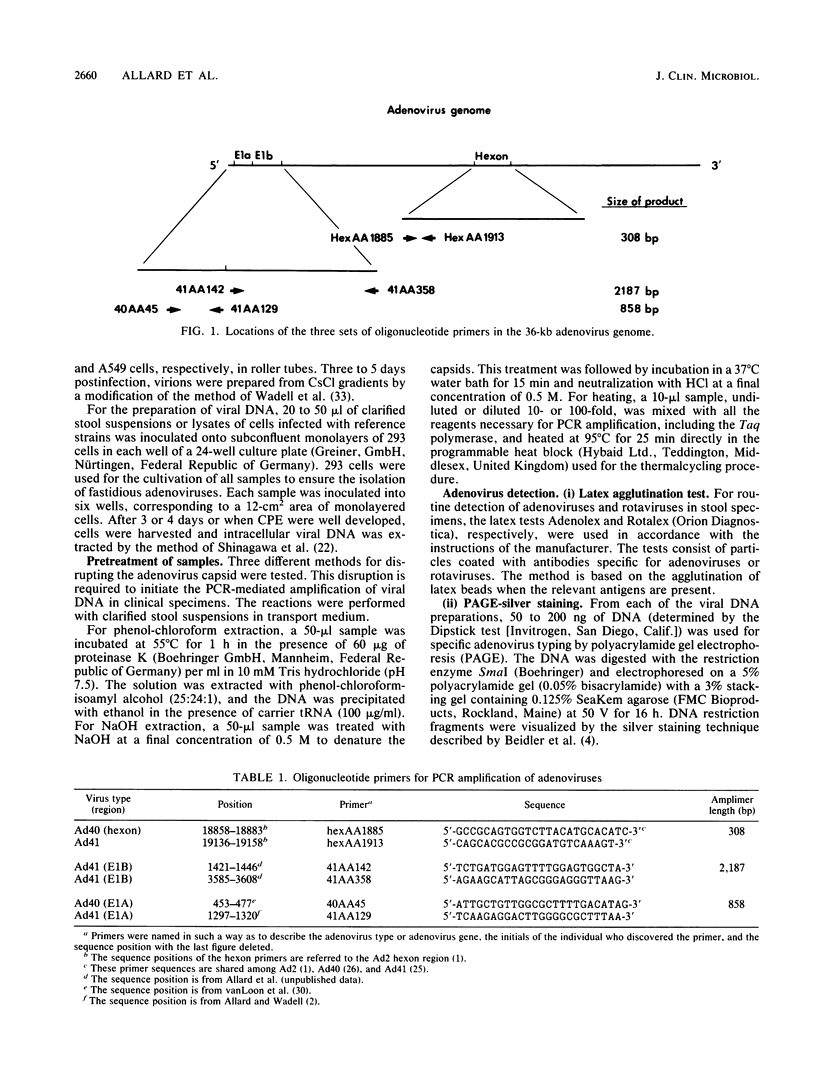
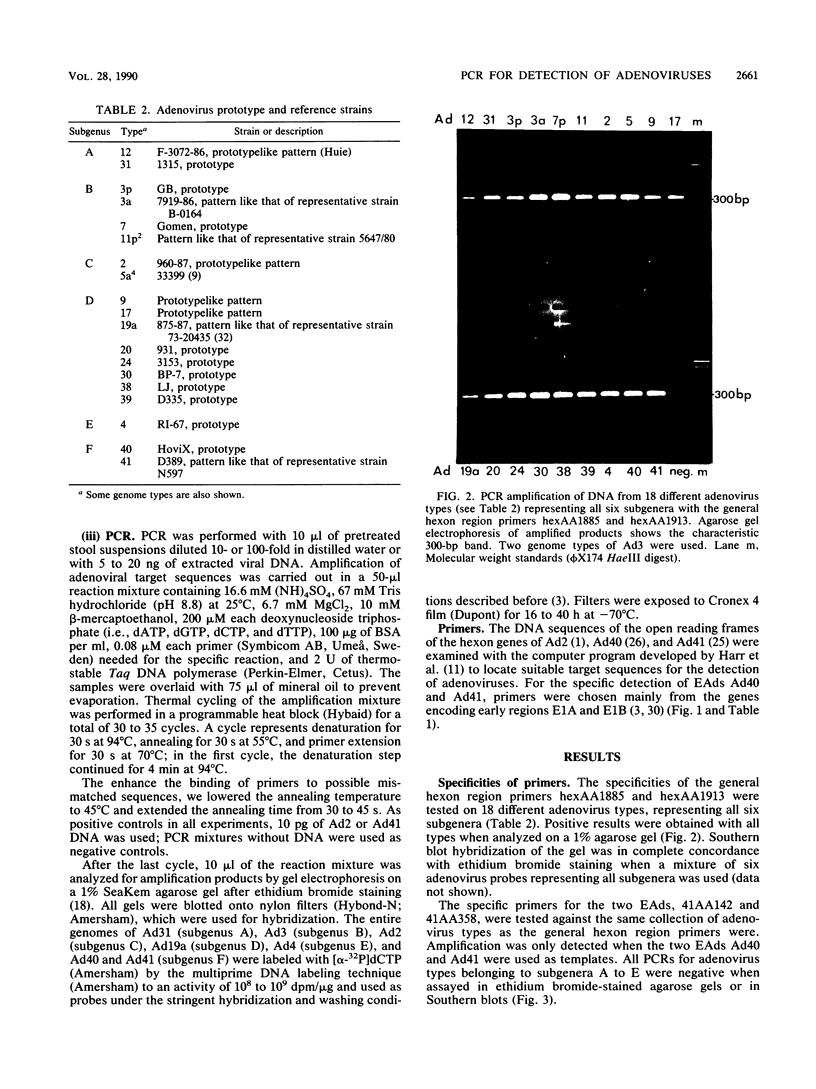
Abstract
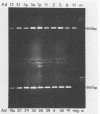
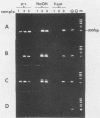
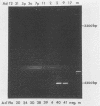
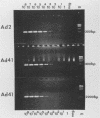
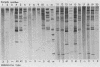
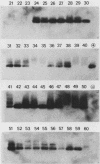
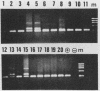
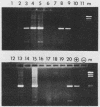
The usefulness of the polymerase chain reaction (PCR) method for diagnosing adenovirus infections was investigated. Several primers, including primers specific for the hexon-coding region and for enteric adenovirus types 40 and 41, were evaluated. The PCR method was validated against cell culturing in routine diagnostic work and against restriction enzyme analysis of viral DNA. Sixty diagnostic specimens were selected for evaluation by the PCR method. Twenty of the 60 specimens were found positive on the basis of cytopathic effects and latex agglutination (Adenolex [Orion Diagnostica, Helsinki, Finland]), and 16 were identified and typed as adenoviruses by polyacrylamide gel electrophoresis. PCR was performed on all 60 specimens in parallel directly on diluted stool samples and on viral DNA extracted from cells inoculated with the same stool samples. When the general hexon primers were used 51 of the 60 specimens from infected cell cultures were found positive by PCR, whereas only 13 specimens were found positive when PCR was performed directly on stool samples. With the use of selective primers for enteric adenoviruses 16 of the 60 cell cultures were found to exhibit amplification products by PCR, whereas 4 were detected in stool samples. None of the 60 specimens were found positive by PCR when an adenovirus type 40-specific primer pair was used. PCR was found to be a fast, sensitive, and reliable method for the detection of adenoviruses in diarrheal disease, provided the amplifications were performed directly on diluted stool samples.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Aleström P., Pettersson M., Lager M., Jörnvall H., Pettersson U. The gene for the adenovirus 2 hexon polypeptide. J Biol Chem. 1984 Nov 25;259(22):13976–13979. [PubMed] [Google Scholar]
- Allard A. K., Wadell G., Evander K. M., Lindman G. K. Specific properties of two enteric adenovirus 41 clones mapped within early region 1A. J Virol. 1985 Apr;54(1):145–150. doi: 10.1128/jvi.54.1.145-150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard A., Wadell G. Physical organization of the enteric adenovirus type 41 early region 1A. Virology. 1988 May;164(1):220–229. doi: 10.1016/0042-6822(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Beidler J. L., Hilliard P. R., Rill R. L. Ultrasensitive staining of nucleic acids with silver. Anal Biochem. 1982 Nov 1;126(2):374–380. doi: 10.1016/0003-2697(82)90530-9. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Gardner M. K., Parrott R. H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985 Mar;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Rodriguez W. J., Kim H. W., Arrobio J. O., Jeffries B. C., Parrott R. H. Rapid presumptive recognition of diarrhea-associated adenoviruses. J Clin Microbiol. 1984 Nov;20(5):1008–1009. doi: 10.1128/jcm.20.5.1008-1009.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandien M., Pettersson C. A., Svensson L., Uhnoo I. Latex agglutination test for adenovirus diagnosis in diarrheal disease. J Med Virol. 1987 Dec;23(4):311–316. doi: 10.1002/jmv.1890230402. [DOI] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson P. A., Johansson M. E., Wadell G. Identification of an enteric adenovirus by immunoelectroosmophoresis (IEOP) technique. J Med Virol. 1979;3(4):307–312. doi: 10.1002/jmv.1890030409. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Uhnoo I., Kidd A. H., Madeley C. R., Wadell G. Direct identification of enteric adenovirus, a candidate new serotype, associated with infantile gastroenteritis. J Clin Microbiol. 1980 Jul;12(1):95–100. doi: 10.1128/jcm.12.1.95-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Uhnoo I., Svensson L., Pettersson C. A., Wadell G. Enzyme-linked immunosorbent assay for detection of enteric adenovirus 41. J Med Virol. 1985 Sep;17(1):19–27. doi: 10.1002/jmv.1890170104. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Cosgrove B. P., Brown R. A., Madeley C. R. Faecal adenoviruses from Glasgow babies. Studies on culture and identity. J Hyg (Lond) 1982 Jun;88(3):463–474. doi: 10.1017/s0022172400070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H., Harley E. H., Erasmus M. J. Specific detection and typing of adenovirus types 40 and 41 in stool specimens by dot-blot hybridization. J Clin Microbiol. 1985 Dec;22(6):934–939. doi: 10.1128/jcm.22.6.934-939.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H., Rosenblatt A., Besselaar T. G., Erasmus M. J., Tiemessen C. T., Berkowitz F. E., Schoub B. D. Characterization of rotaviruses and subgroup F adenoviruses from acute summer gastroenteritis in South Africa. J Med Virol. 1986 Feb;18(2):159–168. doi: 10.1002/jmv.1890180208. [DOI] [PubMed] [Google Scholar]
- Morris C. A., Flewett T. H., Bryden A. S., Davies H. Epidemic viral enteritis in a long-stay children's ward. Lancet. 1975 Jan 4;1(7897):4–5. doi: 10.1016/s0140-6736(75)92370-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. M., White J. L., Grütter M. G., Burnett R. M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986 May 30;232(4754):1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shinagawa M., Matsuda A., Ishiyama T., Goto H., Sato G. A rapid and simple method for preparation of adenovirus DNA from infected cells. Microbiol Immunol. 1983;27(9):817–822. doi: 10.1111/j.1348-0421.1983.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Tiemessen C. T., Wegerhoff F. O., Erasmus M. J., Kidd A. H. Infection by enteric adenoviruses, rotaviruses, and other agents in a rural African environment. J Med Virol. 1989 Jul;28(3):176–182. doi: 10.1002/jmv.1890280313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood C. I., Hay R. T. DNA sequence of the adenovirus type 41 hexon gene and predicted structure of the protein. J Gen Virol. 1988 Sep;69(Pt 9):2291–2301. doi: 10.1099/0022-1317-69-9-2291. [DOI] [PubMed] [Google Scholar]
- Toogood C. I., Murali R., Burnett R. M., Hay R. T. The adenovirus type 40 hexon: sequence, predicted structure and relationship to other adenovirus hexons. J Gen Virol. 1989 Dec;70(Pt 12):3203–3214. doi: 10.1099/0022-1317-70-12-3203. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev Biol Stand. 1983;53:311–318. [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Olding-Stenkvist E., Ekwall E., Mölby R. Aetiology and epidemiology of acute gastro-enteritis in Swedish children. J Infect. 1986 Jul;13(1):73–89. doi: 10.1016/s0163-4453(86)92348-0. [DOI] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Varsanyi T. Incomplete virus particles of adenovirus type 16. J Gen Virol. 1973 Sep;20(3):287–302. doi: 10.1099/0022-1317-20-3-287. [DOI] [PubMed] [Google Scholar]
- Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980 Feb;27(2):292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. J., Bijlsma K., de Jong J. C., Tonkin C. Evaluation of a commercial monoclonal antibody-based enzyme immunoassay for detection of adenovirus types 40 and 41 in stool specimens. J Clin Microbiol. 1989 Jun;27(6):1155–1158. doi: 10.1128/jcm.27.6.1155-1158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. J., Longhurst D., Killough R. I., David T. J. One-year prospective cross-sectional study to assess the importance of group F adenovirus infections in children under 2 years admitted to hospital. J Med Virol. 1988 Dec;26(4):429–435. doi: 10.1002/jmv.1890260410. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- van Loon A. E., Ligtenberg M., Reemst A. M., Sussenbach J. S., Rozijn T. H. Structure and organization of the left-terminal DNA regions of fastidious adenovirus types 40 and 41. Gene. 1987;58(1):109–126. doi: 10.1016/0378-1119(87)90034-5. [DOI] [PubMed] [Google Scholar]