biomass. The total 14C in corms was then divided by the total 14C in leaves to give an indication of the fraction of the 14C that reached the corm at each chase period and at each phenological stage. Based on these estimates of total 14C incorporated by the plants, between 8.8% and 22.0% of the initial 50 μCi of 14C present in the bag was fixed by the 12/8 °C plants and between 5.9 and 21.4% by the 18/14 °C plants throughout the season.
Corm carbohydrate concentration
The water-soluble and digested starch fractions were analysed for carbohydrate concentration by using an ion exchange chromatography system (Metrohm, Herisau, Switzerland) consisting of the following: 818 IC pump, 837 IC combi degasser, 830 IC Interface, 820 IC separation centre, 817 Bioscan containing the column and conductivity detector, pulsed ampliometric detection (PAD), MSM (metrohm suppressor module) suppressor, and Metrodata 2.3 software. The soluble carbohydrates and the digested starch samples were separated on a Metrosep Carb-250 column (250 cm×4 mm) using 0.1 mM NaOH as isocratic eluent and a flow rate of 1 ml min−1 (20 min per sample), and quantified using standard curves obtained by diluting stock standards and running dilution series.
Statistical analyses
A linear regression model was used to determine the slope of each growth curve. Then these slopes were compared with an analysis of variance (ANOVA). Two-way ANOVA was used to compare the total 14C incorporated in leaves per g FW, with growth temperature and phenological stages as fixed factors (1 h chase period only). Similarly, carbohydrate concentrations were compared using two-way ANOVAs where growth temperature and phenological stages were used as fixed factors. Data from all chase periods were pooled for these last analyses. The partitioning into the different fractions in percent of total 14C in the corm as well as the total 14C measured in corms were compared using three-way ANOVAs, with growth temperature, phenological stages, and chase time as fixed factors. F, degree of freedom of the factor and of the error term, and the P values are reported for the factors or interactions that are significant. All statistical analyses were performed with Statistix 8.0 (Analytical Software, Tallahassee, Florida).
Results
Plant growth
Growth temperature was found to exert a strong effect on the final dry mass of C. vernus plants (Fig. 1A). The lower temperature regime of 12/8 °C (day/night) resulted in much higher biomass accumulation in the new corms, with a final corm dry weight that was 88% higher than that of the warmer grown plants (Fig. 1B; 10.5±0.9 g at 18/14 °C versus 19.7±1.3 g at 12/8 °C; t20=5.89; P <0.001). The rate of biomass increase was also higher at 12/8 °C than at 18/14 °C (0.18 g d−1 at 18/14 °C versus 0.25 g d−1 at 12/8 °C; F1,107=4.89, P=0.03). Most of the loss in biomass of the old corm occurred early in the growth season, probably to support shoot development and the initiation of the new corms (Fig. 1C). Biomass loss from the old corms was faster at 18/14 °C than at 12/8 °C, but reached a similar final size at both temperatures. Growth at the lower temperature also prolonged leaf life, as reported (Badri et al., 2007), and decreased the initial rate at which senescence occurred (Fig. 1D). At the higher temperature, leaf senescence was complete by day 67, at which point the plants grown at 12/8 °C had only just entered senescence. Complete leaf senescence at 12/8 °C occurred after 90 d. Total leaf area increased more rapidly with the higher temperature regime but reached almost identical maximum values at both temperatures just before leaf senescence was induced (Fig. 1E; t10=0.11, P=0.91). On the other hand, shoot biomass growth was essentially similar at both temperatures up to 32 d (Fig. 1F). However, shoot mass continued to increase for another 14 d at 12/8 °C while shoot mass started to decrease at 18/14 °C due to leaf senescence. Maximum shoot mass reached just before leaf senescence therefore differed between the two growth temperatures (t10=2.52, P=0.03). Thus, it was the growth of the storage corm that was mainly affected by growth temperature not the final size of the leaves.
Incorporation of 14C in leaves
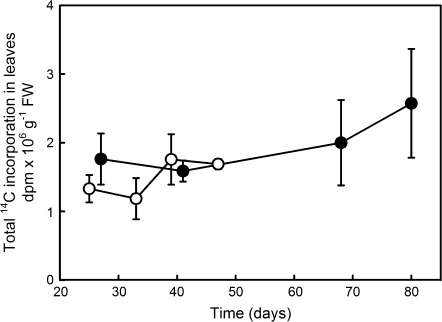
Plants were 14C labelled at four specific times during the growth period that corresponded to different phenological stages of C. vernus (Fig. 1A, D, labels 1–4). Plants were labelled at a single time point during the photoperiod (morning). Photosynthetic rates of spring ephemerals grown in controlled conditions are constant throughout the day (Gutjahr and Lapointe, 2008) and it has been shown that C translocation rates were similar between morning and afternoon (Badri et al., 2007). Based on preliminary measures of photosynthetic rates (MA Badri, unpublished data), plant total leaf area, and initial CO2 concentrations within the head space (17.5 l), it was estimated that between 135 ppm and 250 ppm of CO2 was still present in the head space at the end of the labelling period, indicating that CO2 depletion over the short (30 min) labelling period should not have been sufficient to affect C partitioning, especially during the longer chase periods. Incorporation of 14C into leaves after a chase period of 1 h was used to assess the effect of temperature on photosynthetic activity (Fig. 2). No significant differences were detected in the amount of 14C assimilated by the plants grown at either 12/8 °C or 18/14 °C (F1,21=1.77, P=0.20 for log-transformed data). The amount of carbon incorporated on a green leaf FW basis remained constant throughout the whole growth period at both temperatures (F3,21=0.85, P=0.48 for log-transformed data).
Fig. 2.
Total incorporation of 14CO2 into leaves throughout the epigeous growth period after a chase period of 1 h, in plants grown at either 12/8 °C (filled circles) or 18/14 °C (empty circles). Each point represents the mean (±SE) of 3–4 different plants. Only the green portions of the leaves were analysed for 14C. Labelling occurred in the growth chamber at the growth conditions of light and temperature.
Translocation of newly fixed 14C to developing corms
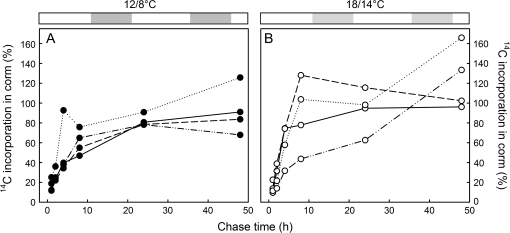
In order to determine the effect of growth temperature on the amount of newly fixed carbon translocated to the corm, the initial incorporation in leaves (data from Fig. 2 on a total leaf FW) was compared with the incorporation into corms up to 48 h after the labelling pulse was given (Fig. 3 on a total corm biomass). The initial rate (up to 8 h) at which the newly fixed carbon was translocated to the corm was slightly higher in plants grown at 18/14 °C than at 12/8 °C (11.4% h−1 and 6.0% h−1, respectively) (Fig. 3A, B). However, comparisons of regression curves indicated no statistical differences in terms of rate of 14C translocation between the two growth temperatures. Translocation of the pulse-labelled carbon into the developing corms continued in plants grown at 12/8 °C for up to 24 h at all phenological stages. In contrast, during early and active phases of corm development at 18/14 °C, translocation of the pulse-labelled carbon into the corm was completed within 8 h. The initiation of senescence at 18/14 °C prolonged the period during which the pulse-labelled carbon from the leaves was translocated to the corm to up to 48 h. Total 14C measured in corms indicated that, in general, more 14C reached the corm at 18/14 °C than at 12/8 °C (Fig. 4D and H; temperature×stage interaction: F3,136=8.51, P <0.001). It was only during the early stage of corm development that more 14C reached the corm at the lower than at the higher temperature regime (first labelling date). However, this high labelling of corm material was associated with a high rate of 14C fixation in the leaves as well, so that the fraction reaching the corm was not higher overall during this phenological stage than later on under the lower temperature regime (Fig. 3A). Therefore, while the rate of C uptake was similar at the two temperature regimes, the proportion of assimilated C delivered to the growing corm was, in general, greater at the higher temperature regime.
Fig. 3.
Fraction of the 14C incorporated in the leaves that was translocated to the corms of plants grown at 12/8 °C (A) or 18/14 °C (B) after 1–48 h of the chase period. Plants were labelled at four different phenological stages throughout the epigeous growth period: at the beginning of corm growth (solid lines), during the active phase of corm growth (dashed lines), at the beginning of leaf senescence (dash-dotted lines), and at mid leaf senescence (dotted lines). Total 14C in leaves is obtained from digestion of whole green leaf tissue while total 14C in corms is the summation of the 14C counts measured in each chemical fraction.
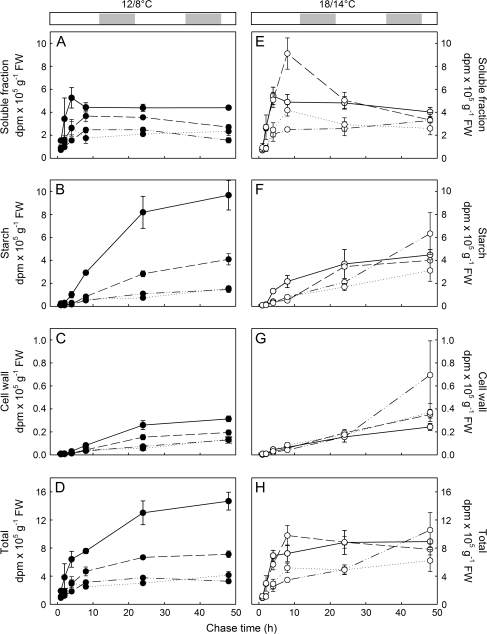
Fig. 4.
Total counts of 14C in the different fractions of the digested corm from plants grown at 12/8 °C (A–D) or 18/14 °C (E–H), after 1–48 h of chase. Plants were labelled at four different phenological stages throughout the epigeous growth period: at the beginning of corm growth (solid lines), during the active phase of corm growth (dashed lines), at the beginning of leaf senescence (dash-dotted lines), and at mid leaf senescence (dotted lines). Each point represents the mean (±SE) of 3–4 different plants.
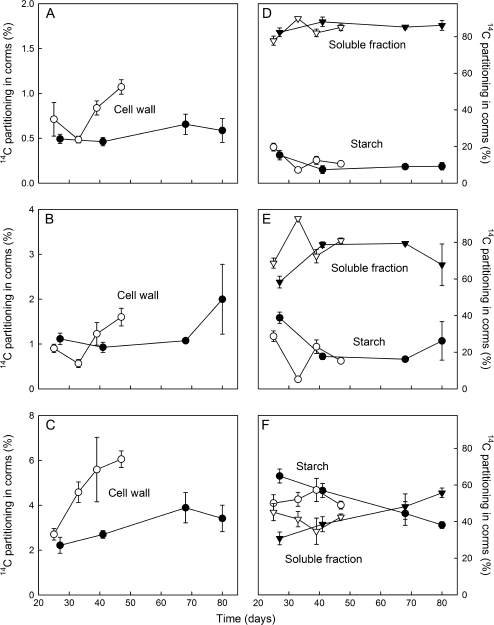
Partitioning of 14C in the corm
Due to the slow incorporation of 14C into the other fractions, most of the 14C translocated to the corm was found in the soluble fraction 4 h into the chase period (Fig. 5D). This soluble fraction contained essentially glucose, fructose, and sucrose, with retention times of 9.8, 12.3, and 14 min, respectively, on the Metrosep Carb-250 column. Accumulation of 14C label into the soluble fraction continued between 4 h and 8 h after labelling, after which it either remained constant or slowly decreased with time (Fig. 4A, E). After 4 h, the incorporation of 14C into other fractions exceeded the translocation of new 14C into the soluble fraction, causing the proportion of labelled carbon in the soluble fraction to decrease over time (Fig. 5E, F). This decrease over time was similar at both temperatures (temperature×chase period interaction: F5,136=1.97, P=0.09). While the soluble fraction reached similar maximum percentages at either temperature (Fig. 5D), the minimum values after 48 h of chase time differed between growth temperatures and between phenological stages (Fig. 5F; temperature×stage×time interaction: F15,136=2.35, P=0.005). Partitioning of 14C into the soluble fraction 48 h after labelling was higher at 18/14 °C than at 12/8 °C during the initial growth of the corm, after which it reached similar values during the active growth phase, finally to become higher at 12/8 °C than at 18/14 °C during senescence. In fact, the minimum was similar throughout the growth period at 18/14 °C (between 35% and 45%) while it steadily increased with time at 12/8 °C from 31% in the early stage of corm growth to 56 % at mid leaf senescence. Thus, 14C labelling in the soluble fraction did not fluctuate during the growth season at the higher temperature regime, while it increased over time at the lower temperature regime.
Fig. 5.
Partitioning of 14C as a percentage of the total amount of 14C in the corm of plants grown at either 12/8 °C (filled symbols) or 18/14 °C (open symbols) after 4 (A and D), 8 (B and E), and 48 h (C and F) of chase. In the left panel, cell wall fraction (circles); in the right panel, soluble (triangles) and starch fractions (circles). Plants were labelled at four different phenological stages throughout the epigeous growth period. Each point represents the mean (±SE) of 3–4 samples.
14C-labelled carbon began to appear in the starch fraction 8 h after the pulse labelling (Fig. 4B, F) and continued to increase up to 48 h. The amount of labelled starch increased more quickly at 12/8 °C between 4 h and 8 h, so that the percentage of 14C in this fraction after 8 h was higher at 12/8 °C than at 18/14 °C (Fig. 5E; temperature×chase period: F5,136=2.53, P=0.032 for rank-transformed data). However, there was a strongly significant interaction between growth temperature and phenological stage on the fraction of 14C in starch (temperature×stage interaction: F3,136=10.64, P <0.001 for rank-transformed data). Labelled starch represented a larger fraction of the total l4C in the corm at 12/8 °C than at 18/14 °C during the early growth period (first two labelling periods), while it represented a larger fraction at 18/14 °C than at 12/8 °C during the later growth period (last two labelling periods; Fig. 5F). At 12/8 °C, 65±4% of the 14C in the corm was present as starch in the early development of the corm, but as time progressed there was a continuous decrease in partitioning towards starch so that after 75 d of growth it had dropped down to 38±2%. The partitioning into starch in plants grown at 18/14°C was stable throughout the growth period, with 50±5% being converted to starch at the first sampling point (day 25) and 49±2% at the last sampling point (day 47). These temporal changes in the proportion located in the starch fraction mirrored the changes in the proportion of 14C located in the soluble fraction after 48 h of chase period: stable at the higher temperature regime and increasing with time at the lower temperature regime.
Similarly to starch, incorporation of the pulse-labelled carbon into cell walls also appeared after 8 h and increased up to 48 h (Fig. 4C, G), while the lipid fraction was labelled as soon as 1 h into the chase period and continued to accumulate 14C over the next 48 h (data not shown). The labelled fraction in the cell wall increased faster at 18/14 °C than at 12/8 °C so that it represented a larger fraction of the total 14C after 24 h and 48 h at 18/14 °C than at 12/8 °C (Fig. 5A–C) (temperature×chase period: F5,136=3.67, P=0.004 for log-transformed data). Both groups of plants allocated an increasing fraction of 14C into corm cell walls as the season progressed but the increase was much higher in the 18/14 °C plants than in the 12/8 °C plants (Fig. 5C; temperature×stage: F3,136=8.95, P <0.001 for log-transformed data): it varied from 2.2% in the early growth phase up to 3.9% during leaf senescence in the 12/8 °C plants, whereas it varied from 2.7% up to 6.1% in the 18/14 °C plants. The percentage of total 14C that was incorporated into the lipid fraction was similar at both temperatures and increased from 2.0% at the beginning of the season up to 2.6% towards the end of the season (data not shown). Investment into cell wall was thus more important at the higher than at the lower temperature regime, although investment in the cell wall increased with time at both temperatures.
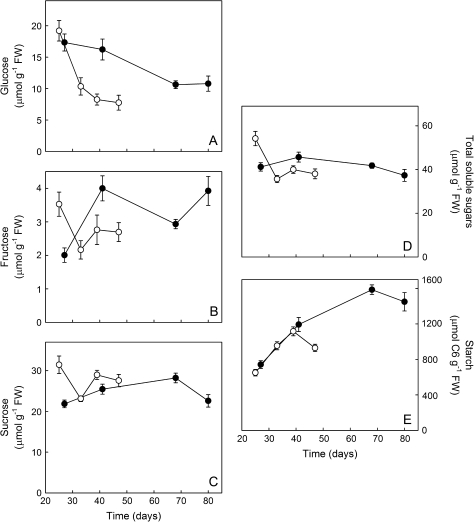
Carbohydrate concentration in the corm
On average, total soluble sugars and sucrose concentrations were similar within each phenological stage between the two temperatures, except for the very first stage where it was higher at the warmer temperature (Fig. 6C, D; temperature×stage interaction: F3,171=8.59, P <0.001 for total soluble sugars and F3,171=7.02, P <0.001 for sucrose; both variables were square root transformed). The total amount of soluble sugars decreased slightly with time and the decrease was faster at the warmer temperature regime. This decrease in total soluble sugars over time was mainly due to changes in glucose concentrations. Glucose dropped during the growth period and the decrease was much faster and also earlier at the warmer temperature regime than at 12/8 °C (Fig. 6A; temperature×stage interaction: F3,171=3.19, P=0.025 for square root-transformed data). Of all the sugars, glucose was the one that exhibited the largest changes in concentration over time, especially at 18/14 °C. Fructose concentration was low compared with glucose and sucrose concentrations (Fig. 6A–C). At 12/8 °C, the concentration was low during the initial growth stage of the corm (first labelling week) and then exhibited an increase, while at 18/14 °C it was the opposite, i.e. the fructose concentration decreased after the initial stage of corm development (temperature×stage interaction: F3,171=12.88, P <0.001 for log-transformed data). Thus the ratios of hexoses to sucrose decreased rapidly at the higher temperature regime and more slowly at the lower temperature regime (data not shown).
Fig. 6.
Carbohydrate concentration in the corm of plants grown at either 12/8 °C (filled circles) or 18/14 °C (empty circles). Plants were harvested at four different phenological stages throughout the epigeous growth period. Each value represents the mean (±SE) of 24 samples (data from all chase times averaged out per date).
Starch was by far the main form of carbohydrate that accumulated in the developing corm of C. vernus (Fig. 6E). Temperature had no effect on the concentration of starch in the corm for the first 40 d. After 40 d, which represents the third phenological stage for plants grown at 18/14 °C and the second stage for plants grown at 12/8°C, the concentration in the warmer grown plants decreased as the leaves were senescing. The corm of plants grown at the lower temperature regime continued to accumulate starch until it also reached the stage where half of the leaves had senesced, which occurred after ∼70 d of growth. This resulted in a much higher starch concentration of the corm in the plants grown at 12/8 °C than in that of those grown at 18/14 °C during the two last phenological stages (temperature×stage interaction: F3,167=4.44, P=0.005 for square root-transformed data). Although starch concentration decreased during leaf senescence under both temperature regimes, the decrease was statistically significant only at 18/14 °C.
Discussion
Growth temperature plays a significant role in determining the growth and biomass accumulation of the perennial organ of C. vernus, and ultimately survival and reproductive success. A new corm is produced every spring and a large amount of starch is accumulated in order to sustain the development of new roots and buds that occurs during the following autumn. Large corms develop more than one bud and thus more than one new corm will form during the following spring, leading to greater reproductive success. Low growth temperatures have a positive effect on leaf longevity and the final biomass of the corms in this species as reported previously (Badri et al., 2007). This increased growth at low temperature is an unusual trait rarely displayed in other temperate higher plants, with the exception of a few other species such as some cultivars of onion, although for onion while growth rate increased with temperature final bulb yield was higher at lower temperature regimes (Daymond et al., 1997).
The present study confirmed that Crocus plants grown at 12/8 °C produce larger corms, with up to 88% more biomass than those of plants grown at 18/14 °C (Fig. 1B). The increase was correlated with higher rates of biomass accumulation in the corms, but no significant increase in the amount of carbon assimilated by the leaves could be observed, based on the amount of 14C detected in the leaves after 1 h of chase period. The amount of carbon fixed by the leaves remained constant throughout the growth period (Fig. 2). Furthermore, the leaves continued to increase in biomass until the first visible sign of senescence appeared at the tip of the leaves. The continued production of new tissue could therefore compensate for the decrease in photosynthetic rates that can be expected with leaf ageing and explain the constant fixation of 14C per g of FW seen over time. Nevertheless, the smaller corm size at the higher growth temperature cannot be explained by restrictions in photosynthetic capacity or by a reduction in leaf area. Growth of another spring ephemeral, E. americanum, under high CO2 concentrations confirmed that under the 18/14 °C regime, bulb growth is not limited by the amount of C fixed by the leaves (Gutjahr and Lapointe, 2008).
Translocation of newly fixed carbon to the corm was found to be slightly faster at 18/14 °C, but there was no statistical difference between the two temperatures. On average the total percentage of carbon redirected to the corm after 48 h was higher in the 18/14 °C plants compared with plants grown at 12/8 °C (Fig. 3). Restrictions in export at low temperature have been reported in other species; however, it is usually associated with a short-term cold shock, and export generally recovers as the plant acclimates to the cold (Lundmark et al., 2006). Translocation of stored carbon from the old corm, based on biomass reduction over time, was also faster at the higher temperature (Fig. 1C), but most of the reserves were mobilized early in the season (first 20 d), while the new corms are still being initiated, and thus did not contribute to the accumulation of biomass in these new organs. Previous data using 11C showed that the amount of carbon translocated to the corm decreased over time in C. vernus and that the decrease took place earlier at the higher temperature regime (Badri et al., 2007). However, Badri et al. (2007), only measured the translocation of 11C during the first 2 h after labelling (half-life of 20 min), at which time only small amounts of 14C were detected in the corm. It was concluded from the 11C data that temperature per se did not influence translocation rates and that the reduction in corm growth at the higher temperature regime could not be explained by a restriction in carbon supply (Badri et al., 2007). In the present study, the chase period was extended up to 48 h. The reduction in the amount of C translocated as the season progresses was not as clear with the 14C data as it was for the shorter chase periods used with 11C. Furthermore, the longer chase periods showed that translocation is greater at the higher temperature regime during most of the season and cannot explain the reduced corm growth observed.
Differences in leaf life duration could only partially explain the larger corms (88% bigger) of the 12/8 °C plants; they also exhibited higher growth rates than the plants grown at the warmer temperature. It was estimated that plants grown at 12/8 °C would be 39% bigger than those grown at the warmer temperature regime if both senesced at the same time (90 d). However, leaf life duration can also be modulated by sink activity (van Doorn, 2008). In a previous study on C. vernus, it was shown that soil temperatures had more impact than air temperatures on corm growth but also on leaf life duration (Badri et al., 2007). Such results strongly suggest that the reduction in leaf life duration at warmer temperature is not mainly a response to faster leaf metabolism at warmer temperature but rather related to below-ground activity that is controlled by soil temperatures. Leaf life duration of C. vernus appears to be modulated by sink strength and thus does not explain the differences observed in final plant size.
The partitioning of the newly fixed carbon allocated to the corm was also influenced by the growth temperature (Figs 4, 5). During the first two phenological stages, the plants grown at 12/8 °C partitioned more of the translocated carbon into starch after a 48 h chase period, while during the later growth stages more of the carbon was redirected towards the soluble fraction (Fig. 5D–F). In contrast, the plants grown at higher temperature constantly allocated a higher percentage of the total labelled carbon pool towards starch than into the soluble fraction, and it remained near 50% throughout the epigeous growth period (Fig. 5D–F). Nevertheless, the rate of starch accumulation in the corm was similar between the two temperatures up to 40 d (Fig. 6E). These results show that the slower growth rate and the restriction in the final size of the perennial organ at 18/14 °C is not due to any limitation in carbon supply, since the plant was able to fix similar amounts of carbon, redirect more of the fixed carbon to the developing corm, and accumulate starch at a rate equal to that of the plants grown at cooler temperatures. The limitation may instead be connected to a differential C partitioning between the different sugar forms within the corm.
Glucose concentrations decreased abruptly very early in the development of the corm at 18/14 °C while it decreased slowly over time at 12/8 °C. Sucrose concentrations exhibited smaller and less consistent changes than glucose over time (Fig. 6A, C). Hexoses are known to stimulate cell division and elongation, whereas an increase in sucrose induces cell differentiation (Kock, 2004). The cells in the corm would thus be held in the elongation phase for a more extended period at 12/8 °C whereas cells at 18/14 °C would enter the differentiation phase much earlier in the season. This model is consistent with the fact that final cell size was reached earlier at 18/14 °C than at 12/8 °C (Badri et al., 2007) and could explain why the overall period of corm growth was extended at the lower temperature regime, allowing cells to reach larger final sizes than at warmer temperature. As accumulation of carbon for storage occurs concurrently with cell elongation in spring ephemerals (Badri et al., 2007), a reduction in the duration of cell elongation caused by a reduction in the ratio of hexoses to sucrose would limit the capacity for storage of photoassimilates within the perennial organ at 18/14 °C. An earlier and faster increase in C partitioning to cell wall material at higher temperatures might also reflect a more rapid shift from cell elongation to cell differentiation (Fig. 5C). Such an inverse relationship between cell growth and C allocation to cell wall material has also been reported in maize where double mutants of sucrose synthase (SuSy) genes, with reduced root tip growth and smaller root cell size, allocated more 13C to cell wall synthesis than wild-type maize at least during the first 25 h (Alonso et al., 2007). More specific measurements of both cell wall thickness and composition are required to understand fully how changes in C allocation to cell wall material are related to cell growth.
It is concluded that the reduced final corm biomass in plants grown at 18/14 °C is not a result of a restriction in carbon supply to the corm. The data presented suggest instead that sugar signalling could be involved and that more rapid decreases in the ratio of hexoses to sucrose induced by the warmer growth temperature could shorten the cell elongation period, leading to reductions in final corm biomass. Furthermore, this transition from cell growth to cell differentiation would reduce the carbohydrate storage capacity and thus the sink strength of the corm, which could be the triggering factor inducing leaf senescence. An earlier reduction in sink demand would cause a negative feedback that restricts photosynthesis and in turn leads to the induction of leaf senescence. It is also plausible that the prolonged leaf life seen at low temperature is, in part, an effect of a reduced sensibility to sugar signals, as a result of the adjustments made in order to cope with the increased sugar accumulation in leaves associated with growth at low temperature (Strand et al., 1997; Stitt and Hurry, 2002). However, more biochemical studies are needed in order to determine if leaf senescence is indeed directly controlled by sink limitation in C. vernus. Because the reduced growth at warmer temperatures appears to be related to the impact of temperature on carbon metabolism and cell growth in the corm, soil temperature should have a major impact on the growth of such species. These data suggest, therefore, that the rate at which soils will warm up in the spring under an increasingly warmer climate could be a determinant for the growth and survival of these species. Spring ephemerals could be amongst the first species to be lost in response to climate warming in the temperate forests in which they occur.
Acknowledgments
This research was supported by research grants from the Natural Sciences and Engineering Research Council of Canada to LL, and from the Swedish Council for Forestry and Agricultural Research to VH.
References
- Alonso AP, Raymond P, Hernould M, et al. A metabolic flux analysis to study the role of sucrose synthase in the regulation of the carbon partitioning in central metabolism in maize root tips. Metabolic Engineering. 2007;9:419–432. doi: 10.1016/j.ymben.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Badri MA, Minchin PEH, Lapointe L. Effects of temperature on the growth of spring ephemerals: Crocus vernus (L.) Hill. Physiologia Plantarum. 2007;130:67–76. [Google Scholar]
- Daymond AJ, Wheeler TR, Hadley P, Ellis RH, Morison JIL. The growth, development and yield of onion (Allium cepa L.) in response to temperature and CO2. Journal of Horticultural Science. 1997;72:135–145. [Google Scholar]
- De Hertogh A, Le Nard M. The physiology of flower bulbs. A comprehensive treatise on the physiology and utilization of ornamental flowering bulbous and tuberous plants. Amsterdam: Elsevier; 1993. [Google Scholar]
- Farrar JF. Carbon partitioning. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and production in a changing environment: a field and laboratory manual. London: Chapman & Hall; 1993. pp. 232–246. [Google Scholar]
- Fujita K, Kai Y, Takayanagi M, El-Shemy H, Adu-Gyamfi JJ, Mohapatra PK. Genotypic variability of pigeonpea in distribution of photosynthetic carbon at low phosphorus level. Plant Science. 2004;166:641–649. [Google Scholar]
- Gibson SI. Control of plant development and gene expression by sugar signalling. Current Opinion in Plant Biology. 2005;8:93–102. doi: 10.1016/j.pbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gutjahr S. Erythronium americanum. 2006. Modulation de la force des sources et des puits de carbone sur la croissance du bulbe de l’érythrone d'Amérique. MSc thesis, Laval University, Québec, Canada. [Google Scholar]
- Gutjahr S, Lapointe L. Carbon dioxide enrichment does not reduce leaf longevity or alter accumulation of carbon reserves in the woodland spring ephemeral Erythronium americanum. Annals of Botany. 2008;102:835–843. doi: 10.1093/aob/mcn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isherwood FA. Starch–sugar interconversion in Solanum tuberosum. Phytochemistry. 1973;12:2579–2591. [Google Scholar]
- Kock K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology. 2004;7:235–246. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Körner C, Larcher W. Plant life in cold climates. Symposium of the Society for Experimental Biology. 1988;42:25–57. [PubMed] [Google Scholar]
- Lapointe L. How phenology influences physiology in deciduous forest spring ephemerals. Physiologia Plantarum. 2001;113:151–157. doi: 10.1034/j.1399-3054.2001.1130201.x. [DOI] [PubMed] [Google Scholar]
- Lapointe L, Lerat S. Annual growth of the spring ephemeral Erythronium americanum as a function of temperature and mycorrhizal status. Canadian Journal of Botany. 2006;84:39–48. [Google Scholar]
- Leonardos ED, Savitch LV, Huner NPA, Öquist G, Grodzinski B. Daily photosynthetic and C-export patterns in winter wheat leaves during cold stress and acclimation. Physiologia Plantarum. 2003;117:521–531. doi: 10.1034/j.1399-3054.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- Lundmark M, Cavaco AM, Trevanion S, Hurry V. Carbon partitioning and export in transgenic Arabidopsis thaliana with altered capacity for sucrose synthesis grown at low temperature: a role for metabolite transporters. Plant, Cell and Environment. 2006;29:1703–1714. doi: 10.1111/j.1365-3040.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- McKenna MF, Houle G. Why are annual plants rarely spring ephemerals? New Phytologist. 2000;148:295–302. [Google Scholar]
- Menzel A. Trends in phenological phases in Europe between 1951 and 1996. International Journal of Biometeorology. 2000;44:76–81. doi: 10.1007/s004840000054. [DOI] [PubMed] [Google Scholar]
- Nault A, Gagnon D. Ramet demography of Allium tricoccum, a spring ephemeral, perennial forest herb. Journal of Ecology. 1993;81:101–119. [Google Scholar]
- Nielsen TH, Veierskov B. Regulation of carbon partitioning in source and sink leaf parts of sweet pepper (Capsicum annuum L.) plants—a role of fructose 2,6-bisphosphate. Plant Physiology. 1990;93:637–641. doi: 10.1104/pp.93.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Lawlor DW, Driscoll S. The effect of temperature on photosynthesis and carbon fluxes in sunflower and rape. Journal of Experimental Botany. 1990;41:547–555. [Google Scholar]
- Stitt M, Hurry V. A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology. 2002;5:199–206. doi: 10.1016/s1369-5266(02)00258-3. [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry VM, Gustafsson P, Gardestrom P. Development of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. The Plant Journal. 1997;12:605–614. doi: 10.1046/j.1365-313x.1997.00605.x. [DOI] [PubMed] [Google Scholar]
- van Doorn WG. Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? Journal of Experimental Botany. 2008;59:1963–1972. doi: 10.1093/jxb/ern076. [DOI] [PubMed] [Google Scholar]
- Yoshie F, Fukuda T. Effects of growth temperature and winter duration on leaf phenology of Erythronium japonicum, a forest spring geophyte. Oecologia. 1994;97:366–368. doi: 10.1007/BF00317326. [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T. Life under pressure: hydrostatic pressure in cell growth and function. Trends in Plant Science. 2007;12:90–97. doi: 10.1016/j.tplants.2007.01.006. [DOI] [PubMed] [Google Scholar]