Abstract
Anti-tumor immune responses can be stimulated by interfering with regulatory T cell (Treg) function. However this effect is short-lived unless T cell memory to tumor antigens can be generated. Our recent studies show that Treg cells not only limit primary responses to tumor/self antigens in tumor-bearing hosts, but also prevent the natural generation of T cell memory to such antigens. Here we discuss the role of regulatory T cells in suppressing T cell memory after surgical excision of tumors, and the potential clinical benefits of overcoming this suppression.
Keywords: T cell memory, tumors, regulatory T cells, CD8 T cells, surgery, melanoma
Background
The generation of CD8 T cell memory is one of the major goals of tumor immunotherapy. For years, studies in infectious disease models have demonstrated that memory T cells are required for pathogen clearance and protection against re-infection. More recently, these lessons are being applied to cancer models. While surgery currently remains the leading cure for solid tumors, memory T cell responses may be required for the durable prevention of tumor recurrence and metastasis following surgery. In vitro-generated memory CD8 T cells have also been shown to be highly effective at treating large established melanomas (1, 2). However, in stark contrast with infectious disease models, most human tumors are poorly-immunogenic, and a majority of tumor antigens are unaltered self proteins. This presents a significant challenge, as mechanisms of central and peripheral tolerance prevent the priming of T cell responses against self antigens. Even if tolerance is broken, T cells remain exposed to self antigens in the periphery, which may lead to the development of functionally-impaired memory, as observed with chronic viral infections (3, 4).
Despite these challenges, some vaccination strategies have been capable of inducing long-lived protective T cell responses against poorly-immunogenic tumors. In one of the earliest examples, Engelhard and co-workers showed that a CD40L-matured dendritic cell vaccine could generate CD8 T cell recall responses against the melanocyte differentiation antigen tyrosinase, as well as long-term protection against melanoma (5). Cytokines can also drive the development of memory in vivo, as shown by the development of a durable and protective central and effector memory CD8 T cell response following administration of a DNA vaccine encoding the tumor antigen Fra-1 and the cytokine IL-18 (6). Co-stimulatory molecules may also play an important role. A xenogeneic DNA vaccine encoding gp100, which typically induces only short-term immunity, induced long-lived T cell responses and tumor protection when coupled with a stimulatory antibody to GITR (7). However, aside from these studies, there are few examples of active immunotherapy inducing durable T cell memory to tumor/self antigens. More frequently, memory is demonstrated following the immune-mediated rejection of primary tumors. For example, regression of B16 melanoma, induced by a GM-CSF-producing tumor cell vaccine and CTLA-4 blockade, protected mice from tumor challenge as long as 100 days post-vaccination (8). Such studies illustrate that some aspect of active tumor rejection may lead to the development of immunological memory.
Interestingly, historical data show that progressive tumors themselves can induce functional T cell memory. In the 1980’s North found that protective T cell memory resulted after surgical excision of a highly-immunogenic methylcholanthrene-induced tumor (9). This memory arose naturally in response to tumor growth and without a need for vaccination. However it was crucial to excise primary tumors when they were small to prevent the generation of “suppressor” cells that would attenuate the response. Notably, post-surgical immunity was only a phenomenon of highly-immunogenic tumors, and was not observed in hosts bearing poorly-immunogenic cancers (10).
In hindsight, the “suppressor” cells identified by North may likely have been tumor-induced regulatory T cells (Treg). Treg cells are crucial mediators of peripheral tolerance (11). They possess a CD4+CD25+Foxp3+ phenotype, and arise both in the thymus and through the conversion of Foxp3− CD4+ T cells in the periphery (11, 12). Treg cells suppress the development of CD8 T cell memory in infectious disease models (13), but until recently their role in preventing memory against poorly-immunogenic tumors had not been shown.
Many studies have illustrated that Treg cells prevent primary T cell responses against poorly-immunogenic cancers (11, 12). We previously demonstrated that CD4+CD25+ Treg cells suppress the de novo priming of CD8 T cells in response to growth of the poorly-immunogenic B16 melanoma (14). If Treg cells were depleted during growth of the melanoma, mice primed CD8 T cells against differentiation antigens expressed by both the tumor cells and normal melanocytes. Melanoma tumor-bearing mice that lacked Treg cells also developed concomitant immunity, evidenced by the rejection of a secondary melanoma inoculated at a different site. Thus, Treg cells functioned early to suppress the de novo priming of immunity against this poorly-immunogenic tumor. However whether such tumor/self antigen-specific T cells could develop into functional T cell memory remained unknown.
Removing Treg cells during tumor growth drives the natural development of T cell memory
We recently asked whether tumor growth and Treg depletion could induce functional T cell memory by studying immunity following curative surgery in mice bearing B16 melanoma (15). As in our previous work (14), mice were inoculated with B16 melanoma, and then Treg cells were eliminated with a CD4 depleting antibody. This strategy eliminates CD4+CD25+ T cells, as well as any CD4+ precursors of induced Treg cells. Following Treg depletion, intradermal primary tumors were surgically excised to attenuate T cell priming and to extend the lifespan of the mice. Defining T cell memory was challenging because classical memory T cells are defined based on their ability to persist following the clearance of antigen (4). Because tumor/self antigens are never cleared, we chose to employ an operational definition of memory as a functional T cell response present at least 1 month following surgery.
To assess the development of T cell memory, mice were challenged with B16 tumor cells in the flank 1 month after surgery. Not surprisingly, mice that had received surgical treatment alone were overtaken by secondary tumors. Mice that had received CD4 depletion alone, but no primary tumor, also succumbed to the second tumors. However, 40–60% of mice that had been depleted of CD4 T cells during growth of their primary tumors were protected against secondary tumors given as long as 2 months after surgery. Moreover, these mice developed systemic immunity, evidenced by their rejection of lung tumors inoculated intravenously. Lung tumors that were already established at the time of surgery were also rejected, indicating a potential control of metastatic disease. Importantly, the depletion of CD8 T cells abrogated this long-lived tumor protection, providing evidence of CD8 T cell memory.
These data established that growth of a poorly-immunogenic tumor could induce functional T cell memory, although the specificity of these memory T cells was not known. Our previous work had shown that short-term CD8 T cell responses in Treg-depleted, B16 melanoma-bearing mice were specific for tumor/self antigens (14). Among these antigens were the melanosomal membrane proteins TRP-2/DCT and gp100 (14). However, due to immunological tolerance and antigen persistence, it seemed unlikely that T cells against melanosomal proteins would be sustained following surgery. Because of this, we were surprised to find memory CD8 T cells specific for both TRP-2/DCT and gp100 in mice with post-surgical immunity. TRP-2/DCT-specific T cells were present at least 30 days post-surgery, and IFN-γ and IL-2-producing transgenic T cells specific for gp100 were found as long as 150 days following surgery. Thus tumor-growth in the absence of Treg cells induced durable T cell memory against self antigens expressed by the tumor.
Because little was known about T cell memory against tumor/self antigens, it was also important to characterize these T cells with regards to their phenotype and localization. Based on infectious disease models, the memory CD8 T cell compartment can be divided into two phenotypically and functionally-distinct subpopulations: central (TCM) and effector (TEM) memory (16). In vitro generated TCM have been shown to be more potent than TEM for mediating tumor rejection (2). However, it was unknown whether tumor antigen-specific TCM could be generated in hosts with persistent peripheral self antigen. Interestingly, we found that mice with post-surgical immunity developed a mixed population of antigen-specific TEM and TCM. TEM dominated the population and were found in lung as well as lymphoid tissues, whereas TCM represented a smaller population that was only found in lymphoid tissues. These data illustrated that T cells recognizing tumor/self antigens can develop into long-lived populations of TEM and TCM, even in the face of persistent antigen.
Finally, we observed that a high proportion of Treg-depleted tumor-excised mice also developed an autoimmune response against normal melanocytes. This was evidenced by the outgrowth of white hair (on black mice) beginning at the surgery site, and progressing to other locations with time. This demonstrated that melanoma growth can induce an immune response against normal host melanocytes, and that such autoimmunity is normally prevented by Treg cells. Melanocyte-specific autoimmunity also provided further confirmation of a potent post-surgical immune response against shared tumor/self antigens.
Implications
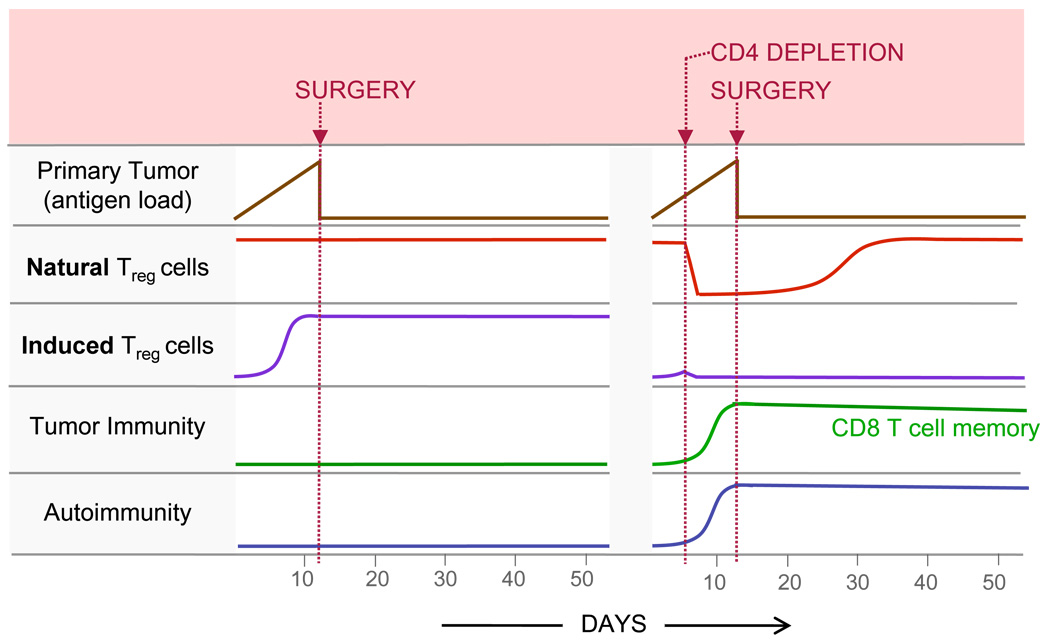
The study of post-surgical immunity has demonstrated that Treg cells are a fundamental obstacle to the development of T cell memory in hosts bearing poorly-immunogenic tumors (Figure 1). As seen in most patients with cancer, our model shows that surgery alone does not induce protection against poorly-immunogenic tumors (Fig. 1; left). However in our model, surgery in conjunction with the depletion of CD4+ Treg cells enables the development of long-lived tumor protection and CD8 T cell memory, as well as melanocyte-specific autoimmunity (Fig. 1; right). The relative contribution of naturally-occurring and tumor-induced Treg cells to the suppression of priming remains unknown, and future work will be required to characterize the kinetics of Treg responses in tumor-bearing mice (Fig. 1). However, based on the robust and undiminished tumor protection that we observe following surgery, one could speculate that the Treg cells that re-emerge in tumor-excised hosts are less suppressive than those that arise in tumor-bearing hosts.
Figure 1. Model for the generation of post-surgical tumor protection and T cell memory in hosts bearing poorly-immunogenic tumors.
Surgery alone is insufficient for providing immunity against poorly-immunogenic tumors which do not naturally prime functional CD8 T cell responses, and may instead induce Treg development (left panel). However, the depletion of CD4 T cells eliminates natural and induced populations of Treg cells, thereby enabling the priming of protective anti-tumor immunity during growth of a poorly-immunogenic tumor (right panel). This tumor-primed immune response develops into functional CD8 T cell memory against tumor/self antigens following surgical excision of the primary tumor (right panel). CD4 depletion in tumor bearing mice also leads to concurrent autoimmunity against the normal tissue counterpart of the tumor (right panel). This model demonstrates that Treg depletion in hosts bearing poorly-immunogenic tumors is sufficient for the generation of CD8 T cell memory following surgical tumor excision
Treg depletion several days prior to surgery could potentially be modeled in the clinic for the treatment of minimal residual disease. The best strategy for Treg depletion in vivo remains to be determined, because cell-surface markers specific for Treg cells have remained elusive. Total CD4 depletion is one possibility, as it clearly induces durable protective memory despite the temporary loss of CD4 helper T cells which have been thought to be crucial for the development of memory (17). Targeting CD25 with an IL-2-diptheria toxin fusion protein is another option that has already proven effective for the depletion of Treg cells in the peripheral blood of cancer patients (18). Alternatively, GITR stimulation and CTLA-4 blockade, which have been used in conjunction with vaccines to induce T cell memory (7, 8) may act directly or indirectly to attenuate Treg function (11). Importantly, in this model of post-surgical immunity, the tumor itself serves as the source of antigen, presumably priming T cell responses against a multitude of tumor antigens. While our studies analyzed memory responses against tumor/self antigens, it is likely that T cells specific for many antigens collectively provide tumor protection. Such a diverse T cell repertoire may help to prevent the emergence of antigen loss variants, in contrast to immunotherapies that target only single antigens.
Following Treg depletion and surgery, we observe long-lived tumor protection and persistent populations of functional TCM and TEM. This was somewhat unexpected in light of studies with chronic viral infections, where a prolonged exposure to high loads of antigen leads to dramatic functional impairments within the memory T cell compartment. Such responding CD8 T cells progressively lose functions and are eventually deleted (3, 19). However, it is important to note that this is not always the case. For example we have previously observed in murine gammaherpesvirus (MHV-68) infection, that most antiviral effector functions are intact (20). In fact, CD8 T cells from MHV-68 persistently infected mice mediate more efficient control of a challenge infection compared to cells from mice that have cleared the virus (20). Therefore some memory cells generated in the face of persisent antigen may actually be better adapted for long-term immune surveillance. Memory T cells in mice with post-surgical immunity might also possess this capability.
In summary, there is now convincing evidence that functional CD8 T cell memory can be generated against tumor/self antigens. In contrast to studies which employ active immunization, our study shows that poorly-immunogenic tumors themselves can induce tumor-specific T cell memory after the hurdle of Treg suppression is overcome. This work stresses the importance of exploring immunotherapies in conjunction with Treg depletion and the surgical treatment of cancer to provide long-lived and meaningful control of recurrent and metastatic disease.
Acknowledgments
Support for this work was provided by the NIH (R01 CA120777), and the Melanoma Research Foundation (New Investigator Award to MJT). In addition, EJU receives support from NIH grant CA103642.
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, Blattman JN, Sette A, Ahmed R. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology. 2003;315:93–102. doi: 10.1016/j.virol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Y, Zhou H, Mizutani M, Mizutani N, Liu C, Xiang R, Reisfeld RA. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 2005;65:3419–3427. doi: 10.1158/0008-5472.CAN-04-3120. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, Sakaguchi S, Houghton AN. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bursuker I, North RJ. Immunological consequences of tumor excision: from active immunity to immunological memory. Int J Cancer. 1986;37:275–281. doi: 10.1002/ijc.2910370216. [DOI] [PubMed] [Google Scholar]
- 10.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 11.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Cote AL, de Vries VC, Usherwood EJ, Turk MJ. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007;67:6468–6476. doi: 10.1158/0008-5472.CAN-07-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 17.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 20.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J Virol. 2006;80:8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]