Abstract
Objective
Modulation of growth of intrauterine growth retarded (IUGR) newborns causes either adult obesity or normalization of body weight and fat. We investigated the impact of rapid versus delayed catch-up growth of IUGR offspring on glucose and lipid profiles.
Study Design
From 10d to term gestation and through lactation, control pregnant rats received ad libitum food, whereas study rats were 50% food-restricted. Glucose and lipid profiles were determined in offspring at ages, 1 day, 3 weeks and 9 months.
Results
Food-restriction during pregnancy produced hypoglycemic IUGR pups. Those permitted rapid catch-up growth demonstrated adult obesity with insulin-resistance (hyperglycemia/hyperinsulinemia) and hypertriglyceridemia. Conversely, IUGR exhibiting delayed catch-up growth demonstrated normal adult body weight and insulin-deficiency (hyperglycemia/hypoinsulinemia) and elevated cholesterol levels.
Conclusion
The timing and rate of IUGR newborn catch-up growth causes markedly altered adult phenotypes. Although delayed newborn catch-up growth may be beneficial in prevention of adult obesity, there may be significant adverse effects on pancreatic function.
Keywords: Intrauterine growth retardation, glucose intolerance, lipids, obesity, catch-up growth
INTRODUCTION
Currently, greater than 55% of adults in the United States are overweight, and one in five are obese (BMI > 30kg/m2), representing a modern health crisis. Concomitantly, type II diabetes has dramatically increased in Western society.1,2 The World Health Organization (WHO) predicts a 170% rise in the prevalence of type II diabetes from 1995-2025 with a total of 84-228 million persons in the industrializing world.3 In conjunction with these rates of obesity and diabetes, the incidence of hypertension is increasing. Among adults in the United States, nearly 30% of the population is hypertensive and 25% of the population exhibit metabolic syndrome as defined by 3 of the 5 following criteria: obesity, hypertriglyceridemia, low high density lipoprotein, hypertension and high fasting glucose.4,5 Perhaps of greater concern in the rapid rise in incidence of obesity, type 2 diabetes and metabolic syndrome in children and adolescents.6,7
There is increasing evidence that the in utero environment impacts on fetal development and alters a diversity of adult regulatory mechanisms contributing to the expression of metabolic syndrome. Recent human studies indicate that a striking 25-63% of adult diabetes, hypertension, and coronary heart disease can be attributed to low birth weight, with subsequent accelerated newborn to adolescent weight gain.8-10 Animal models have confirmed the association of low birth weight with offspring metabolic syndrome. Low birth models include maternal rats, which when undernourished during select periods of pregnancy produce intrauterine growth restricted (IUGR) pups.11-13 In our previous studies, we demonstrated that maternal 50% food restriction during pregnancy (e10-e21) resulted in growth restricted pups with reduced newborn plasma leptin levels. When nursed by normally nourished lactating dams, these pups exhibited rapid catch-up growth by three weeks of age. Subsequent ad libitum feeding of normal rat chow resulted in nine month old adults with increased body weight, increased percent body fat, and elevated plasma leptin levels. As an alternative paradigm, continued nutrient restriction of the growth restricted pups during the first three weeks of life resulted in further growth restriction at three weeks of age. However, by nine months, these pups attained normal body weight, percent body fat, and plasma leptin levels. These results suggest that accelerated newborn growth of IUGR pups resulted in the development of offspring obesity, while prevention of rapid newborn weight accretion appeared to prevent the increased body weight, body fat, and plasma leptin.14
In view of the potential therapeutic effect of limiting the rapid weight gain of growth restricted newborns, we sought to examine the impact of maternal food restriction during pregnancy and/or lactation on offspring glucose homeostasis and plasma lipid profiles.
MATERIAL AND METHODS
Maternal Rat Diets
Studies were approved by the Animal Research Committee of the Los Angeles BioMedical Research Institute at Harbor-UCLA (LABioMed), and were in accordance with the American Association for Accreditation of Laboratory Care (AALC), and National Institutes of Health (NIH) guidelines. The rat model utilized for maternal food restriction during pregnancy and lactation has been previously described.14 Briefly, first time pregnant Sprague Dawley rats (Charles River Laboratories, Hollister, California) were housed in a facility with constant temperature and humidity and controlled 12:12 hour light/dark cycle. At 10 days of gestation, rats were provided either an ad libitum (AdLib; n=12) diet of standard laboratory chow (Lab Diet 5001, Brentwood, MO), or a 50% food restricted (FR; n=12) diet determined by quantification of normal intake in ad libitum fed rats. These diets were provided from day 10 of pregnancy to term, and throughout the 21 day lactation period. At day one of birth, pups were limited to eight per liter (four males and four females) to normalize rearing.
Offspring
Among the IUGR offspring born to FR dams, pups were allocated to one of two groups of dams during lactation. One half of the IUGR pups were nursed after cross fostering to ad libitum fed dams (FR/AdLib), whereas the other half were nursed by their own FR dams (FR/FR). Similarly, one half of the normal weight pups born to ad libitum fed dams were continued to be nursed by these dams (AdLib/Adlib), while the other half were cross fostered to FR dams (AdLib/FR). At three weeks of age, offspring in all four groups were housed individually, and weaned to ad libitum standard laboratory chow. The results of body weight, body composition, and food intake have been previously reported.14,15
Offspring Studies
At 1 day of age, pups were decapitated and blood and tissues collected. At 3 weeks and 9 months of age, one male and one female from each litter were fasted overnight, and blood was collected via cardiac puncture into heparinized tubes for determination of plasma insulin, triglycerides, cholesterol, and LDL-cholesterol. Additionally, 9 month old offspring underwent glucose tolerance test (GTT) as follows: After an overnight fast, D-glucose (1mg/g body weight) was injected intraperitoneally in conscious rats. Blood was taken from tail vein prior to (time 0) and 15, 30, 60, 120 and 180 min after glucose injection.
Plasma insulin levels were measured at time 0 and 180 min. Blood glucose was determined using Hemocue B-Glucose Analyzer (HemoCue Inc, Mission Viejo, CA) and plasma insulin was measured by RIA (Rat Insulin RIA Kit, Linco Research, St. Charles, MO). Plasma lipid levels were measured using reagents from Raichem, Inc. (San Diego, CA) and run on an automated Cobas-Mira Chemistry Analyzer (Roche Diagnostic Systems Inc., Sommerville, NJ). Plasma triglycerides (Cat No. 80008) and cholesterol (Cat No. 80015) concentrations were analyzed using Raichem Enzymatic Reagents (with control serum level 1 #83082 and control serum level 2 #83083). HDL- and LDL cholesterol direct (Cat No. 85510) were analyzed using Raichem lipid calibrator (Cat No.85528). The inter- and intra-assay variabilities were 4.9% and 3.0%, respectively for triglycerides; 1.0 and 1.3%, respectively, for total cholesterol; 0.6 and 1.3%, respectively, for HDL cholesterol; and 2.1 and 2.6%, respectively for LDL cholesterol.
Statistical Analysis
Differences between control and experimental groups were compared using either unpaired t-test (1-day-old neonate), repeated measures of ANOVA (GTT), or ANOVA with Dunnett post hoc tests (glucose, lipids and insulin). Area under the curve (AUC) was computed for the GTT. At ages of 1 day and 3 wk, combined data for males and females are shown, since no sex differences were evident. At the age of nine months, sex differences were evident and results are presented according to sex. Values are expressed as means ± SE.
RESULTS
Newborn pups
One day old pups of FR dams were growth retarded and had significantly reduced wet weight of pancreas and liver as compared to the pups from AdLib dams. However, when expressed relative to the body weight, the organ weights were comparable between the two groups (Table 1). Additionally, the growth retarded 1 day old FR pups had significantly reduced blood glucose (93.6 ± 3.2 vs. 102.0 ± 4.1 mg/dl, P < 0.01) and plasma triglyceride levels (80.2 ± 4.0 vs. 93.5 ± 5.6 mg/dl, p < 0.05) compared with controls.
Offspring at 3 weeks
At 3 weeks of age, FR/AdLib offspring were significantly heavier (50±1 vs. 45±1 g, p < 0.01) but demonstrated significantly decreased fasting blood glucose, plasma insulin and triglyceride levels as compared to the controls. However, plasma cholesterol levels were similar to controls (Figure 1). Conversely, AdLib/FR offspring which were significantly smaller (28±1 g), and demonstrated normal blood glucose, plasma insulin, triglycerides and cholesterol levels comparable to the controls. In contrast, despite FR/FR offspring being markedly smaller (24±1 g) than controls, they had significantly lower blood glucose and plasma insulin levels. Furthermore, though the plasma triglyceride levels were similar to the controls, the plasma cholesterol levels were significantly increased in the FR/FR offspring (Figure 1).
Figure 1.
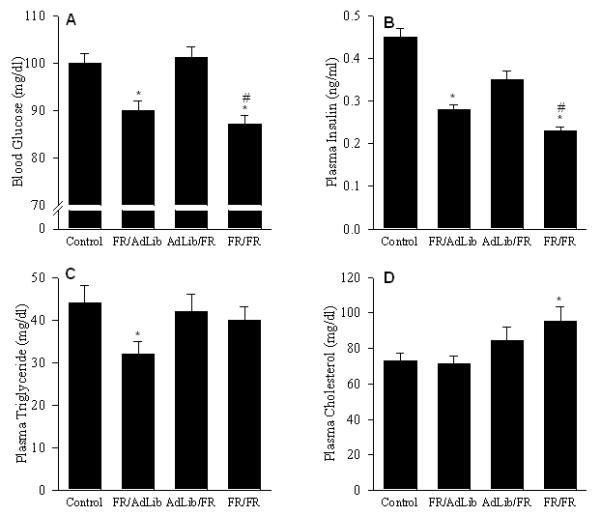
Blood glucose (A), and plasma insulin (B), triglyceride (C) and cholesterol (D) levels in 3 week old offspring. Because no sex difference were evident, combined data of males (n=6) and females (n=6) from 6 litters in the 4 groups are shown. * P < 0.01 vs. control offspring.
Offspring at 9 months
Food restriction during pregnancy alone (FR/Adlib)
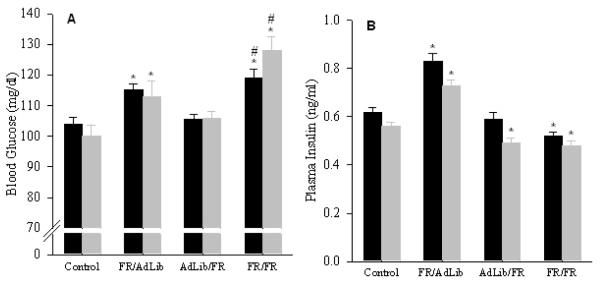
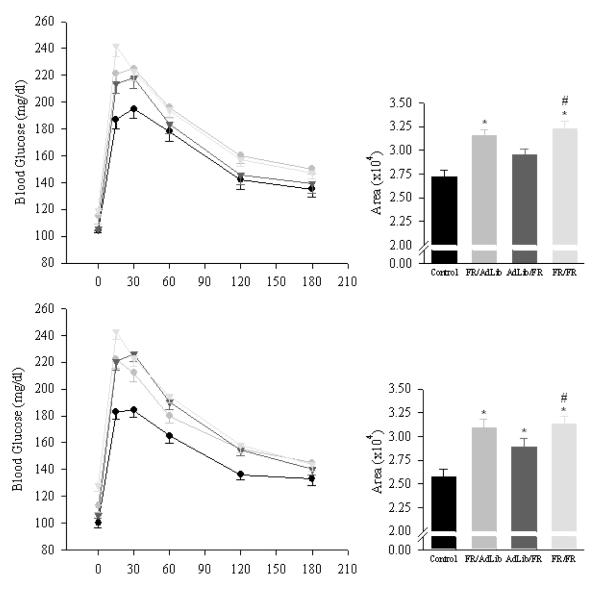
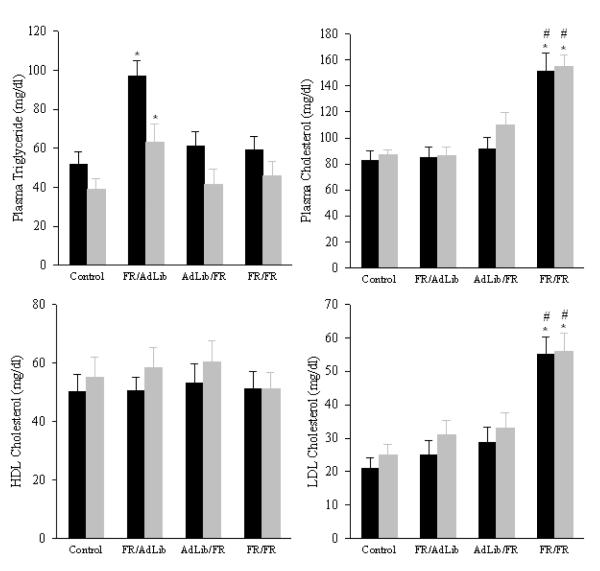
FR/AdLib male and female offspring remained significant heavier than controls (male: 742±17 vs. 647±18 g; female: 420±16 vs. 350±14 g, p < 0.01) but exhibited significantly increased fasting blood glucose and increased plasma insulin levels (Figure 2). The GTT demonstrated that the FR/AdLib offspring had significantly increased basal and peak glucose levels. Glucose levels remained significantly elevated throughout the 180 min study, resulting in a significantly increased area under the curve (Figure 3). Furthermore, the elevated plasma insulin level noted during the basal period was still evident at 180 min (male, 2.38 ± 0.12 vs. 1.68 ± 0.12 ng/ml; female, 2.01 ± 0.11 vs. 1.06 ± 0.09 ng/ml; p < 0.01). Analogous to the glucose profile, male and female plasma triglycerides changed from a hypo- to hyper state from 3 weeks to 9 months. No changes were evident in plasma cholesterol, HDL-cholesterol and LDL-cholesterol levels (Figure 4).
Figure 2.
Blood glucose (A), and plasma insulin (B) levels in 9 month old offspring. Since sex difference was evident, data for males (solid bars) and females (shaded bar) are shown separately. Number of animals studied per group was 6 males and 6 females from 6 litters. * P < 0.01 vs. control offspring.
Figure 3.
Glucose tolerance test and area under the curve in 9 month old offspring. Data are shown of 6 males (upper panel) and 6 females (lower panel) from 6 litters from the control (solid circle), FR/AdLib (shaded circle), AdLib/FR (solid triangle) and FR/FR (shaded triangle) groups. * P < 0.01 vs. control offspring.
Figure 4.
Plasma triglycerides (A), cholesterol (B), HDL cholesterol (C), and LDL cholesterol (D) levels in 9 month old offspring. Since sex difference was evident, data for males (solid bars) and females (shaded bar) are shown separately. Number of animals studied per group was 6 males and 6 females from 6 litters. * P < 0.01 vs. control offspring.
Food restriction during lactation alone (AdLib/FR)
AdLib/FR male and female basal blood glucose levels were comparable to the controls. In response to the GTT, AdLib/FR females had significantly higher blood glucose levels at 15, 30, 60 and 120 min and greater area under the curve (Figure 3). However in AdLib/FR females, the plasma insulin levels during the basal period (Figure 2) and at 180 min of the GTT (0.81 ± 0.06 vs. 1.06 ± 0.09 ng/ml, p < 0.01) were significantly decreased. In contrast, AdLib/FR males demonstrated normal basal and stimulated blood glucose and insulin values (Figure 3). Both male and female AdLib/FR exhibited normal plasma triglycerides, cholesterol, HDL-cholesterol and LDL-cholesterol levels (Figure 4).
Food restriction during pregnancy and lactation (FR/FR)
FR/FR male and female offspring showed hyperglycemia with decreased plasma insulin levels (Figure 2). The GTT revealed significantly elevated peak glucose values throughout the 180 min test with increased area under the curve (Figure 3). Reduced plasma insulin levels were still evident at 180 min (male, 0.77 ± 0.08 vs. 1.68 ± 0.12 ng/ml; female, 0.82 ± 0.05 vs. 1.06 ± 0.09 ng/ml; p < 0.01). Notably, the FR/FR offspring demonstrated no change in plasma triglyceride and HDL-cholesterol levels, though plasma cholesterol and LDL-cholesterol levels were significantly elevated (Figure 4).
Male/Female comparison
In general, at 9 months the male offspring of all groups had significantly higher blood glucose, plasma insulin and triglyceride levels as compared with female offspring. In contrast, plasma cholesterol, HDL-cholesterol and LDL-cholesterol levels were significantly lower in males than females.
COMMENT
Previous studies on animal models of intrauterine perbutations induced by placental insufficiency, maternal undernutrition, maternal diabetes and glucocorticoid exposure in utero, demonstrate abnormalities in the secretion and action of insulin in adult offspring.16-19 In the present study, the marked variance in plasma glucose, insulin and lipid values among IUGR offspring of food restricted dams is dependent on the pattern of offspring growth during the first three weeks of life. In particular, (i) growth retarded pups demonstrate significant hypoglycemia and hypotriglyceridemia at birth; (ii) rapid catch-up growth of IUGR pups during the nursing period (i.e., FR/ADLib offspring) results in adult obesity with evidence of hyperglycemia and insulin resistance, in presence of hypertriglyceridemia and normal plasma cholesterol, whereas (iii) delayed catch-up growth during nursing (i.e., FR/FR offspring) results in normal adult body weight, hyperglycemia and insulin insufficiency associated with hypercholesterolemia and normal plasma triglycerides. Thus, the timing and mechanisms of this metabolic transition may be dependent upon the rate of newborn weight gain. Further, in IUGR pups hyperglycemia develops with adult maturity, and this is independent of diet or growth rate during the nursing period, though with markedly different mechanisms of insulin resistance vs insulin insufficiency. Similar to FR/FR, the control offspring nursed by food restricted dams (AdLib/FR) also demonstrated normal adult body weight, hyperglycemia and insulin insufficiency, though the glucose changes were evident only in the female offspring. Additionally, AdLib/FR offspring had normal lipid profile.
The hypoglycemia evident in growth retarded pups is consistent with previous observations,20 and may occur as a result of suboptimal hepatic glycogen stores21 or impaired gluconeogenesis.22 In the present study, maternal food restriction during the last half of pregnancy resulted in growth retarded pups with proportionately reduced pancreas and liver weight. These pups transit from relative hypoglycemic state at 3 weeks of age to hyperglycemia at 9 months of age. At early stage of postnatal life, the increased insulin sensitivity as assessed by glucose to insulin ratio is consistent with previous reported studies.16,23 Nevertheless, our study emphasizes that transition to hyperglycemic state with adult maturity occurs by discernibly different mechanisms of insulin resistance vs insulin insufficiency, which is primarily dependent upon the diet and growth rate during the nursing period. For instance, we have previously reported that FR/AdLib offspring at 3 weeks of age are significantly heavier than controls with comparable relative weights of pancreas and liver. Conversely, FR/FR offspring have lower body weights with reduced relative weight of pancreas and liver. At 9 months of age, the FR/AdLib offspring are obese with increased percentage body fat whereas the FR/FR have normal adult body weights and percentage body fat. Control offspring nursed solely during the lactation period (AdLib/FR) demonstrate growth patterns similar to FR/FR.14 Hence, these results suggest that hyperglycemia due to insulin insufficiency may occur as a result of permanent alteration in pancreatic β-cell development while that due to insulin resistance may be associated with development of obesity and increased body fat.24,25
The findings on glucose/insulin are consistent with the epidemiology studies26 and are reminiscent of the differing results of the effects of wartime famine in Holland27 and Leningrad.28 Men and women exposed to the Dutch famine while they were in utero had impaired glucose tolerance, mainly as a result of insulin resistance, whereas those exposed to Leningrad famine had unaltered glucose-insulin metabolism. This was attributed to the timing and intensity of famine. The ‘Dutch famine’ was a brief period of intense deprivation in a well-nourished population whose level of nutrition was immediately restored after the famine. Children exposed to famine in utero were, therefore, well-nourished in childhood and likely experienced accelerated weight gain (akin to FR/AdLib). Unlike the Dutch famine, the siege of Leningrad occurred over a prolonged period in a previously malnourished population who remained malnourished after the siege was lifted. Children in this case probably did not experience accelerated weight gain (akin to FR/FR). Indeed this concept is supported by further epidemiologic studies which have shown that adult obesity augment intrauterine effects, such that the highest prevalence of type 2 diabetes and impaired glucose tolerance is seen in people who were small at birth but obese as adults.26
Lipid homeostasis is regulated primarily by the liver and adipose tissue, and abnormalities in lipid metabolism are associated with the metabolic syndrome, type 2 diabetes and coronary heart disease.29 The underlying mechanisms for altered plasma triglyceride and cholesterol concentrations may include either altered hepatic expression of key genes/enzymes relevant to triglyceride and cholesterol metabolism,30 changes in bile acid synthesis, secretion and absorption, especially in view of the fact that these develop during the final third of gestation and continue to mature after birth,31 modified hepatic growth, proliferation, and morphology,32,33 or altered milk composition as a result of maternal nutrient restriction. Although we have not delineated the precise mechanism, our results accentuate the importance of postnatal nutrition and growth in modulating specific adverse changes in lipid indices of IUGR offspring. This is illustrated by the fact that FR/AdLib offspring with increased percent body fat have elevated plasma triglyceride but normal cholesterol levels, whereas FR/FR offspring without adult obesity have normal plasma triglyceride but elevated cholesterol levels. Animal studies have shown that overfeeding during early postnatal life in rats has a long-term impact on plasma cholesterol in adulthood. The important influence of the sucking period is further emphasized by studies, in which culling rat litters (typically to 4–6 pups from 10–16) increases milk availability per pup and results in offspring with dyslipidaemia,34 increased body mass index and an altered growth trajectory.35
Our findings of hypertriglyceridemia and normal cholesterol in FR/AdLib offspring concur with the data from the Dutch Famine study36 which found no association between birth weight and subsequent total blood cholesterol. Similarly, a prospective study on small-for-gestational age infants showed no changes in cholesterol levels at 1 year of age but, interestingly, noted a trend towards higher triglycerides and fasting insulin levels.37 Nonetheless, there is conflicting data on the association between early nutrition and raised serum concentrations of total and LDL-cholesterol. Barker and colleagues have noted that specifically small abdominal circumference at birth is associated with raised serum concentrations of total and LDL-cholesterol in adulthood and have suggested that nutritionally impaired growth, particularly of the liver, during late gestation could result in permanent changes in cholesterol metabolism which persist until adult life.38 Other studies have highlighted the critical role of infant nutrition in influencing cholesterol metabolism in later life.39 Recent evidence from a randomized trial of preterm infants also suggests that infant nutrition determines the lipoprotein profile later in life.40 It appears that poor growth in utero might interact with postnatal growth to influence lipid metabolism and risk for coronary heart disease.
The overall gender differences seen in glucose and lipid profile, namely that the males exhibit higher blood glucose, plasma insulin and triglyceride levels than females, is consistent with prior human and rat data.8,23,41 The disparity in gender response to maternal food restriction was evident only in the offspring exposed to maternal food restriction exclusively during the nursing period (AdLib/FR). This was apparent only in the stimulated glucose/insulin levels with the females exhibiting hyperglycemia in the presence of reduced insulin levels. Although the cause for sex-dependent response is unknown, previous studies have noted differential impact of early undernutrition on male vs females.15,41
In conclusion, these results demonstrate that intrauterine growth retarded offspring which experience rapid catch-up growth evidence complementary findings consistent with metabolic syndrome. Specifically, elevated plasma glucose and insulin and the GTT results are consistent with manifestations of type II diabetes (insulin resistance) while elevated plasma triglycerides are likely a reflection of increased percent body fat and obesity. In our original studies of nutrient restriction, offspring which were nursed by food restricted dams, demonstrated a normalization of body weight and percent body fat as adults. Although these results suggested a beneficial effect of limiting newborn rapid weight gain, the present results of the glucose/insulin and lipid values suggests adverse consequences of this paradigm. Finally, despite the similar GTT area under the curve in both, AdLib/FR and FR/FR offspring, the differing phenotype of insulin resistance vs insulin insufficiency emphasize the importance of both pre- and postnatal growth on glucose and lipid metabolism.
ACKNOWLEDGMENTS
The authors acknowledge Linda Day, Stacy Behare and Glenda Calvario for technical assistance.
Financial support: This work was supported by the National Institutes of Health K01 DK 063994, the American Heart Association 0455117Y and March of Dimes Birth Defects Foundation
Footnotes
Condensation Nutrient restriction solely during pregnancy causes adult hypertriglyceridemia, insulin resistance and obesity whereas nutrient restriction during pregnancy and lactation causes hypercholesterolemia, insulin insufficiency without obesity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sturm R. Increases in clinically severe obesity in the United States, 1986 2000. Arch Intern Med. 2003;163:2146–48. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 2.Xavier F, Sunyer PI. The obesity epidemic: Pathophysiology and consequences of obesity. Obes Res. 2002;10:97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 3.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9:228S–33S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 4.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9:228S–33S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 5.Isganaitis E, Lustig RH. Fast food, central nervous system insulin resistance, and obesity. Arterioscler Thromb Vasc Biol. 2005;25:2451–62. doi: 10.1161/01.ATV.0000186208.06964.91. [DOI] [PubMed] [Google Scholar]
- 6.Vivian EM. Type 2 diabetes in children and adolescents--the next epidemic? Curr Med Res Opin. 2006;22:297–306. doi: 10.1185/030079906X80495. [DOI] [PubMed] [Google Scholar]
- 7.Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab. 2005;19:405–19. doi: 10.1016/j.beem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–9. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Jaquet D, Deghmoun S, Chevenne D, Collin D, Czernichow P, Levy-Marchal C. Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia. 2005;48:849–55. doi: 10.1007/s00125-005-1724-4. [DOI] [PubMed] [Google Scholar]
- 11.Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev. 1997;72:329–48. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- 12.Vickers MH, Reddy S, Ikenasio BA, Breier BH. Dysregulation of the adipoinsular axis: A mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J Endocrinol. 2001;170:323–32. doi: 10.1677/joe.0.1700323. [DOI] [PubMed] [Google Scholar]
- 13.Ross MG, Desai M. Gestational Programming: Population Survival Effects of Drought and Famine during. Am J Physiol Regul Integr Comp Physiol. 2005;288:R25–R33. doi: 10.1152/ajpregu.00418.2004. [DOI] [PubMed] [Google Scholar]
- 14.Desai M, Gayle DA, Jooby B, Ross MG. Programmed Obesity in Intrauterine Growth Restricted Newborns: Modulation by Newborn Nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 15.Desai M, Gayle DA, Jooby B, Ross MG. Permanent Reduction in Heart and Kidney Organ Growth in Offspring of Undernourished Rat Dams. Am J Obstet Gynecol. 2005;93:1224–32. doi: 10.1016/j.ajog.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans. 1996;24:341–50. doi: 10.1042/bst0240341. [DOI] [PubMed] [Google Scholar]
- 17.Reusens B, Remacle C. Programming of the endocrine pancreas by the early nutritional environment. Int J Biochem Cell Biol. 2006;38:913–22. doi: 10.1016/j.biocel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Seckl JR. Prenatal glucocorticoids and long term programming. Eur J Endocrinol. 2004;151:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 19.Garg M, Thamotharan M, Rogers L, Bassilian S, Lee WN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2006;290:E1218–26. doi: 10.1152/ajpendo.00474.2005. [DOI] [PubMed] [Google Scholar]
- 20.Holtrop PC. The frequency of hypoglycemia in full-term large and small for gestational age newborns. Am J Perinatol. 1993;10:150–54. doi: 10.1055/s-2007-994649. [DOI] [PubMed] [Google Scholar]
- 21.Bussey ME, Finley S, LaBarbera A, Ogata ES. Hypoglycemia in the newborn growth-retarded rat: delayed phosphoenolpyruvate carboxykinase induction despite increased glucagon availability. Pediatr Res. 1985;19:363–67. [PubMed] [Google Scholar]
- 22.Oh W, D’Amodio MD, Yap LL, Hohenauer L. Carbohydrate metabolism in experimental intrauterine growth retardation in rats. Am J Obstet Gynecol. 1970;108:415–21. doi: 10.1016/0002-9378(70)90424-2. [DOI] [PubMed] [Google Scholar]
- 23.Desai M, Crowther N, Ozanne SE, Lucas A, Hales CN. Adult glucose and lipid metabolism may be programmed during fetal life. Biochem Soc Trans. 1995;23:331–35. doi: 10.1042/bst0230331. [DOI] [PubMed] [Google Scholar]
- 24.Berney DM, Desai M, Palmer DJ, Greenwald S, Brown A, Hales CN, Berry CL. The effects of maternal protein deprivation on the fetal rat pancreas: major structural changes and their recuperation. J Pathol. 1997;183:109–115. doi: 10.1002/(SICI)1096-9896(199709)183:1<109::AID-PATH1091>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Holeman K, Aerts L, Van Assche FA. Lifetime consequences of abnormal fetal pancreatic development. J Physiol. 2003;547:11–20. doi: 10.1113/jphysiol.2002.036582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey KM, Barker DJP. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–52S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 27.Ravelli ACJ, van der Meulen JHP, Michels RPJ, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to the Dutch famine. Lancet. 1998;351:173–77. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 28.Stanner SA, Bulmer K, Andres C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ. 1997;315:1342–48. doi: 10.1136/bmj.315.7119.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taskinen MR. Hyperinsulinism and dyslipidemias as coronary heart disease risk factors in NIDDM. Adv Exp Med Biol. 1993;334:295–301. doi: 10.1007/978-1-4615-2910-1_23. [DOI] [PubMed] [Google Scholar]
- 30.Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. Br J Nutr. 1996;76:605–12. doi: 10.1079/bjn19960066. [DOI] [PubMed] [Google Scholar]
- 31.Lester R. Bile acid metabolism in the fetus and newborn. Ciba Found Symp. 1979;18:99–115. doi: 10.1002/9780470720530.ch6. [DOI] [PubMed] [Google Scholar]
- 32.Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, Germain JP, Going TCH, Bailey RA. Gluconeogenesis, glucose handling and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Invest. 1997;100:1768–74. doi: 10.1172/JCI119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai M, Byrne CD, Zhang J, Petry CJ, Lucas A, Hales CN. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am J Physiol. 1997;272:G1083–90. doi: 10.1152/ajpgi.1997.272.5.G1083. [DOI] [PubMed] [Google Scholar]
- 34.Hahn P. Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents. J Nutr. 1984;114:1231–34. doi: 10.1093/jn/114.7.1231. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A. The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disord. 2001;25:1168–74. doi: 10.1038/sj.ijo.0801669. [DOI] [PubMed] [Google Scholar]
- 36.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–06. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 37.Soto N, Bazaes RA, Peña V, Salazar T, Ávila A, Iñiguez G, Ong KK, Dunger DB, Mericq MV. Insulin Sensitivity and Secretion Are Related to Catch-Up Growth in Small-for-Gestational-Age Infants at Age 1 Year: Results from a Prospective Cohort. J Clin Endocrinol Metabolism. 2003;88:3645–50. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJP, Martyn CN, Osmond C, Hales CN, Fall CHD. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993;307:1524–27. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortaz M, Fewtrell MS, Cole TJ, Lucas A. Birth weight, subsequent growth, and cholesterol metabolism in children 8-12 years old born preterm. Arch Dis Child. 2001;84:212–17. doi: 10.1136/adc.84.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singhal A, Cole TJ, Fewtrell M, Lucas A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomized study. Lancet. 2004;363:1571–78. doi: 10.1016/S0140-6736(04)16198-9. [DOI] [PubMed] [Google Scholar]
- 41.Freedman DS, Jacobsen SJ, Barboriak JJ, Sobocinski KA, Anderson AJ, Kissebah AH, Sasse EA, Gruchow HW. Body fat distribution and male/female differences in lipids and lipoproteins. Circulation. 1990;81:1498–506. doi: 10.1161/01.cir.81.5.1498. [DOI] [PubMed] [Google Scholar]