Abstract
Background
Hyperhomocysteinemia may be a modifiable risk factor for the prevention of arteriosclerotic outcomes in chronic kidney disease (CKD). Few clinical trials of homocysteine lowering have been conducted in persons with CKD prior to reaching end-stage renal disease. Kidney transplant recipients are considered individuals with CKD.
Objectives
To describe the baseline characteristics of renal transplant recipients (RTRs) enrolled in a clinical trial of homocyteine lowering with a standard multivitamin containing high doses of folic acid, vitamins B6 and B12 aimed at reducing arteriosclerotic outcomes. Factors considered were level of kidney function, total homocysteine (tHcy) concentrations, and the prevalence of diabetes and previous cardiovascular disease (CVD).
Study Design
Cross sectional survey within a randomized controlled trial (RCT) cohort.
Setting and Participants
Participants were recruited from kidney transplant clinics in the U.S., Canada, and Brazil. Eligible participants had elevated levels of homocysteine (≥12.0 μmol/L in men and ≥11.0 μmol/L in women) and kidney function measured by Cockroft Gault estimated creatinine clearance of 30 mL/min or greater.
Results
Among 4,110 randomized participants 38.9% had diabetes, and 19.5% had previous CVD. Mean (± standard deviation) tHcy concentrations were 17.1 ± 6.3 μmol/L, while the mean (± standard deviation) creatinine clearance was 66.4 ± 23.2 mL/min. Approximately 90% of the trial cohort had an estimated glomerular filtration rate (eGFR) consistent with stage 2-3 CKD (i.e., eGFR 30-89 mL/min).
Limitations
Analysis is based on cross-sectional data from a RCT, self-report of co-morbid illnesses, and level of kidney function was estimated.
Conclusions
A large population of stable RTRs who are at high risk for the development of CVD (both de novo and recurrent) has been recruited into FAVORIT and are likely to experience a sufficient number of events to address the primary hypothesis of the trial.
Index Words: chronic kidney disease, renal transplantation, hyperhomocysteinemia, creatinine clearance, estimated GFR, arteriosclerosis, diabetes
Introduction
Chronic kidney disease (CKD) increases substantially the risk of cardiovascular disease (CVD). The increase in risk can be attributed, in part, to a higher prevalence of established arteriosclerotic risk factors, including older age, hypertension, diabetes, dyslipidemia, and physical inactivity. However, these established risk factors do not account adequately for the excess burden of CVD observed in the CKD population. 1 This observation has led to the search for non-traditional risk factors that may also contribute to this excess CVD risk. 2 One non-traditional risk factor, hyperhomocysteinemia or elevated concentrations of homocysteine, a sulfur-containing amino acid by-product of methionine metabolism, has received considerable attention over the past decade.
A number of prospective observational studies of persons with CKD prior to kidney failure, which have been conducted throughout the world, have revealed a linear trend for increased CVD risk, i.e., per μmol/L increase or across quantiles of total homocysteine (tHcy). As expected, the greatest relative risk was confined to persons with higher levels of tHcy. 3, 4 Whether mild to moderate hyperhomocysteinemia is a risk factor for arteriosclerotic outcomes in these populations or is only a surrogate for the apparent relationship between kidney function and clinical CVD has not yet been resolved. Although a substantial number of randomized controlled clinical trials of tHcy-lowering treatment have been undertaken in different at-risk populations to evaluate efficacy for reducing CVD events, 5-13 including persons with CKD, 14 none have demonstrated a significant reduction in CVD risk.
The renal transplant patient population remains a suitable group to enroll in clinical trials to test the “homocysteine-lowering hypothesis” since they have a high rate of both incident and recurrent cardiovascular disease, 2 and continue to display an excess prevalence of hyperhomocysteinemia despite the fortification of cereal grain flour with folic acid. 3 Importantly, these patients are not routinely treated with supplemental folic acid, and are able to “normalize” their tHcy levels with combined folic acid, vitamin B12, and vitamin B6 treatment. 15, 16 In contrast, hyperhomocysteinemia in end-stage renal disease (ESRD) patients persists with such vitamin therapy,17 albeit at slightly lower tHcy levels. Furthermore, at the 2004 Kidney Disease: Improving Global Outcomes (KDIGO) International Controversies Conference on Definition and Classification of Chronic Kidney Disease in Adults, the expert consensus was to “consider all kidney transplant recipients to have CKD, irrespective of GFR level or presence or absence of markers of kidney damage.” 18 Although there is great heterogeneity among causes of CKD, many of the complications of CKD in renal transplant recipients are similar to those experienced by persons with CDK of their native kidneys. 19 These observations led to the design and conduct of a randomized clinical trial of homocysteine lowering in renal transplant recipients, the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial. 20
The primary objective of the FAVORIT trial is to determine whether lowering total homocysteine levels in clinically stable renal transplant recipients with a multivitamin containing high doses of folic acid (5.0 mg), vitamin B6 (pyridoxine, 50 mg) and vitamin B12 (cyanocobalamin, 1.0 mg) will reduce their risk of CVD compared to treatment with a “low dose” multivitamin devoid of folic acid and with estimated average requirement (EAR) amounts of vitamins B6 (1.4 mg) and B12 (2.0 μg). We describe the major demographic and clinical characteristics at baseline for participants enrolled into the trial.
Methods
Details of the FAVORIT study design have been published previously. 20 Briefly, the trial is being conducted at 30 clinical sites in the United States (27 sites), Canada (2 sites) and Brazil (1 site), with support from an Operations Center, Data Coordinating Center, and Central Laboratory. Study enrollment began in August 2002 and was completed in January 2007; planned follow-up is through July 2011. The primary outcome is a combination of both clinical events and invasive procedures for cardiovascular, peripheral vascular or renovascular disease. A sample size of 4,000 with five years of follow-up is estimated to provide 87% power to detect a 20% treatment effect. Institutional review board approval is maintained by all sites.
Study participants
Kidney transplant recipients were eligible for the study if they were aged 35 to 75 years, had clinically stable kidney function and elevated tHcy levels. Stable kidney function was ascertained by medical chart review to establish that the patient's current graft had been functioning for at least six months post-transplantation and there was no documented clinical indication of renal function deterioration. All enrolled participants had a Cockcroft-Gault estimated creatinine clearance (Ccr) 21 of 30 mL/min or greater and elevated tHcy (≥12.0 μmol/L for men or ≥11.0 μmol/L for women) based on central laboratory analysis of screening specimens. For women recruited after July 2005, the Ccr eligibility criteria was reduced to 25 mL/min or greater in acknowledgment of the lower Ccr distribution routinely observed in women relative to the distribution in men. Individuals with chronic illness limiting life expectancy less than 2 years were excluded, as were those with CVD risk modified because of recent CVD-related events or procedures. Written informed consent was obtained from all study participants.
Screening and baseline data
The screening visit began with the informed consent process for screening, baseline, and follow-up contacts. Eligibility criteria were verified and fasting or non-fasting blood specimens (serum, plasma, buffy coat, and red blood cells) were collected for central analysis of creatinine and total homocysteine, and for specimen banking. Based on the central laboratory determinations, participants meeting laboratory eligibility values for Ccr and tHcy were scheduled for the baseline (randomization) visit.
The study protocol allowed up to 120 days to elapse between screening and randomization. Therefore, eligibility criteria were confirmed just prior to randomization. Participants meeting all study criteria were equally randomly allocated to one of two treatment groups. Data on regular medication use during the past month, blood pressure, height, weight, and medical history were collected. Of note, history of diabetes includes any history, pre- or post-transplant, including diabetes that may have resolved through pancreas transplant. Assessment of diabetes history is a combination of data recorded in available medical records and self-reported medical history. Race and ethnicity were defined in accordance with the National Institutes of Health Policy on Reporting Race and Ethnicity Data. 22 The term ‘African American’ includes African descendents in Brazil. Blood specimens were obtained to assess tHcy, folate, pyridoxal-5′-phosphate (PLP), total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, creatinine and glucose, and for specimen banking. Midstream clean catch urine specimens were also collected for storage.
In spring 2005, the study protocol was modified to incorporate a mechanism to reduce the participant burden of needing to make a special visit to complete the baseline examination. Essentially, the screening visit was expanded to include all of the baseline data collection except for final verification of eligibility and randomization; these could be completed through a telephone contact up to 120 days following screening with baseline data collection.
Laboratory methods for major analytes
Total homocysteine was measured by an HPLC method based on the principles described by Araki and Sako. 23 Serum creatinine was determined by a kinetic adaptation of the Jaffe reaction 24 on a clinical chemistry analyzer, (Cobas Mira Analyzer, Roche Diagnostic Systems, Inc., Indianapolis, IN), according to Larsen 25 and as modified by Roche Diagnostic Systems, Inc. Serum total cholesterol, direct LDL-cholesterol, direct HDL-cholesterol, and triglyceride were measured on a clinical chemistry automated analyzer, Olympus AU400,(Olympus America Inc., 2 Corporate Center Drive, Melville, NY, 11747-3157), using Olympus America, Inc. enzymatic reagents and calibrators, and according to protocol as specified in Olympus AU400 standard operating procedure manual. When serum triglycerides were 400 mg/dL or less, LDL-cholesterol was estimated using the Friedewald formula; 26 if serum triglycerides were greater than 400 mg/dL, direct LDL-cholesterol was measured as indicated above.
Statistical analyses
All analyses except the power calculations in Table 1 were computed using SAS® version 9.1.27 P-values for comparisons by country and history of diabetes were based on chi-squared test for categorical variables when all cell counts were 10 or greater. Otherwise, Fisher's Exact Test was used. The analysis of variance f-test was used to test for a difference in means for continuous variables. Estimated glomerular filtration rate (eGFR) was computed using the Modification of Diet in Renal Disease Study (MDRD) equation based on serum creatinine assays before calibration to isotope dilution mass spectrometry. 28
Table 1. Baseline characteristics by country and history of diabetes.
| Country* | Diabetic Status** | |||||
|---|---|---|---|---|---|---|
| Characteristic | United States n=3000 |
Brazil n= 612 |
Canada n= 498 |
Diabetic n= 1597 |
Non-Diabetic n=2513 |
Overall n=4110 |
| Age | 52 ± 9.4 | 49 ± 8.5 | 53 ± 10.2 | 53 ± 9.3 | 51 ± 9.5 | 52 ± 9.4 |
| Sex | ||||||
| Women | 1145 (38.2 %) | 214 (35.0 %) | 169 (33.9 %) | 579 (36.3 %) | 949 (37.8 %) | 1528 (37.2 %) |
| Men | 1855 (61.8 %) | 398 (65.0 %) | 329 (66.1 %) | 1018 (63.7 %) | 1564 (62.2 %) | 2582 (62.8 %) |
| Ethnicity | ||||||
| Hispanic | 101 (3.4 %) | 612 (100.0 %) | 4 (0.8 %) | 211 (13.2 %) | 506 (20.1 %) | 717 (17.4 %) |
| Non Hispanic | 2879 (96.0 %) | 492 (98.8 %) | 1373 (86.0 %) | 1998 (79.5 %) | 3371 (82.0 %) | |
| Race | ||||||
| White | 2216 (73.9 %) | 431 (70.4 %) | 428 (85.9 %) | 1153 (72.2 %) | 1922 (76.5 %) | 3075 (74.8 %) |
| African American | 577 (19.2 %) | 129 (21.1 %) | 19 (3.8 %) | 326 (20.4 %) | 399 (15.9 %) | 725 (17.6 %) |
| Other or Mixed | 129 (4.3 %) | 52 (8.5 %) | 40 (8.0 %) | 82 (5.1 %) | 139 (5.5 %) | 221 (5.4 %) |
| Graft Source | ||||||
| Living | 1139 (38.0 %) | 379 (61.9 %) | 187 (37.6 %) | 554 (34.7 %) | 1151 (45.8 %) | 1705 (41.5 %) |
| Cadaver | 1842 (61.4 %) | 233 (38.1 %) | 296 (59.4 %) | 1026 (64.2 %) | 1345 (53.5 %) | 2371 (57.7 %) |
| Years Since Transplant | 5 ± 4.9 | 5 ± 3.9 | 7 ± 6.5 | 5 ± 4.6 | 6 ± 5.3 | 5 ± 5.0 |
| Weight (kg) | 88 ± 20.5 | 73 ± 13.8 | 82 ± 18.3 | 87 ± 20.6 | 83 ± 19.6 | 85 ± 20.1 |
| Body Mass Index (kg/m2) | 30 ± 6.5 | 27 ± 4.5 | 28 ± 5.9 | 30 ± 6.6 | 29 ± 5.9 | 29 ± 6.2 |
| Systolic Blood Pressure (mmHg) | 135 ± 18.9 | 145 ± 22.9 | 133 ± 16.8 | 138 ± 20.4 | 135 ± 19.3 | 136 ± 19.7 |
| Diastolic Blood Pressure (mmHg) | 76 ± 10.6 | 91 ± 13.4 | 78 ± 9.4 | 76 ± 11.5 | 80 ± 12.3 | 78 ± 12.2 |
| Medical History | ||||||
| Previous MI/CHD*** | 454 (15.1 %) | 46 (7.5 %) | 50 (10.0 %) | 320 (20.0 %) | 230 (9.2 %) | 550 (13.4 %) |
| Previous Stroke/CBVD*** | 213 (7.1 %) | 45 (7.4 %) | 12 (2.4 %) | 155 (9.7 %) | 115 (4.6 %) | 270 (6.6 %) |
| Previous Abdominal/LEAD*** | 143 (4.8 %) | 8 (1.3 %) | 9 (1.8 %) | 116 (7.3 %) | 44 (1.8 %) | 160 (3.9 %) |
| Previous CVD (any of above) | 646 (21.5 %) | 92 (15.0 %) | 65 (13.1 %) | 469 (29.4 %) | 334 (13.3 %) | 803 (19.5 %) |
| Previous Renal Arterial Revascularization | 28 (0.9 %) | 32 (5.2 %) | 7 (1.4 %) | 33 (2.1 %) | 34 (1.4 %) | 67 (1.6 %) |
| Immunosuppression Therapy | ||||||
| Cyclosporine A | 1492 (49.7 %) | 309 (50.5 %) | 294 (59.0 %) | 771 (48.3 %) | 1324 (52.7 %) | 2095 (51.0 %) |
| Tacrolimus | 1182 (39.4 %) | 221 (36.1 %) | 149 (29.9 %) | 681 (42.6 %) | 871 (34.7 %) | 1552 (37.8 %) |
| Sirolimus | 281 (9.4 %) | 42 (6.9 %) | 23 (4.6 %) | 137 (8.6 %) | 209 (8.3 %) | 346 (8.4 %) |
| Mycophenolate Mofetil | 2075 (69.2 %) | 302 (49.3 %) | 298 (59.8 %) | 1068 (66.9 %) | 1607 (63.9 %) | 2675 (65.1 %) |
| Azathioprine | 428 (14.3 %) | 269 (44.0 %) | 43 (8.6 %) | 240 (15.0 %) | 500 (19.9 %) | 740 (18.0 %) |
| Prednisone | 2705 (90.2 %) | 587 (95.9 %) | 442 (88.8 %) | 1434 (89.8 %) | 2300 (91.5 %) | 3734 (90.9 %) |
| History of Diabetes | 1282 (42.7 %) | 165 (27.0 %) | 150 (30.1 %) | 1597 (38.9 %) | ||
| Laboratory Characteristics | ||||||
| Screening Fast ≥ 8 Hours | 1039 (34.6 %) | 599 (97.9 %) | 102 (20.5 %) | 613 (38.4 %) | 1127 (44.8 %) | 1740 (42.3 %) |
| Screening tHcy (μmol/L) | 17.0 ± 6.3 | 17.1 ± 7.1 | 17.4 ± 4.8 | 17.0 ± 6.3 | 17.1 ± 6.3 | 17.1 ± 6.3 |
| Screening CCr (mL/min) | 68.1 ± 24.0 | 59.7 ± 18.6 | 64.0 ± 22.0 | 68.2 ± 24.1 | 65.2 ± 22.6 | 66.4 ± 23.2 |
| Screening Creatinine (mg/dL) | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 |
| Baseline Fast ≥ 8 Hour | 914 (30.5 %) | 577 (94.3 %) | 97 (19.5 %) | 552 (34.6 %) | 1036 (41.2 %) | 1588 (38.6 %) |
| Total Cholesterol (mg/dL) | 184 ± 43.5 | 192 ± 48.4 | 178 ± 39.4 | 177 ± 42.3 | 189 ± 44.3 | 185 ± 43.9 |
| HDL (mg/dL) | 47 ± 14.2 | 44 ± 12.4 | 47 ± 14.2 | 46 ± 14.2 | 46 ± 13.7 | 46 ± 13.9 |
| Calculated or Direct LDL (mg/dL) | 100 ± 33.9 | 111 ± 39.1 | 94 ± 28.3 | 94 ± 32.2 | 105 ± 35.2 | 101 ± 34.4 |
| Triglycerides (mg/dL) | 203 ± 200.9 | 186 ± 114.6 | 193 ± 131.4 | 199 ± 243.1 | 199 ± 130.4 | 199 ± 182.4 |
Distributions between countries were statistically significant at the p<0.05 level for all variables except sex, screening tHcy and triglycerides.
Distributions between diabetic status were statistically significant at the p<0.05 level for all variables except sex, previous renal arterial revascularization, screening tHcy, screening creatinine, HDL and triglycerides.
CHD=Coronary Heart Disease, CBVD=Cerebrovascular Disease, AAA=Abdominal Aortic Aneurysm, TAA=Thoracic Aortic Aneurysm, LEAD=Lower Extremity Arterial Disease
The post-hoc power estimates provided in Table 1 were calculated based on data taken from published papers and abstracts. Power was computed using the observed event rate in the control group and the attained sample sizes for each study. For the studies reported only in abstract form 5, 6 in which sample sizes by treatment group were not reported, the sample size in each treatment group was assumed to be one half of the total sample size. The power was computed for 10%, 15%, and 20% reduction in events for the treated group using a 5% (two-sided) significance level in STATA version 7.
Results
A total of 7,273 renal transplant recipients met pre-screening criteria and had creatinine and tHcy centrally assessed. As depicted in Figure 1, 4,753 (65%) of the screenees met the trial's eligibility criteria for Ccr and tHcy level and among those 4,110 (86%) were randomized. Although the study did not systematically collect reasons lab-eligible individuals were otherwise ineligible or not randomized, frequently-cited responses included patients wanting to take their own vitamins (usually to insure that folic acid was included), time or transportation issues, and not wanting the additional commitment.
Figure 1. Screening and Enrollment Profile.
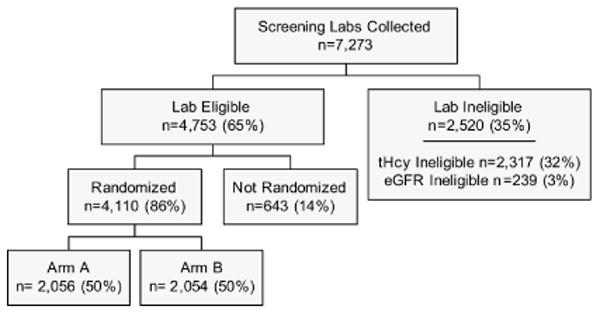
Major clinical and demographic characteristics of the randomized study participants assessed at baseline are presented in Table 2. The 3,000 participants recruited in the United States were similar in sex and donor type distributions but include a smaller proportion of individuals with Hispanic ethnicity than kidney transplant recipients documented in the Organ Procurement and Transplantation Network (Based on OPTN data as of January 18, 2008 from http://www.optn.org/latestData/rptData.asp) for kidney transplants completed from 1988 through 2007 (60.0% male, 34.5% living donor, 11.5% Hispanic). Traditional CVD risk factors varied by country with participants in the United States having a higher mean BMI (30 kg/m2) and a larger proportion with a history of diabetes (43%) than participants in Brazil (27 kg/m2 and 27%, respectively) and Canada (28 kg/m2 and 30%, respectively). Total cholesterol level and systolic blood pressure were highest in Brazil (192 mg/dL and 145 mm Hg) and lowest in Canada (178 mg/dL and 133 mm Hg). A smaller proportion of participants in Canada (4%) were African American than in the United States (19%) or Brazil (21%). The mean tHcy among all study participants at screening was 17.1 μmol/L and the prevalence of diabetes was substantial (39%).
Table 2. Distribution of estimated glomerular filtration rate and CKD stage at screening.
| United States | Brazil | Canada | Overall | |
|---|---|---|---|---|
| eGFR (ml/min) | ||||
| N | 3000 | 612 | 498 | 4110 |
| Mean ± SD | 50 ± 16.5 | 53 ± 16.5 | 50 ± 15.9 | 51 ± 16.5 |
| Range | 16.1 - 152.7 | 17.5 - 116.9 | 18.0 - 104.3 | 16.1 - 152.7 |
| CKD Stage | ||||
| Stage 1 (eGFR 90+) | 67 (2.2 %) | 15 (2.5 %) | 8 (1.6 %) | 90 (2.2 %) |
| Stage 2 (eGFR 60 - 89) | 662 (22.1 %) | 173 (28.3 %) | 117 (23.5 %) | 952 (23.2 %) |
| Stage 3 (eGFR 30 - 59) | 2064 (68.8 %) | 397 (64.9 %) | 335 (67.3 %) | 2796 (68.0 %) |
| Stage 4 (eGFR 15 - 29) | 207 (6.9 %) | 27 (4.4 %) | 38 (7.6 %) | 272 (6.6 %) |
Consistent with their increased risk for the development of clinical arteriosclerosis, participants with diabetes had more than double the prevalence of baseline CVD (29% vs. 13%), compared to participants without diabetes. Moreover, the higher prevalence of risk factors for CVD in participants with diabetes (slightly older, heavier, and higher systolic blood pressure than persons without diabetes) suggests that incident cardiovascular disease will be elevated in this group as well.
The distribution of estimated glomerular filtration rate based on the MDRD formula (eGFR) at screening is provided in Table 3. Approximately 90% of the participants meet criteria for stage 2-3 CKD (i.e., eGFR 30-89 mL/min).
Table 3. Power comparisons for completed clinical trials, and FAVORIT.
| Study | Event rate controls | Power for 10% reduction in events | Power for 15% reduction in events | Power for 20% reduction in events |
|---|---|---|---|---|
| NORVIT7 | 0.182 | 0.16 | 0.33 | 0.54 |
| Hope-29 | 0.198 | 0.46 | 0.81 | 1.00 |
| VISP11 | 0.171 | 0.27 | 0.55 | 0.81 |
| SWISS Heart10 | 0.228 | 0.08 | 0.14 | 0.23 |
| Wrone et al12 | 0.417 | 0.10 | 0.18 | 0.31 |
| CHAOS II*6 | 0.100 | 0.10 | 0.18 | 0.30 |
| ASFAST13 | 0.541 | 0.13 | 0.26 | 0.43 |
| HOST**8 | 0.238 | 0.24 | 0.48 | 0.73 |
| WAFACS*5 | 0.148 | 0.33 | 0.65 | 0.89 |
| FAVORIT***20 | 0.186 | 0.32 | 0.63 | 0.88 |
Note: Event rates in the control group are the observed event rates for all studies except FAVORIT. For FAVORIT, the event rate is the projected event rate from the protocol.
Event rates not given by treatment group; total event rate divided by 2
Event rates for fatal and non-fatal MI, fatal and non fatal stroke, and amputation, pooled
Not observed data; based on design
Discussion
Cardiovascular disease remains the most common cause of morbidity and mortality in persons with CKD. This increased risk along with the high prevalence of hyperhomocysteinemia, the expected infrequency of treatment with supplemental folic acid, and the previously-demonstrated ability to “normalize” tHcy levels in renal transplant recipients with combined folic acid, vitamin B12 and vitamin B6 treatment makes kidney transplant recipients an especially suitable population in which to test the effects of lowering homocysteine.
In order to test this hypothesis we recruited a large sample of stable kidney transplant recipients who are at high risk of CVD. Most of the participants had either Stage 2 or 3 CKD based on the MDRD Study formula. Moreover nearly forty percent had a diagnosis of diabetes, another important risk factor for CVD, exceeding the estimate used in the original power calculation (35%). As expected, a history of cardiovascular disease was more prevalent among the FAVORIT participants with diabetes than among those without diabetes (29% and 13%, respectively). Thus the trial appears to be well poised to accrue a sufficient number of events to address the primary hypothesis of whether homocysteine lowering in stable kidney transplant recipients will significantly reduce arteriosclerotic cardiovascular disease. We are aware of the results of nine tHcy-lowering clinical trials evaluating the potential reduction of cardiovascular disease outcomes among various patient populations 5-13 and none have shown a beneficial effect. However, each of these trials had limitations including failure to normalize tHcy levels, 8, 12, 13 the impact of folic acid fortification in folate “sensitive” non-CKD populations 5, 9, 11 or medication drop-ins who used up to 1 mg/day of folic acid 8 which may have reduced study power.
One recently reported trial of homocysteine lowering in approximately 2,000 ESRD or stage 4 CKD patients, the Homocysteinemia in Kidney and End Stage Renal Disease (HOST) study, reported that even a very high dose B-vitamin regimen for tHcy-lowering (40 mg/day folic acid, 100 mg/day B6, and 2 mg/day B12) failed to reduce crude all-cause mortality during a median of 3.2 years of follow-up. However, there was a trend for fewer myocardial infarctions in the actively treated group compared to placebo. 8 In comparison, a subgroup analysis of participants with CKD (GFR <60 ml/min) in the Heart Outcomes Prevention Evaluation 2 (HOPE-2) Study failed to show a beneficial effect of homocysteine lowering on cardiovascular risk. 14
While the limited or lack of statistical power to detect CVD event rate changes of 20% in the preliminary CKD patient trials, and even the larger HOST trial are clear (see Table 1), we investigated whether the considerably larger non-CKD patient trial cohorts might also have been inadequately powered based on their now reported control event rates. Only two 5, 9 of the five 5-7, 9, 11 large trials completed in predominantly non-renal populations, HOPE-2 9 and Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS), 5 remained well-powered (i.e., >=85%) to detect a 20% reduction in CVD events with active treatment, based on the actual control group event rate. None of the trials are well-powered to detect a 10% or 15% reduction in CVD events. Furthermore, the mean tHcy concentration at baseline in HOPE-2 was only 12.1 μmol/L, and was reduced by just 2.4 μmol/L with active treatment. 9 Comparable data from WAFACS reveal an initial level of 12.2 μmol/L, which was only reduced by 2.2 μmol/L with active treatment. 5 In contrast, the Norwegian Vitamin trial (NORVIT) participants had slightly higher baseline tHcy concentrations (13.1 μmol/L), and achieved a greater absolute reduction (3.5 μmol/L), but when the actual control group event rate was considered, the study lacked adequate statistical power to detect a 20% reduction in CVD event rates. 7 The Cambridge Heart Antioxidant Study-2 (CHAOS-2), 6 and to a lesser extent the Vitamin Intervention for Stroke Prevention trial (VISP),11 may have also suffered from the combined effects of these limitations. In contrast, at screening, FAVORIT participants have a mean tHcy level of 17.1 μmol/L in comparison to a range of baseline levels of 11.2-13.4 μmol/L in these recently completed trials of patients without CKD. 5-7, 9, 11 We have previously shown that 15-17 subsequent to chronic exposure to cereal grain flour products fortified with folic acid, long-term kidney transplant recipients with mild hyperhomocysteinemia who were similar to participants enrolled in FAVORIT, still experienced a nearly one-third (5.0-5.5 μmol/L) decrease during treatment with a folic acid, B12, and B6 regimen, and 50% of these participants maintained their tHcy concentrations below 12 μmol/L. A similar reduction in tHcy of 5-6 μmol/L is expected among the FAVORIT participants receiving the high dose multivitamin. Thus, we anticipate that both the higher observed tHcy levels and expected greater impact of tHcy-lowering vitamin therapy may enhance the likelihood of the FAVORIT study showing a reduction of CVD risk compared to previous studies.
The FAVORIT trial has several potential limitations. As we have shown there are important differences in several baseline CVD risk factors by country that could impact the observed event rate. However, these risk factor differences are not in the same direction. For example, among participants in Brazil, mean BMI is lower whereas mean blood pressure is higher than among participants in the U.S. Additionally, the within-country sample sizes are large and this heterogeneity may increase the generalizability of the findings. Many important, albeit tertiary, research questions will be addressed through analyses stratified by country or within the U.S. subgroup. Another limitation of the baseline data is that history of disease was taken from patient report and medical records without verification or classification using standardized definitions as is being done prospectively with the myocardial infarction, stroke, resuscitated sudden death, and cardiovascular death components of the FAVORIT primary endpoint. While this is a limitation for cross-sectional baseline analyses, it will not affect the validity of the primary trial objective. We also acknowledge that renal function is estimated instead of measured GFR. However data are provided based on two well-established prediction equations 21, 28 for comparison with other scientific literature and clinical practice. However, these equations have not been uniformly validated in kidney transplant recipients with stable renal function. Finally, caution should be exercised if generalizing CKD among RTRs to CKD among other patient groups.
FAVORIT has a number of strengths. The sample size of 4,110 chronic stable renal transplant recipients is large. The planned period of follow-up (4.5 to 9 years) is long. This duration of follow-up may detect a lag in treatment effect, a focus not considered in most previous clinical trials. Screening data confirm that baseline tHcy levels are higher than in trials conducted among non-CKD populations. Finally, with the projected FAVORIT event rate for CVD among the control group, the trial appears to be adequately powered to discern even a moderate 20% reduction in events (if achieved) with active tHcy-lowering treatment. Since FAVORIT includes participants with predominantly stage 2 and stage 3 CKD the findings will provide important information on the clinical benefit of tHcy-lowering with B-vitamin therapy among this group and may indicate a low-cost intervention for the general CKD population.
Acknowledgments
PamLab LLC of Covington, LA, provided the high and low-dose multivitamins.
FAVORIT Principal Investigators: David Conti, MD (Albany Mendical Center); Alfredo Fabrega, MD (Banner Health Good Samaritan); Ajay Singh, MD (Brigham and Women's Hospital); Mike Bunnapradist, MD (Cedars-Sinai Health System); Mysore S. Anil Kumar, MD (Drexel University); Stephen Smith, MD (Duke University); Paul Bolin, Jr., MD (East Carolina University); Bertram Kasiske, MD (Hennepin County Medical Center); Muhammad Sohail Yaqub, MD (Indiana University); Andrew House, MD (London Health Sciences Center); John Vella, MD (Maine Medical Center); Fernando Cosio, MD (Mayo Clinic); Barbara Bresnahan, MD (Medical College of Wisconsin); Lorenzo Gallon, MD (Northwestern University); Todd Pesavento, MD (Ohio State University); Douglas Norman, MD (Oregon Health Sciences University); Andrew Bostom, MD (Rhode Island Hospital); Edward Alfrey, MD (Southern Illinois University); Mariana Markell, MD (SUNY/Downstate Medical Center); Alvaro Pacheco-Silva, MD, PhD (Universidade Federal de Sao Paulo); Clifton Kew, MD (University of Alabama/Birmingham); Deborah Adey, MD (University of California/ San Francisco); Gabriel Danovitch, MD (University of California/Los Angeles); Lawrence Hunsicker, MD (University of Iowa); Matthew Weir, MD (University of Maryland/Baltimore); Akinlolu Ojo, MD (University of Michigan); Arthur Matas, MD (University of Minnesota); Edward Cole, MD (University of Toronto); John Pirsch, MD (University of Wisconsin/Madison); Matthew Koch, MD (Washington University).
Supported by cooperative agreement U01 DK61700 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Support also provided by the Office of Dietary Supplements, NIH
Footnotes
For the FAVORIT Study Investigators
FAVORIT Trial includes thirty clinical sites and three subcontract sites. See Acknowledgements for listing.
A list of the FAVORIT investigators can be found in the acknowledgements.
The FAVORIT Trial is registered at clinicaltrials.gov: Registration Number: NCT00064753
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew G. Bostom, Rhode Island Hospital.
Myra A. Carpenter, University of North Carolina.
Lawrence Hunsicker, University of Iowa.
Paul F. Jacques, Jean Mayer USDA HNRCA.
John W. Kusek, NIDDK.
Andrew S. Levey, Tufts Medical Center.
Joyce L. McKenney, Rhode Island Hospital.
Renee Y. Mercier, Brigham and Women's Hospital.
Marc A. Pfeffer, Brigham and Women's Hospital.
Jacob Selhub, Jean Mayer USDA HNRCA.
References
- 1.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 3.Bostom AG, Culleton BF. Hyperhomocysteinemia in chronic renal disease. J Am Soc Nephrol. 1999;10:891–900. doi: 10.1681/ASN.V104891. [DOI] [PubMed] [Google Scholar]
- 4.Friedman AN, Rosenberg IH, Selhub J, Levey AS, Bostom AG. Hyperhomocysteinemia in renal transplant recipients. Am J Transplant. 2002;2:308–313. doi: 10.1034/j.1600-6143.2002.20404.x. [DOI] [PubMed] [Google Scholar]
- 5.Albert CM, Cook NR, Gaziano JM, et al. A randomized trial of folic acid and B-vitamins in the secondary prevention of cardiovascular events in women: Results from the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) Circulation. 2006;114:2424. abstr. [Google Scholar]
- 6.Baker F, Picton D, Blackwood S, et al. Blinded comparison of folic acid and placebo in patients with ischemic heart disease: An outcome trial. Circulation. 2002;106:II-741. abstr 3642. [Google Scholar]
- 7.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 8.Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 9.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 10.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: The Swiss Heart study: A randomized controlled trial. JAMA. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 11.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 12.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 13.Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 14.Mann JF, Sheridan P, McQueen MJ, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008;23:645–653. doi: 10.1093/ndt/gfm485. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu AJ, Gohh RY, Han H, et al. Enhanced reduction of fasting total homocysteine levels with supraphysiological versus standard multivitamin dose folic acid supplementation in renal transplant recipients. Arterioscler Thromb Vasc Biol. 1999;19:2918–2921. doi: 10.1161/01.atv.19.12.2918. [DOI] [PubMed] [Google Scholar]
- 16.Bostom AG, Gohh RY, Beaulieu AJ, et al. Treatment of hyperhomocysteinemia in renal transplant recipients. A randomized, placebo-controlled trial. Ann Intern Med. 1997;127:1089–1092. doi: 10.7326/0003-4819-127-12-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Bostom AG, Shemin D, Gohh RY, et al. Treatment of mild hyperhomocysteinemia in renal transplant recipients versus hemodialysis patients. Transplantation. 2000;69:2128–2131. doi: 10.1097/00007890-200005270-00029. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 19.Gill JS, Pereira BJ. Chronic kidney disease and the transplant recipient. Blood Purif. 2003;21:137–142. doi: 10.1159/000067871. [DOI] [PubMed] [Google Scholar]
- 20.Bostom AG, Carpenter MA, Kusek JW, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152:448.e1–448.e7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. NIH Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research. 2001. [Google Scholar]
- 23.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 24.Jaffé M. Ueber den Niederschlag welchen Pikrinsaeure in normalen Harn erzeugt und ueber eine neue Reaction des Kreatinins. Z Physiol Chem. 1886;10:391–400. [Google Scholar]
- 25.Larsen K. Creatinine assay by a reaction-kinetic principle. Clinica chimica acta. 1972;41:209–217. doi: 10.1016/0009-8981(72)90513-x. international journal of clinical chemistry. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.SAS Institute. SAS Language Reference: Concepts, Version 8. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]


