Abstract
We prospectively evaluated tumour response and renal function in 12 newly-diagnosed children with high-risk Wilms tumour receiving ifosfamide, carboplatin, and etoposide (ICE) chemotherapy. Two cycles of ICE were followed by 5 weeks of vincristine, dactinomycin, and doxorubicin (Adriamycin) (VDA), and nephrectomy, radiotherapy, additional VDA, and a third ICE cycle. Carboplatin dosage was based on glomerular filtration rate (GFR) to achieve targeted systemic exposure (6 mg/ml × min). Mean GFR (measured by technetium 99m-DTPA clearance) declined by 7% after 2 cycles of ICE and by 38% after nephrectomy; the mean carboplatin dose was reduced 32% after nephrectomy. Mean GFR remained stable after the third ICE cycle. Although urinary β2-microglobulin excretion increased during therapy, no patient had clinically significant renal tubular dysfunction at the end of treatment.
Treatment with ICE, nephrectomy, and radiotherapy significantly reduces GFR, largely as the result of nephrectomy. Adjustment of carboplatin dosage on the basis of GFR and careful monitoring of renal function may alleviate nephrotoxicity.
Keywords: ifosfamide, carboplatin, nephrectomy, renal function, creatinine, glomerular filtration rate, kidney neoplasms
1. Introduction
Over the past 3 decades, the survival of patients with Wilms tumour has dramatically improved through risk-adapted treatment stratification based on tumour stage and histology.1–3 Standard therapy for advanced disease consists of nephrectomy, radiotherapy, and chemotherapy. Patients with unresectable or metastatic Wilms tumour fare worse than patients with localized and resectable tumours.4,5 Preoperative chemotherapy, the standard treatment approach in the International Society of Paediatric Oncology trials,6, 7 facilitates surgical resection of large tumours that may involve vital structures.4 Patients with diffuse anaplastic Wilms tumour, particularly stages III and IV, continue to have poor outcomes2, 8–10 and may benefit from new treatment strategies.
Ifosfamide, carboplatin, and etoposide, used as single agents or in combination, are active against Wilms tumour.11–18 The three-agent combination (ICE) has been used effectively to treat recurrent Wilms tumour,19, 20 but its use in frontline therapy has been limited by concern about potential nephrotoxicity in patients who undergo nephrectomy and abdominal radiotherapy.
In healthy individuals, the remaining kidney shows compensatory hypertrophy and an increased glomerular filtration rate (GFR) after nephrectomy.21, 22 In children, compensatory hypertrophy allows a post-nephrectomy GFR that is 70% to 90% of healthy controls.23 There is concern that cancer chemotherapy and irradiation may inhibit compensatory renal hypertrophy after nephrectomy in patients with Wilms tumour.21 However, although long-term renal function has been studied in survivors of Wilms tumour, no data are available about acute changes in GFR during therapy.
We evaluated renal function in patients (March 1994 to August 1998) with high-risk Wilms tumour receiving chemotherapy that included the ICE regimen given in an “upfront window”. Here we report the study results including response rate to ICE, the toxicity of ICE, and the longitudinal effect of ICE, nephrectomy, radiotherapy, and vincristine, dactinomycin, and doxorubicin (Adriamycin) (VDA) on glomerular and renal tubular function.
2. Patients and methods
2.1 Patients
Tumour was staged according to the National Wilms Tumor Study (NWTS) Group surgical-pathologic staging system.24 Eligibility requirements comprised: age <21 years; previously untreated, histologically proven, unresectable or metastatic Wilms tumour with favorable histology or focal anaplasia or stage II–IV Wilms tumour with diffuse anaplasia; life expectancy ≥6 weeks; Eastern Cooperative Oncology Group performance status <2; baseline white blood cell (WBC) count ≥2,000/μl, absolute neutrophil count (ANC) ≥ 1,000/μl, and platelet count >100,000/μl; adequate liver function (AST and ALT <3 times normal value); and informed consent signed by the patient, parent, or guardian, as appropriate. The study was approved by the Institutional Review Board at St. Jude Children’s Research Hospital.
2.2 Treatment
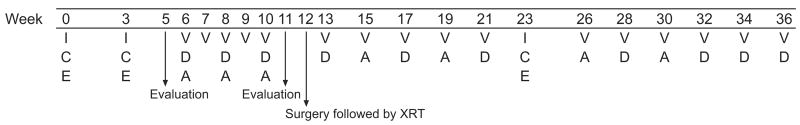
The treatment protocol comprised 2 cycles of ICE followed by 5 weeks of VDA given preoperatively, nephrectomy plus radiotherapy, additional VDA and a third cycle of ICE (36 weeks of treatment) (Fig. 1). To ensure consistent systemic exposure among patients, carboplatin dosage was based on the patient’s GFR as measured by technetium 99m-DTPA clearance.25 The carboplatin dose (a 1-hour infusion) was calculated to target an area under the concentration-time curve (AUC) of 6 mg/m l× min by using the following formula: dose in mg/m2 =6×[(0.93×GFR in ml/min per m2)+15].26, 27 Etoposide (100 mg/m2 per day) was infused IV over 1 hour, and ifosfamide (2 g/m2 per day) over 15 minutes. Mesna (500 mg/m2 per dose) was administered immediately after ifosfamide and 3 and 6 hours later. Granulocyte-colony stimulating factor (GCSF) was administered after each ICE cycle. VDA cycles consisted of vincristine (IV bolus), dactinomycin (IV bolus), and doxorubicin IV over 30 minutes. The cumulative dose of doxorubicin was 175 mg/m2.
Fig 1. Therapy schema.
V=vincristine 1.5 mg/m2 (maximum dose, 2 mg); D=dactinomycin 0.6 mg/m2 (maximum dose, 2 mg); A=doxorubicin (Adriamycin) 25 mg/m2; ICE chemotherapy: C=carboplatin, dosage based on GFR to target an AUC of 6 mg/ml × min on day 1; E=etoposide 100 mg/m2 per day on days 2–4; I=ifosfamide 2 g/m2 per day on days 2–4.
Patients with stable or progressive disease after the first 2 cycles of ICE continued treatment on study but did not receive the third ICE cycle. Patients with local stage III favorable-histology Wilms tumour or stage II–III diffuse anaplastic Wilms tumour received 12 Gy radiation to the whole abdomen or the flank, depending on the extent of disease. Patients with gross residual disease and focal or diffuse anaplasia received a boost radiation dose (cumulative dose, 27 Gy) to the residual tumour volume. Patients with lung metastases received 12.0 Gy whole-lung radiation.
2.3 Patient evaluation
Physical examination and laboratory studies preceded each cycle of chemotherapy. Before each ICE cycle, a complete blood count and serum electrolytes (including phosphorus and magnesium), blood urea nitrogen, creatinine, and bilirubin were obtained. Complete blood counts were performed three times weekly during GCSF treatment, after each ICE cycle. Liver function, blood urea nitrogen, and creatinine were assessed every 6 weeks during treatment. Urinalysis was performed before treatment and during ifosfamide administration.
Disease evaluations were performed at baseline, after the first 2 cycles of ICE, before nephrectomy, before the third ICE cycle, and at the end of treatment. Computed tomography of the chest and abdomen was performed at baseline and after the first 2 cycles of ICE. After completion of treatment, patients were regularly monitored by chest radiography and abdominal ultrasonography for 6 years.
A complete response to ICE was defined as complete disappearance of measurable disease. A partial response was defined as greater than 50% and less than 100% regression in the maximum diameters of all measurable lesions in the absence of new lesions. Stable disease was defined as the absence of complete response, partial response, and progressive disease. Progressive disease was defined as an increase greater than 25% in the maximum diameter of any lesion or the appearance of new lesion(s). Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria (version 1.0).
2.4 Studies of glomerular and tubular function
Glomerular and tubular function were assessed at baseline, after the first 2 cycles of ICE, after nephrectomy (before the third ICE cycle), and at treatment cessation. During therapy, GFR was measured using technetium 99m-DTPA clearance,28 and creatinine clearance (Clcr) was estimated by using the Schwartz formula.29 During follow-up after completion of therapy, glomerular function was assessed using estimated Clcr only; the MDRD formula was used for patients who were 18 years of age or older.30 To assess renal tubular function, phosphorus, magnesium, β2-microglobulin (a low-molecular weight protein and sensitive marker of proximal renal tubular damage), and creatinine were measured in random urine samples. Urinary magnesium excretion was calculated as the ratio of urine magnesium and creatinine concentration values. Urinary phosphate excretion was assessed by calculating tubular reabsorption of phosphate under basal conditions (TMP) normalized by the GFR: TMP/GFR=serum phosphate–(urine phosphate×serum creatinine)/urine creatinine.31
2.5 Statistical methods
Mean GFR and mean estimated Clcr at each time point during therapy were compared with those at baseline by using the paired t-test. The change in GFR and estimated Clcr over time during therapy was modeled by using a linear mixed-effect model accounting for potential within-patient correlation. A Bland-Altman plot was used to assess the agreement between GFR and estimated Clcr.32
3. Results
3.1 Response to ICE and outcome
Twelve children (14 months to 14.3 years; median, 4.5 years) were enrolled (Table 1). The 3 patients with stage III favorable histology Wilms tumour had unresectable tumours at diagnosis. Of the 11 patients with measurable disease before initiation of chemotherapy, 10 had a partial response to ICE and 1 had stable disease. Patient 9 had no measurable disease and therefore was inevaluable for tumour response evaluation. This patient received ICE and VDA and is alive without evidence of disease. Patient 6 completed the protocol without the third ICE cycle because of lack of response to ICE. Patient 12, who had tumour thrombus that extended into the right atrium, was taken off protocol therapy at week 12 because of persistent thrombus in the inferior vena cava and high-risk of tumour resection. This patient died after a subsequent pulmonary relapse. Eleven of the 12 patients survived without evidence of disease for a median of 11.9 years after diagnosis (Table 1).
Table 1.
Patient characteristics, response to ICE, and treatment outcome
| Patient | Age | Race/Sex | Disease stage | Tumour histology | Response to ICE | Outcome, survival duration^ |
|---|---|---|---|---|---|---|
| 1* | 5 y | W/F | IV | Favorable | PR | NED, 13.6 y |
| 2 | 9 y 2 mo | W/F | III | Favorable | PR | NED, 13.4 y |
| 3 | 1 y 2 mo | B/M | III | Favorable | PR | NED, 13.1 y |
| 4 | 2 y 1 mo | B/F | IV | Favorable | PR | NED, 12.3 y |
| 5 | 4 y | W/F | IV/V | Focal anaplasia | PR | NED, 12.3 y |
| 6 | 6 y 4 mo | Hispanic/F | IV | Favorable | SD | NED, 11.9 y |
| 7 | 5 y 8 mo | W/F | IV | Diffuse anaplasia | PR | NED, 11.9 y |
| 8 | 2 y 2 mo | W/M | IV | Favorable | PR | NED, 11.8 y |
| 9* | 14 y 3 mo | B/F | II | Diffuse anaplasia | NE | NED, 11.3 y |
| 10 | 10 y 1 mo | B/F | IV | Favorable | PR | NED, 8 y |
| 11 | 1 y 2 mo | B/F | III | Favorable | PR | NED, 10.8 y |
| 12 | 3 y 9 mo | B/M | III | Diffuse Anaplasia | PR | DOD, 1.9 y |
B = black; W = white; DOD = dead of disease; NE = not evaluable (nephrectomy at diagnosis, no measurable disease); NED = no evidence of disease; PR = partial response; SD = stable disease.
Measured from the time of diagnosis
Nephrectomy at diagnosis
3.2 Toxicity of ICE
Table 2 summarizes the grade 3 and 4 toxicity encountered during 34 cycles of ICE. The main toxicity was hematologic; all 12 patients had grade 3 or 4 toxicity. There were 9 episodes of febrile neutropenia, 2 of documented infection (impetigo and central line infection), and one each of grade 2 proteinuria and grade 1 serum creatinine elevation.
Table 2.
Grade 3 and 4 toxicity during a total of 34 cycles of ICE
| Toxicity | No. courses with toxicity (%) |
|---|---|
| Absolute neutrophil count <1,000/μl | 25 (74%) |
| WBC Count <2,000/μl | 24 (71%) |
| Hemoglobin <8 g/dl | 23 (68%) |
| Platelet count <50,000/μl | 23 (68%) |
| RBC transfusion(s) | 31 (91%) |
| Platelet transfusion(s) | 19 (56%) |
| Grade 3 nausea | 3 (9%) |
| Fever in absence of infection | 2 (6%) |
| Grade 3 allergy to etoposide | 1 (3%) |
3.3 Glomerular function
All patients, except patients 8 and 12, received radiotherapy to the flank or the whole abdomen on study. In the 4 patients who received radiotherapy to the whole abdomen, the remaining kidney received 12 Gy in 3 children and 10.5 Gy in 1. We found no significant difference in glomerular function after nephrectomy between patients who did and did not receive whole-abdomen radiation (P>0.1). Therefore, data for these groups were combined in the analyses because of the limited number of patients. The mean GFR values measured by technetium 99m-DTPA clearance are shown in Table 3. Two patients were excluded because they had nephrectomy at diagnosis before ICE (including one who received whole-abdomen radiation).
Table 3.
Change in glomerular filtration rate during therapy as measured by technetium 99m-DTPA clearance
| Baseline | After 2 ICE cycles | After nephrectomy | End of therapy | |
|---|---|---|---|---|
| No. patients^ | 10 | 10 | 9 | 9 |
| Mean GFR ± SD (ml/min per 1.73 m2) | 122 ± 31 | 113 ± 18 | 76 ± 9 | 72 ± 15 |
| GFR range (ml/min per 1.73 m2) | 74–175 | 74–135 | 55–85 | 52–97 |
| P value* | 0.27 | 0.0018 | 0.0024 | |
| Percentage of baseline GFR | 100 | 93 | 62 | 59 |
GFR = glomerular filtration rate (normal range, 90–130 ml/min per 1.73 m2 for children older than 12 months42).
Two patients who underwent nephrectomy at diagnosis were excluded.
Comparison of mean GFR value with that at baseline.
The linear mixed-effect model showed a significant decrease in mean GFR over the treatment period (P<0.0001). Mean GFR decreased by only 7 % after 2 cycles of ICE (P=0.27) but decreased by 38% after nephrectomy (P=0.0018) and did not decrease further after the third ICE cycle. Because of the reduction in GFR, the mean calculated carboplatin dose was reduced from 525 mg/m2 for the first 2 cycles of ICE (before nephrectomy) to 359 mg/m2 (32% dose reduction) for the third ICE cycle.
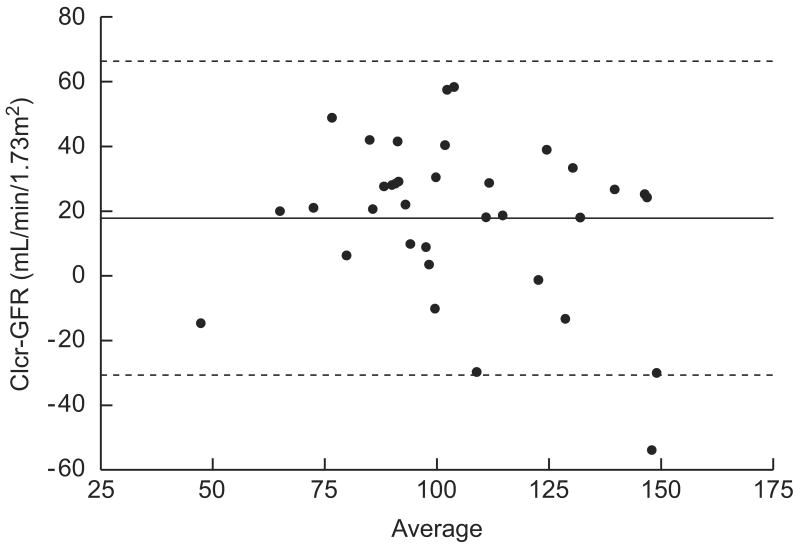
Table 4 summarizes change in the mean estimated Clcr during therapy and follow-up. To allow comparison of estimated Clcr with GFR data, we excluded the 2 patients who had nephrectomy at diagnosis and the patient who was taken off protocol therapy at week 12. The linear mixed-effect model showed that estimated Clcr declined significantly over the treatment period (P=0.0003) but remained stable during follow-up. Correlation analysis of GFR values at the same time points (n=35), either measured by technetium 99m-DTPA clearance or estimated from Clcr, showed a Pearson’s correlation coefficient of 0.61 (P<0.0001). However, a Bland-Altman plot revealed wide variation in the difference between the two measures (estimated Clcr – GFR, bias=17.8, CI=−30.6–66.3) (Fig. 2). That is, the estimated Clcr overestimated the GFR by 17.8 ml/min per 1.73 m2.
Table 4.
Change in estimated creatinine clearance during therapy and follow-up
| Baseline | After 2 cycles of ICE |
After nephrectomy |
After nephrectomy and radiation |
End of therapy |
Follow-up 1 year |
Follow-up 2 years |
Follow-up 3 years |
Follow-up 4 years |
Follow-up 5 years |
|
|---|---|---|---|---|---|---|---|---|---|---|
| No. patients^ | 9 | 9 | 9 | 8 | 9 | 9 | 9 | 8 | 8 | 9 |
| Mean Clcr ± SD (ml/min per 1.73 m2) |
120 ± 21 | 135 ± 18 | 103 ± 16 | 102 ± 29 | 97 ± 11 | 100 ± 21 | 96 ± 22 | 96 ± 22 | 88 ± 19 | 98 ± 25 |
| Clcr range (ml/min per 1.73 m2) |
94–159 | 102–159 | 78–131 | 40–133 | 75–106 | 70–119 | 52–119 | 57–120 | 58–112 | 60–134 |
| P value | 0.032* | 0.005* | 0.013* | 0.020* | NS** | NS** | NS** | NS** | NS** |
Clcr = creatinine clearance; NS = Not statistically significant (P ≥ 0.05).
Two patients who underwent nephrectomy at diagnosis and one who completed only 12 weeks of protocol therapy were excluded.
Comparison of mean Clcr values with that at baseline.
Comparison of mean Clcr values with that at end of therapy.
Fig 2.
Bland-Altman plot to assess agreement between GFR and estimated creatinine clearance (n = 35 pairs). The solid line represents the bias of the difference between pairs (17.8 ml/min per 1.73 m2) and the dashed lines represent the 95% confidence interval.
The mean estimated Clcr at the end of therapy (94±12 ml/min per 1.73 m2) did not differ significantly from that at last follow-up, 5 years after completion of therapy (92±26 ml/min per 1.73 m2), in the 11 surviving subjects (P=0.73). However, the estimated Clcr indicated reduced GFR (<90 ml/min per 1.73 m2) in 3 of 11 patients (27%) at the end of therapy and in 5 patients (45%) at one year of follow-up. The estimated Clcr remained consistently below 90 ml/min per 1.73 m2 during the 5 years of follow-up in these 5 patients (including the 2 who underwent up-front nephrectomy). We were unable to perform a parallel comparison of GFR values during follow-up, because the technetium 99m-DTPA clearance test was done only during therapy. Three patients were treated for hypertension: 1 during therapy and 2 after completion of therapy. One of these patients showed diminished Clcr and marked proteinuria as a result of focal segmental glomerulosclerosis (FSGS), detected through retrospective histologic study of non–tumour involved sections of the removed kidney.
Extended follow-up at a median of 11.2 years (range, 7.3–12.8 years) after completion of therapy revealed that 6 of the 11 long-term survivors (aged 12 to 25.6 years; median, 18.6 years) had an estimated Clcr <90 ml/min per 1.73 m2. The median estimated Clcr was 86 ml/min per 1.73 m2 (range, 10–169 ml/min per 1.73 m2). Two patients had hypertension, 2 had trace proteinuria, and 1 (the patient with FSGS) had undergone renal transplantation.
3.4 Renal tubular function
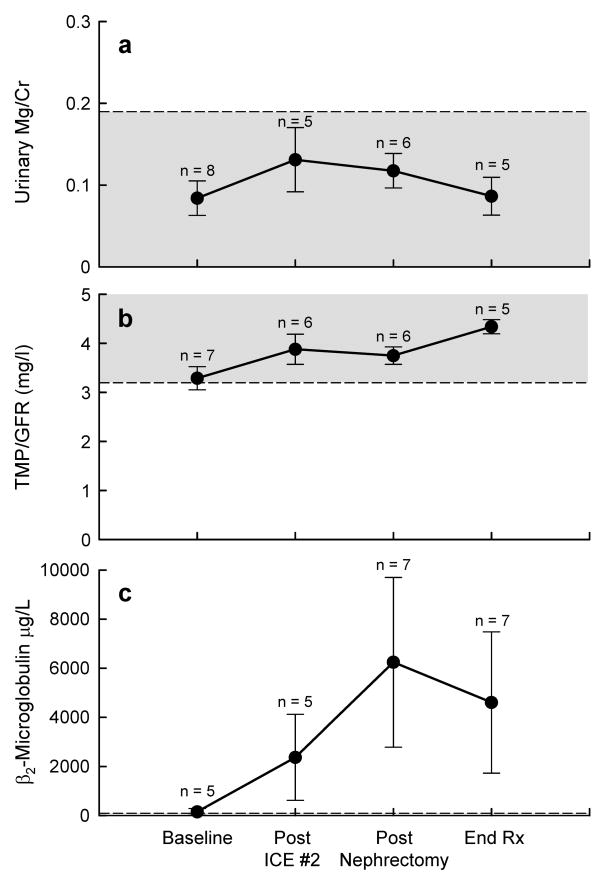
Urinary magnesium wasting was not observed (Fig. 3a); urinary magnesium-to-creatinine ratios were within the age-adjusted normal range33 and no magnesium supplementation was required.
Fig 3.
Renal tubular function was longitudinally assessed by measuring urinary magnesium excretion (a), renal tubular threshold for phosphate (b), and urinary β2-microglobulin (c) in patients with Wilms tumour treated with ICE. Shaded areas (a, b) represent the normal range.
Mean urinary TMP/GFR values were obtained prior to chemotherapy cycles and nephrectomy, and remained within the normal range throughout therapy (Fig. 3b).31, 34 Transient hypophosphatemia was observed after ICE therapy, but resolved before the next cycle. Five patients required phosphate supplementation (2 of 12 after the first ICE cycle and 3 of 10 after the third ICE cycle) for a median duration of 10 days (range, 1–38 days) after their serum phosphorus concentration reached 2.7–3.1 mg/dl. No child required chronic supplementation.
Five children required potassium supplementation (1 after the first ICE cycle, 3 after the second cycle, and 2 after the third cycle) for a median duration of 9.5 days (range, 1–14 days). One child required potassium supplementation after both the second and third ICE cycles. One child required phosphate, potassium, and bicarbonate supplementation after the third ICE cycle; only this patient required bicarbonate supplementation. No child required chronic potassium supplementation.
Mean β2-microglobulin excretion increased during therapy, peaking after nephrectomy and remaining elevated at the end of treatment (Fig. 3c).
At the time of extended follow-up (median of 11.2 years after completion of therapy), no patient had evidence of tubular dysfunction based on serum levels of electrolytes and phosphorus or required electrolyte or mineral supplementation.
4. Discussion
To our knowledge, this is the first longitudinal study of GFR during therapy for Wilms tumour. Our patients’ mean GFR did not decline significantly after 2 cycles of ICE. GFR was substantially reduced after nephrectomy but did not decline further after the third ICE cycle.
Most studies of renal function in Wilms tumour survivors were performed months to years after nephrectomy and completion of therapy. One study reported no statistically significant difference in GFR (measured by inulin clearance) between children who underwent nephrectomy for Wilms tumour or neuroblastoma (median post-nephrectomy follow-up, 12 months and 9 months, respectively) and children of comparable age who underwent nephrectomy for non-malignant disease (median post-nephrectomy follow-up, 23 months).35 At least 50% of the children in that study had chronic renal insufficiency (defined as GFR <90 ml/min per 1.73 m2). In another study, Wilms tumour patients were stratified according to whether treatment had included radiotherapy, GFR (measured a median of 13 months post-nephrectomy) was lower in irradiated (73% of normal) versus non-irradiated (95% of normal) patients.36 In that study, 34% of all subjects were considered to have chronic renal insufficiency (GFR 1.5 SD or more below the mean for age-matched controls).
In a study that examined GFR and renal compensatory growth 5 or more years after nephrectomy, 22 children with Wilms tumour who had received abdominal radiation were compared to 15 children who had undergone nephrectomy for congenital hydronephrosis.23 Kidney size increased by 25% to 29% in the Wilms tumour group, but by 42% in the hydronephrosis group. In addition, mean GFR as measured by inulin clearance was significantly lower in the Wilms tumour group (82% vs. 92%, respectively, of the healthy control mean), of which 73% had chronic renal insufficiency. The authors concluded that renal compensatory growth was retarded by chemotherapeutic agents and/or radiotherapy in children with Wilms tumour. In another long-term follow-up study, 10 of 53 survivors of Wilms tumour had a GFR <80 ml/min per 1.73 m2.37 Low GFR was associated with higher doses of radiation and less renal hypertrophy as measured by ultrasonography.37 Our study was not designed to evaluate renal hypertrophy and lacked the statistical power to discern whether radiation contributed to the decrease in glomerular function after nephrectomy.
We were surprised by the low incidence of chronic renal tubular dysfunction in our patients although the regimen included only 3 cycles of ICE. Ifosfamide-induced renal injury is characterized by tubular wasting of glucose, potassium, bicarbonate, phosphate, amino acids, and low molecular–weight proteins such as β2-microglobulin.38 None of our patients experienced significant chronic tubular wasting or required long-term supplementation of potassium, phosphorus, or bicarbonate. The rate of chronic renal insufficiency was within the range (40% to 73%) reported in patients with Wilms tumour who did not receive nephrotoxic drugs.23, 36, 37 Ifosfamide nephrotoxicity appears to be dose-dependent and more likely to occur in younger children or those who have undergone nephrectomy.39,40 Previous or subsequent administration of other nephrotoxic agents, most notably cisplatin, increases the likelihood of renal toxicity with ifosfamide treatment.38,39 A subset of patients will experience not only the acute effects of ifosfamide but also chronic renal dysfunction manifested by tubular wasting syndromes or reduced GFR. The low incidence of chronic renal tubular dysfunction in our study could be partially explained by the relatively low cumulative dose of ifosfamide (<20 g/m2).
If serum creatinine or Clcr is used to identify patients with decreased GFR, the incidence of glomerular dysfunction will be underestimated. Ashraf and colleagues41 reported that 7 of 20 patients (35%) had abnormal GFR values determined by using radiolabeled chromium-EDTA (an exogenous substrate), yet none had an abnormal Clcr rate based on plasma creatinine values. In our study, estimated Clcr overestimated measured GFR by 17.8 ml/min per 1.73 m2. We observed no progressive decline in mean estimated Clcr after completion of therapy, but the number of our patients with abnormally low estimated Clcr increased over time.
Adjustment of the carboplatin dosage according to the measured GFR may have prevented severe renal tubular toxicity, particularly in the cycle of ICE administered after nephrectomy. Although treatment with ICE resulted in transient tubulopathy and evidence of subclinical tubular damage (increased urinary β2-microglobulin), no clinically significant tubular dysfunction was noted at the end of treatment. However, a subset of survivors experienced chronic renal insufficiency, which may have been related to sub-optimal compensatory hypertrophy after nephrectomy.
Our study showed ICE chemotherapy to be highly active against Wilms tumour, with non-renal toxicity consisting primarily of moderate myelosuppression. The NWTS-5 trial (1995–2002) used VDA to treat children with stage III and IV Wilms tumour with favorable histology or focal anaplasia, and the combination of vincristine, cyclophosphamide, doxorubicin, and etoposide (Regimen I) to treat children with stage II–IV diffuse anaplastic Wilms tumour.2 Although outcomes of patients with diffuse anaplastic Wilms tumour treated with Regimen I were superior to those of historical controls, disease recurrence remained problematic. Because the combination of cyclophosphamide, carboplatin, and etoposide may have activity similar to that of ICE and may be less nephrotoxic, the Children’s Oncology Group is currently investigating this combination in frontline treatment of high-risk renal tumours.
Our study was limited by the small number of patients and the lack of GFR assessment by technetium 99m-DTPA clearance at follow up. However, our data suggest that ICE can be safely used in patients with high-risk renal tumours by adjusting the carboplatin dosage to the GFR and carefully monitoring renal function, especially after nephrectomy. Importantly, our findings document the feasibility of designing protocols that incorporate carboplatin, ifosfamide, or both for the treatment of renal tumours.
Acknowledgments
We thank Sharon Naron, ELS, and Donald Samulack, PhD for editorial review. Total 25 text pages, 4 tables, and 3 figures
Supported in part by United States Public Health Service Cancer Center Support Grant CA21765, Program Project Grant CA23099, and by the American Lebanese Syrian Associated Charities (ALSAC). Presented in part at the Thirty-fifth Annual Meeting of the American Society of Clinical Oncology, May 15–18, 1999.
Footnotes
Conflict of Interest Statement No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green DM. The treatment of stages I–IV favorable histology Wilms’ tumor. J Clin Oncol. 2004;22(8):1366–72. doi: 10.1200/JCO.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24(15):2352–8. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 3.Tournade MF, Com-Nougue C, de Kraker J, et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms’ tumor in children older than 6 months: results of the Ninth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study. J Clin Oncol. 2001;19(2):488–500. doi: 10.1200/JCO.2001.19.2.488. [DOI] [PubMed] [Google Scholar]
- 4.Ritchey ML, Kelalis PP, Haase GM, Shochat SJ, Green DM, D’Angio G. Preoperative therapy for intracaval and atrial extension of Wilms tumor. Cancer. 1993;71(12):4104–10. doi: 10.1002/1097-0142(19930615)71:12<4104::aid-cncr2820711249>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Ritchey ML, Pringle KC, Breslow NE, et al. Management and outcome of inoperable Wilms tumor. A report of National Wilms Tumor Study-3. Ann Surg. 1994;220(5):683–90. doi: 10.1097/00000658-199411000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemerle J, Voute PA, Tournade MF, et al. Effectiveness of preoperative chemotherapy in Wilms’ tumor: results of an International Society of Paediatric Oncology (SIOP) clinical trial. J Clin Oncol. 1983;1(10):604–9. doi: 10.1200/JCO.1983.1.10.604. [DOI] [PubMed] [Google Scholar]
- 7.Tournade MF, Com-Nougue C, Voute PA, et al. Results of the Sixth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study: a risk-adapted therapeutic approach in Wilms’ tumor. J Clin Oncol. 1993;11(6):1014–23. doi: 10.1200/JCO.1993.11.6.1014. [DOI] [PubMed] [Google Scholar]
- 8.Bonadio JF, Storer B, Norkool P, Farewell VT, Beckwith JB, D’Angio GJ. Anaplastic Wilms’ tumor: clinical and pathologic studies. J Clin Oncol. 1985;3(4):513–20. doi: 10.1200/JCO.1985.3.4.513. [DOI] [PubMed] [Google Scholar]
- 9.D’Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms’ tumor. Results of the Third National Wilms’ Tumor Study. Cancer. 1989;64(2):349–60. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1994;12(10):2126–31. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 11.Tournade MF, Lemerle J, Brunat-Mentigny M, et al. Ifosfamide is an active drug in Wilms’ tumor: a phase II study conducted by the French Society of Pediatric Oncology. J Clin Oncol. 1988;6(5):793–6. doi: 10.1200/JCO.1988.6.5.793. [DOI] [PubMed] [Google Scholar]
- 12.Pein F, Pinkerton R, Tournade MF, et al. Etoposide in relapsed or refractory Wilms’ tumor: a phase II study by the French Society of Pediatric Oncology and the United Kingdom Children’s Cancer Study Group. J Clin Oncol. 1993;11(8):1478–81. doi: 10.1200/JCO.1993.11.8.1478. [DOI] [PubMed] [Google Scholar]
- 13.de Camargo B, Melaragno R, Saba e Silva, et al. Phase II study of carboplatin as a single drug for relapsed Wilms’ tumor: experience of the Brazilian Wilms’ Tumor Study Group. Med Pediatr Oncol. 1994;22(4):258–60. doi: 10.1002/mpo.2950220409. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger LJ, Gaynon PS, Krailo MD, et al. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Childrens Cancer Group. Cancer. 1994;73(4):1297–301. doi: 10.1002/1097-0142(19940215)73:4<1297::aid-cncr2820730427>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Cairo MS, Shen V, Krailo MD, et al. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: a children’s cancer group report. J Pediatr Hematol Oncol. 2001;23(1):30–8. doi: 10.1097/00043426-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tannous R, Giller R, Holmes E, et al. A Ccg-4921/Pog-9445 Study Report. 19. 2000. Intensive Therapy for High Risk (HR) Relapsed Wilms’ Tumor (WT) p. 588a. [Google Scholar]
- 17.Kung FH, Desai SJ, Dickerman JD, et al. Ifosfamide/carboplatin/etoposide (ICE) for recurrent malignant solid tumors of childhood: a Pediatric Oncology Group Phase I/II study. J Pediatr Hematol Oncol. 1995;17(3):265–9. doi: 10.1097/00043426-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Malogolowkin M, Cotton CA, Green DM, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Oncol Rep. 2008;50(2):236–41. doi: 10.1002/pbc.21267. [DOI] [PubMed] [Google Scholar]
- 19.Marina NM, Wilimas JA, Meyer WH, Jones DP, Douglass EC, Pratt CB. Refining therapeutic strategies for patients with resistant Wilm’s tumor. Am J Pediatr Hematol Oncol. 1994;16(4):296–300. [PubMed] [Google Scholar]
- 20.Abu-Ghosh AM, Krailo MD, Goldman SC, et al. Ifosfamide, carboplatin and etoposide in children with poor-risk relapsed Wilms’ tumor: a Children’s Cancer Group report. Ann Oncol. 2002;13(3):460–9. doi: 10.1093/annonc/mdf028. [DOI] [PubMed] [Google Scholar]
- 21.Hayslett JP. Effect of age on compensatory renal growth. Kidney Int. 1983;23(4):599–602. doi: 10.1038/ki.1983.64. [DOI] [PubMed] [Google Scholar]
- 22.Potter DE, Leumann EP, Sakai T, Holliday MA. Early responses of glomerular filtration rate to unilateral nephrectomy. Kidney Int. 1974;5(2):131–6. doi: 10.1038/ki.1974.17. [DOI] [PubMed] [Google Scholar]
- 23.Wikstad I, Pettersson BA, Elinder G, Sokucu S, Aperia A. A comparative study of size and function of the remnant kidney in patients nephrectomized in childhood for Wilms’ tumor and hydronephrosis. Acta Paediatr Scand. 1986;75(3):408–14. doi: 10.1111/j.1651-2227.1986.tb10222.x. [DOI] [PubMed] [Google Scholar]
- 24.Green DM, Coppes MJ, Breslow NE, et al. Wilms tumor. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 3. Philadelphia: Lippincott - Raven; 1997. pp. 733–59. [Google Scholar]
- 25.Rodman JH, Maneval DC, Magill HL, Sunderland M. Measurement of Tc-99m DTPA serum clearance for estimating glomerular filtration rate in children with cancer. Pharmacotherapy. 1993;13(1):10–6. [PubMed] [Google Scholar]
- 26.Marina NM, Rodman J, Shema SJ, et al. Phase I study of escalating targeted doses of carboplatin combined with ifosfamide and etoposide in children with relapsed solid tumors. J Clin Oncol. 1993;11(3):554–60. doi: 10.1200/JCO.1993.11.3.554. [DOI] [PubMed] [Google Scholar]
- 27.Marina NM, Rodman JH, Murry DJ, et al. Phase I study of escalating targeted doses of carboplatin combined with ifosfamide and etoposide in treatment of newly diagnosed pediatric solid tumors. J Natl Cancer Inst. 1994;86(7):544–8. doi: 10.1093/jnci/86.7.544. [DOI] [PubMed] [Google Scholar]
- 28.Murry DJ, Synold TW, Pui CH, Rodman JH. Renal function and methotrexate clearance in children with newly diagnosed leukemia. Pharmacotherapy. 1995;15(2):144–9. [PubMed] [Google Scholar]
- 29.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–63. [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994;8(2):250–1. doi: 10.1007/BF00865491. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 33.Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997;131(2):252–7. doi: 10.1016/s0022-3476(97)70162-8. [DOI] [PubMed] [Google Scholar]
- 34.Stark H, Eisenstein B, Tieder M, Rachmel A, Alpert G. Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron. 1986;44(2):125–8. doi: 10.1159/000184216. [DOI] [PubMed] [Google Scholar]
- 35.Schell M, Cochat P, Hadj-Aissa A, Bouffet E, Dubourg L, Brunat-Mentigny M. Renal function following unilateral nephrectomy for neuroblastoma and Wilms’ tumour. Pediatr Nephrol. 1995;9(5):579–82. doi: 10.1007/BF00860940. [DOI] [PubMed] [Google Scholar]
- 36.de Graaf SS, van Gent H, Reitsma-Bierens WC, van Luyk WH, Dolsma WV, Postma A. Renal function after unilateral nephrectomy for Wilms’ tumour: the influence of radiation therapy. Eur J Cancer. 1996;32A(3):465–9. doi: 10.1016/0959-8049(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 37.Levitt GA, Yeomans E, Dicks MC, Breatnach F, Kingston J, Pritchard J. Renal size and function after cure of Wilms’ tumour. Br J Cancer. 1992;66(5):877–82. doi: 10.1038/bjc.1992.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DP, Chesney RW. Renal toxicity of cancer chemotherapeutic agents in children: ifosfamide and cisplatin. Curr Opin Pediatr. 1995;7(2):208–13. doi: 10.1097/00008480-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Rossi R, Godde A, Kleinebrand A, et al. Unilateral nephrectomy and cisplatin as risk factors of ifosfamide-induced nephrotoxicity: analysis of 120 patients. J Clin Oncol. 1994;12(1):159–65. doi: 10.1200/JCO.1994.12.1.159. [DOI] [PubMed] [Google Scholar]
- 40.Skinner R, Sharkey IM, Pearson AD, Craft AW. Ifosfamide, mesna, and nephrotoxicity in children. J Clin Oncol. 1993;11(1):173–90. doi: 10.1200/JCO.1993.11.1.173. [DOI] [PubMed] [Google Scholar]
- 41.Ashraf MS, Brady J, Breatnach F, Deasy PF, O’Meara A. Ifosfamide nephrotoxicity in paediatric cancer patients. Eur J Pediatr. 1994;153(2):90–4. doi: 10.1007/BF01959214. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ. Clinical assessment of renal function. In: Kher KK, Schnapper HW, Makker SP, editors. Clinical Pediatric Nephrology. 2. Taylor and Francis; Boca Raton, FL: 2007. pp. 71–93. [Google Scholar]