Abstract
Background
There is conflicting evidence about comorbid personality pathology in depression treatments.
Aims
To test the effects of antidepressant drugs and cognitive therapy in depressed patients distinguished by presence/absence of personality disorder.
Method
Random assignment of 180 depressed outpatients to 16 weeks of antidepressant medications or cognitive therapy. Random assignment of medication responders to continued medication or placebo, and compared to cognitive therapy responders over a 12-month period.
Results
Personality disorder status led to differential response at 16 weeks; 66% vs. 44% (antidepressants vs. cognitive therapy) for patients with personality disorder, and 49% vs. 70% (antidepressants vs. cognitive therapy) for patients without personality disorder. For patients with personality disorder, sustained response rates over the 12-month follow-up were nearly identical (38%) in the prior-cognitive-therapy and continuation-medication conditions. Patients with personality disorder withdrawn from medication evidenced the lowest sustained response rate (6%). Despite the poor response to cognitive therapy of personality disordered patients, nearly all those who did respond sustained their response.
Conclusions
Comorbid personality disorder was associated with differential initial response rates and sustained response rates for two well-validated treatments for depression.
Although current American Psychiatric Association treatment guidelines1 state that “cognitive behavioral therapy may be more effective than other treatments for depressed individuals with personality disorders,” this statement appears to be largely based on a misunderstanding of data from the Treatment of Depression Collaborative Research Program (TDCRP).2,3 The TDCRP did not reveal a personality-disorder (PD)-by-treatment interaction, but rather a nonsignificant trend whereby patients with no comorbid personality disorder (Non-PD) responded more poorly to cognitive therapy (CT) than did patients with PD. Subsequent studies have not supported the claim that the presence of PD predicts favorable response to CT.e.g.,4 Moreover, the conclusions from two recent meta-analyses reflect the controversy regarding whether comorbid personality pathology affects response to treatment for depression.5,6 One reported that depressed patients with comorbid PD experienced poorer response when receiving either CT or pharmacotherapy.6 The other, which included only trials of antidepressant medications, reported no difference in response as a function of personality pathology.5
We present data drawn from a multi-site randomized controlled trial comparing CT and paroxetine for individuals diagnosed with moderate-to-severe depression.7,8 We focus on whether the presence of comorbid PD predicts differential response to CT and pharmacotherapy, and we explore the effect of comorbid PD on relapse once treatment is terminated.
Methods
The sample characteristics, treatment protocols, and main treatment outcome findings have been reported elsewhere.7,8 Briefly, the sample consisted of 240 depressed outpatients (measured using the Structured Clinical Interview for DSM-IV Diagnosis9) who registered a score of 20 or higher on the modified 17-item version of the Hamilton Rating Scale for Depression.10 Personality pathology was assessed at intake using the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II).11 Among the entire sample, 48% of individuals met criteria for at least one comorbid personality disorder (PD). As was done in the TDCRP, patients with antisocial (n=3) and schizotypal (n=1) personality disorders were excluded from the trial.2 Patients meeting criteria for borderline personality disorder (n=8) were also excluded. The treatments under investigation were judged to be either ill-suited too brief for depressed patients with these 3 disorders. The distributions of personality disorders, displayed in Table 1, were similar between the treatment conditions and resemble those found in other depressed outpatient samples.12 The Institutional Review Boards of the University of Pennsylvania and Vanderbilt University approved the study’s protocols. All participants provided written informed consent.
Table 1.
The distribution of personality disorders in the sample
| Total (N = 240) | ADM (N = 120) | CT (N = 60) | Placebo (N = 60) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Personality Disoder | N | % | N | % | N | % | N | % | X2 | p |
| Cluster C | 74 | 30.8% | 37 | 30.8% | 17 | 28.3% | 20 | 33.3% | 0.35 | 0.84 |
| Avoidant | 45 | 18.8% | 24 | 20.0% | 9 | 15.0% | 12 | 20.0% | 0.74 | 0.69 |
| Dependent | 7 | 2.9% | 3 | 2.5% | 2 | 3.3% | 2 | 3.3% | 0.15 | 0.93 |
| Obsessive-Compulsive | 38 | 15.8% | 17 | 14.2% | 10 | 16.7% | 11 | 18.3% | 0.56 | 0.75 |
| Cluster A | 8 | 3.3% | 3 | 2.5% | 3 | 5.0% | 2 | 3.3% | 0.78 | 0.68 |
| Paranoid | 8 | 3.3% | 3 | 2.5% | 3 | 5.0% | 2 | 3.3% | 0.78 | 0.68 |
| Schizoid | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | . |
| Cluster B | 8 | 3.3% | 4 | 3.3% | 1 | 1.7% | 3 | 5.0% | 1.03 | 0.60 |
| Histrionic | 2 | 0.8% | 1 | 0.8% | 0 | 0.0% | 1 | 1.7% | 1.01 | 0.60 |
| Narcissistic | 7 | 2.9% | 3 | 2.5% | 1 | 1.7% | 3 | 5.0% | 1.32 | 0.52 |
| NOS | 35 | 14.6% | 16 | 13.3% | 9 | 15.0% | 10 | 16.7% | 0.37 | 0.83 |
| Any Axis-II | 116 | 48.3% | 59 | 49.2% | 27 | 45.0% | 30 | 50.0% | 0.37 | 0.83 |
Treatments
Participants were randomly assigned to receive cognitive therapy (CT, N=60), antidepressant medication treatment with paroxetine (ADM, N=120), or pill-placebo (N=60). After 8 weeks of the 16-week acute treatment phase, the placebo arm was terminated and patients who had been receiving placebo were offered antidepressant medication treatment at no cost. Because 8 weeks of treatment is generally not regarded as sufficient to treat patients with comorbid PDs, comparisons at the 8-week point, and therefore those including the placebo arm, were not included in the analyses below.
The criterion for response at 16 weeks was an HRSD score of 12 or lower. In order to limit the effect of transient mood fluctuations on the response designation, additional constraints required patients to have scored 14 or less at week 14, or 12 or less at weeks 10 and 12. Patients who scored higher than 12 at week 16 were still considered responders if they had scored 12 or below at weeks 12 and 14 and again scored 12 or below at an additional evaluation at 18 weeks. Response at 16 weeks also required the completion of acute treatment.
Of the 180 patients assigned to ADM or CT, 104 patients met the response criteria at 16 weeks and were entered into the 12-month continuation phase of the study. Half of the responders from the ADM group (n=35) were randomly assigned to be withdrawn from medication onto pill placebo (continuation-placebo) while the other half (n=34) were continued on medications (continuation-medication) throughout the one-year continuation phase. In the CT condition, regular therapeutic contact ceased at the end of the acute phase of treatment. Treatment responders (n=35) could use up to 3 one-hour booster sessions throughout the one-year continuation phase. When ADM patients were randomized to continuation-placebo or continuation-medication at the beginning of the continuation phase, the process was triple blind - patients, pharmacotherapists, and evaluators did not know which patients were receiving active medication.
Outcome Measures
The primary outcome measure was the 17-item version of the HRSD. During the acute phase, assessments with evaluators blind to treatment condition were held weekly for the first four weeks and biweekly from week 6 to week 16. During the continuation phase, assessments were conducted during each of the first two weeks, biweekly through the end of the second month, and monthly thereafter. Relapse was defined as score of 14 or greater on the HRSD during two consecutive weeks (ad hoc assessments were scheduled as needed to confirm this temporal component). If a patient experienced a worsening of symptoms in the interval between assessments, the timing of relapse was ascertained using the Longitudinal Interval Follow-up Evaluation,13 conducted at the next assessment. Of the 45 patients who were judged to have relapsed, 11 were ascertained using this instrument.
Statistical Analyses
The primary statistical analyses examined whether there were differences in efficacy between the two treatments as a function of PD-status, by examining: a) the percent of patients meeting response criteria, b) change in average depression severity scores, and c) the percent who met response criteria and did not relapse post-treatment (sustained response). Data from all patients randomized to treatment were included in all three sets of (intent-to-treat) analyses. In the case of dropouts, all data collected prior to dropout were included. In each analysis, the treatment-by-PD-status interaction term was of primary interest.
For the acute phase data, Cochran-Mantel-Haenszel (CMH) tests were used to assess differential response/nonresponse as a function of treatment and PD status, stratified across the two sites.14 Odds-ratios, confidence bands, and interaction effects were assessed using a logistic regression model based on the Likelihood Ratio Chi-square statistic.15 Continuous data were examined with multiple regression techniques using a last observation carried forward (LOCF) approach and with hierarchical linear modeling (HLM). The HLM approach adjusts for repeated measures with nested random effects.16,17 Using this approach, each subject’s growth curve and true HRSD score at the end of treatment can be estimated from a collection of patient-specific parameters.18 For all HLM analyses reported, an unstructured covariance structure was assumed in order to model random intercepts and slopes. Two baseline scores were obtained for all participants, allowing for a full intent-to-treat analysis while at the same time covarying for each patient’s initial baseline depression severity score. All models were performed using SAS Version 9.0, PROC MIXED for HLM analyses, PROC GLM for multiple regression analyses, PROC FREQ for CMH tests, and PROC GENMOD for Logistic Regression (SAS Institute Inc, Cary, NC).
The Cox Proportional Hazards Model was used to estimate dropout rates and relapse rates.19 Because a differential response rate for PD and Non-PD patients emerged between the two treatments (see below), the Cox Proportional Hazards technique is inappropriate on its own to estimate survival rates during the continuation phase. Any indication of differential relapse might be an artifact of a differential sieve through which the patients who would be at greatest relapse risk already had failed to respond to a particular treatment,20 and therefore did not enter the continuation phase. To address this concern, estimated survival curves from Cox Proportional Hazards regression models were weighted by the proportion of patients who responded to treatment in each condition. This procedure estimates the percentage of patients who both responded to treatment and maintained that response throughout the one-year continuation phase. In the original publication, 4 possibly confounding covariates (dysthymia, atypical depression, number of prior episodes, and gender) were entered in the survival analyses;8 these were included in the present models as well. These models were fit in the SAS procedure, PROC PHREG.
Results
Attrition
The overall rate of patient attrition was detailed in the original publication.7 Although survival analyses revealed no overall difference between the PD and Non-PD groups, χ2(1)=0.32, p=.57, a statistically non-significant trend level PD-status-by-treatment interaction did emerge, χ2(1)=2.72, p<.10. This effect was driven in large part by the fact that the dropout rate was lower for PD (12%) than for Non-PD (21%) patients in the ADM condition and lower for Non-PD patients (12%) than for PD (22%) patients in the CT condition.
Outcome of Acute Treatment
Categorical Response Analyses
There was a significant interaction between treatment condition and PD status in acute treatment response, χ2(1)=6.77, p=.009. As displayed by the narrow bars in Figure 2, in the PD group there was a non-significant trend in favor of ADM; 66±12% met response criteria compared to 44±19% in the CT condition, CMH χ2(1)=3.42, p=.06 (Odds Ratio=2.42, 95%CI:0.96--6.28). The reverse pattern was observed, as a non-significant trend, for the Non-PD group, with 70±16% meeting response criteria in the CT condition, compared to 49±13% in ADM, CMH χ2(1)=3.20, p=.07 (OR=2.28, 95%CI:0.94--5.81). The tests of the main effects of treatment, PD-status, and the three way site-by-treatment-by-PD-status interaction were nonsignificant, all χ2s<.43, all ps>.51.
Figure 2.
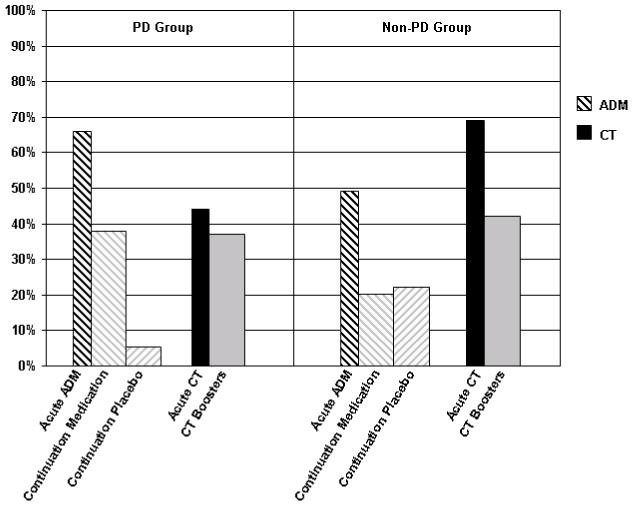
The percentage of patients in each treatment condition meeting response and sustained response criteria.
The narrow bars display the proportion of patients who met response criteria in the two treatments (ADM and CT) at the end of the 16-week acute phase. The wide bars represent the estimated proportion of patients who survived the 12-month follow-up period without a relapse. Bars on the left half of the figure represent patients diagnosed with a comorbid personality disorder; bars on the right represent patients who did not have a comorbid Axis-II diagnosis.
Continuous Response Analyses
In the original publication of the treatment outcome data, the authors reported a significant site-by-treatment interaction in the HLM analyses;7 this interaction term was added to the models described below. Controlling for initial depression severity, the test of the treatment-by-PD-status interaction on estimates of week 16 HRSD scores was significant in the LOCF, F(1,173)=4.18, p=.04, and HLM analyses, F(1,173)=4.32, p=.04; Cohen’s d=.76±.44. Follow-up HLM analyses within the PD and non-PD groups yielded a nonsignificant trend in favor of ADM in the PD group, t(173)=-1.69, p=.09 and a nonsignificant treatment effect in the non-PD group, t(173)= 1.24, p=.22. The test of the treatment-by-PD-status interaction on the linear slope estimates of symptom change, though in the same direction as those obtained in the LOCF and HLM intercept analyses, was not significant, F(1,171)=1.98, p=.16 (Cohen’s d=.40±.44).
Potential Confounds
To address the possibility that patients with PDs differed from those without personality pathology in other important ways, we examined 8 history of illness variables, 2 depression subtype variables, 4 composite axis-I comorbidity variables, 7 demographic variables and, for patients in the medication condition, dosage and augmentation, to determine whether the PD and Non-PD patients differed at the p<.10 level. As shown in Table 2, 9 of these variables differed between the two groups. All 9 variables were entered simultaneously into each of the models reported above. For the model predicting categorical response, as well as the HLM model predicting end of treatment HRSD scores, the PD-status-by-treatment interaction remained significant even with the simultaneous addition of all 9 variables (χ2[1]=5.59, p=.02 for the categorical analysis; F[1, 164]=4.13, p=.04 for the HLM model). In the LOCF analysis, the PD-status-by-treatment interaction became a non-significant trend, F(1,164)=3.54, p=.06.
Table 2.
Differences between the PD and Non-PD groups on potentially confounding variables
| Non-PD | PD | ||||
|---|---|---|---|---|---|
| Potential Confound | Mean / % | SD | Mean / % | SD | t / X2 |
| History of illness | |||||
| Onset Age | 25.0 | 12.8 | 19.2 | 10.8 | 3.3 * |
| Number of Previous Treatments | 1.2 | 1.6 | 1.9 | 1.8 | -2.9 * |
| Number of Previous Hospitalizations | 0.1 | 0.3 | 0.3 | 0.8 | -2.5 † |
| Duration of Illness (months) | 34.1 | 45.1 | 59.0 | 88.6 | -2.4 † |
| Dysthymia | 19.2% | 34.9% | 5.7 † | ||
| Number of Prior Episodes | 2.1 | 2.7 | 2.9 | 2.8 | -2.0 ‡ |
| Chronic MDD | 45.7% | 57.0% | ns | ||
| Recurrent MDD | 69.2% | 76.7% | ns | ||
| Depression Subtype | |||||
| Met DSM-IV specifiers for atypical features | 17.0% | 30.2% | 4.9 † | ||
| Met DSM-IV specifiers for melancholia | 26.6% | 36.1% | ns | ||
| Axis-I Comorbidities | |||||
| Any anxiety disorder | 44.7% | 59.3% | 3.8 † | ||
| Any other Axis-I disorder | 1.1% | 8.1% | Fisher’s Exact Test † | ||
| Any substance use disorder | 28.7% | 31.4% | ns | ||
| Any eating disorder | 17.0% | 16.3% | ns | ||
| Demographics | |||||
| Married | 38.3% | 29.1% | ns | ||
| Female | 62.8% | 53.5% | ns | ||
| Employed | 81.9% | 84.9% | ns | ||
| Caucasian | 80.9% | 84.9% | ns | ||
| Age | 40.1 | 12.3 | 39.7 | 10.7 | ns |
| Education (years) | 14.5 | 2.5 | 14.7 | 2.3 | ns |
| Income (in thousands USD) | 32.1 | 31.4 | 35.2 | 35.7 | ns |
| Medication | |||||
| Dosage (mg) | 34.4 | 8.8 | 34.1 | 8.6 | ns |
| Augmentation | 52.1% | 42.3% | ns | ||
p < .01
p < .05
p < .10
Individual Personality Disorder Diagnoses
The results reported thus far were achieved by comparing all patients with at least one PD diagnosis to patients with no PD diagnosis. In order to better determine what was driving the observed effects, we conducted exploratory analyses examining the relations for individual personality disorders. The strongest pattern occurred for patients diagnosed with PD Not-Otherwise-Specified, wherein 12 of 16 (75%) patients responded to ADM and 3 of 9 (33%) responded to CT (O.R.=6.09, 95%CI:1.09--42.87, in favor of ADM over CT). In order to examine this effect further, we assigned patients diagnosed with PD Not-Otherwise-Specified to the personality disorder cluster in which their highest concentration of symptoms was observed. For example, a broad-band Cluster C category was formed by combining PD Not-Otherwise-Specified patients for whom the highest concentration of symptoms fell in cluster C with those patients who actually received a Cluster C diagnosis. The effect of PD-Status was the strongest for individuals in the broad-band Cluster B category. Of the 16 patients in the broad-band Cluster B grouping, 6 of the 9 patients responded to ADM, whereas only 1 of 7 responded in CT (O.R.=15.03, 95% CI:1.36—515.92). For patients in the broad-band Cluster C group, 32 of 47 (68%) responded to ADM, whereas 10 of 19 (53%) responded to CT (OR=1.97, 95%CI:0.65--5.98). For those in the broad-band Cluster A group, 2 of 4 responded to CT, versus 1 of 4 in ADM (OR=3.28, 95%CI:0.16—137.50).
Sustained Response through the 12-Month Continuation Phase
For the 12-month continuation phase, the survival rates of the 3 treatment conditions (prior CT, continuation-placebo, and continuation-medication) were estimated for the PD and Non-PD groups. Sustained response estimates for each group were then calculated by computing the product of these survival estimates and the group’s treatment response rate (e.g., for patients in the PD group who had received ADM treatment, survival estimates for both the continuation-medication and continuation-placebo were multiplied by the percent of PD patients who responded to acute ADM treatment). Analysis of these estimates revealed a significant treatment-by-PD-status interaction in the percentage of patients who showed a sustained response through the end of the 12 months, χ2(2)= 6.13, p=.047. For patients in the PD group, a significant main effect of treatment emerged, χ2(2)=11.94, p=.003. The wider bars in Figure 2 show that despite the fact that a higher percentage of the PD patients responded to treatment (and hence entered the continuation phase) in the ADM (66%) compared to the CT (44%) conditions, an estimated 38±18% of patients initially randomized to CT evidenced sustained response. With a higher relapse rate in the continuation-medication condition, a nearly identical estimate of the proportion of patients with sustained response was obtained in this group (38±17%). Patients with PD who had previously received ADM but were withdrawn onto pill-placebo (continuation-placebo) tended to relapse at an extremely high rate. Only 6±9% of these patients exhibited sustained response. Specific contrasts revealed that, for the patients with PD, prior CT and continuation-medication were each superior to continuation-placebo on the sustained response variable (χ2[1]=9.80, p=.002 for continuation-medication versus continuation-placebo, and χ2[1]=9.15, p=.003 for prior CT versus continuation-placebo). There was no difference in estimated sustained response rates between prior CT and continuation-medication for this group, χ2[1]=.002, p=.97.
For patients in the Non-PD group, the main effect of treatment was not significant in the analysis of sustained response, χ2(2)=4.54, p=.103. Figure 2 shows that for patients initially randomized to receive CT, 43±17% exhibited sustained response, compared to 23±15% for those_initially randomized to receive ADM treatment who were then assigned to continuation-placebo, and 21±14% who were assigned to ADM treatment and then were assigned to continuation-ADM.
Discussion
The primary purpose of this investigation was to determine whether personality pathology predicts differential response to two generally effective treatments for depression. In this sample of moderate-to-severely depressed patients, short-term cognitive monotherapy proved relatively ill-suited for patients with comorbid personality disorders, a pattern of results consistent with findings from the Nottingham Study of Neurotic Disorder in which cognitive therapy and self-help treatment were less effective for patients with comorbid personality disorder than for those without Axis-II pathology.21 Indeed, in the present study, fewer than half of personality disordered patients responded to CT. Those who did respond, however, tended to sustain their response throughout the ensuing 12-month continuation phase.
ADM, on the other hand, worked particularly well to reduce depressive symptoms for patients diagnosed with comorbid personality pathology. Although the personality-disordered and non-personality disordered groups differed in several respects, including the incidence of comorbid anxiety disorders, none of these factors accounted statistically for the differential treatments effects. Results from the continuation phase of the study further support the conclusion that the medications had potent effects in this group in that nearly all of the personality-disordered patients who were withdrawn from medications relapsed. For depressed patients who did not have diagnosed personality pathology, there was a suggestion in the data that CT was particularly effective at reducing depressive symptoms, relative to ADM.
Limitations
The pattern of findings in regard to acute treatment response was consistent across the three different analytical methods, and the test of significance for the interaction of interest was met in all three. However, in the analysis that employed hierarchical linear modeling (HLM), the interaction of interest was significant only in the prediction of 16-week scores and not in the prediction of symptom change over time. This inconsistency may have arisen due to the fact that HLM analyses can lead to misleading estimates of improvement rates in datasets containing nonrandom attrition.22,23 Consistent with the observation that PD patients assigned to CT, and Non-PD patients assigned to ADM, fared more poorly than the other two groups, these groups also tended to drop out at higher rates. This is another indication that ADM was better-suited to PD patients in this trial, and that CT was better suited to patients without PD. In addition, between-treatment differences in outcome within the PD and Non-PD subgroups were at the level of non-significant trends. Because these tests were associated with relatively low power, these comparisons will be most useful to the field as constituents of future meta-analyses that will combine the current findings with attempts to replicate these results.
A second limitation involves the duration of the active treatments. The differences between CT and ADM in relation to PD status may have depended, in part, on the fact that the treatments were brief, relative to the durations recommended for treating depressed patients who have comorbid PD. Given more time, CT might have been as effective as medications for patients with PDs, and ADM might have been as effective as CT for patients without PDs. Nevertheless, short-term treatment did lead to response percentages of upwards of two-thirds for the PD patients in ADM, as well for the Non-PD patients in CT.
Third, prospective patients with diagnosed schizotypal, antisocial, or borderline personality disorders were excluded from the trial. Thus these results do not, strictly speaking, generalize to the population that includes patients with these comorbidities. However, only 5% of otherwise eligible patients were excluded because they received one of these diagnoses, and all but one of these patients was excluded because of a Cluster B diagnosis. Patients in this trial whose highest concentration of PD symptoms occurred in Cluster B showed a particularly strong response to ADM, relative to CT. This suggests that the pattern we observed might be even stronger in a sample that includes all depressed patients with Axis II comorbidity.
Future Directions
Because the primary medication used in this study, paroxetine, has demonstrated efficacy in treating depression with comorbid anxiety disorders, it might have been expected that ADM’s relative efficacy for PD patients was due to its superiority for patients with Cluster C PDs.24 However, the numerical advantage for ADM was strongest for patients with Cluster B personality pathology. One possible explanation for this result is suggested by research demonstrating that patients with Cluster B personality disorders display deficits in cortical regions thought to underlie the regulation of emotion and the inhibition of impulsive aggression25 and from studies that demonstrate that SSRIs can reduce mood lability, anger, and impulsive behavior in humans and animals.26-28 Future studies might attempt to further investigate these mechanisms in depressed patients with this type of personality pathology. In addition, the surprisingly high rate of relapse among PD patients withdrawn from medications should be examined in future research, as the mechanism driving this effect is currently unclear.
Conclusions
The pattern of results from this study suggests possible prescriptive recommendations regarding short-term treatment for patients with moderate to severe depression as a function of comorbid personality pathology. Evidence from this study, consistent with findings from other investigations, suggests that for depressed patients with a comorbid PD, paroxetine treatment is more likely than CT to alleviate their depressive symptoms in the short term. Due to a higher relapse rate for patients receiving continuation ADM, short-term CT may produce rates of sustained response roughly equivalent to those achieved with sustained ADM, provided that booster sessions are given to the CT patients. Given the relatively low relapse rate among PD patients who responded to CT, the combination of ADM and CT might be especially valuable for these patients. Patients without a PD diagnosis fared better during acute treatment and exhibited a higher sustained response rate in CT, compared to ADM. Indeed, these patients appeared to be equally susceptible to relapse following acute treatment with ADM regardless of whether they were continued on medication or withdrawn onto placebo. This pattern, if replicated, could lead to the consideration of CT as a first-line treatment for patients with depression who do not have a personality disorder.
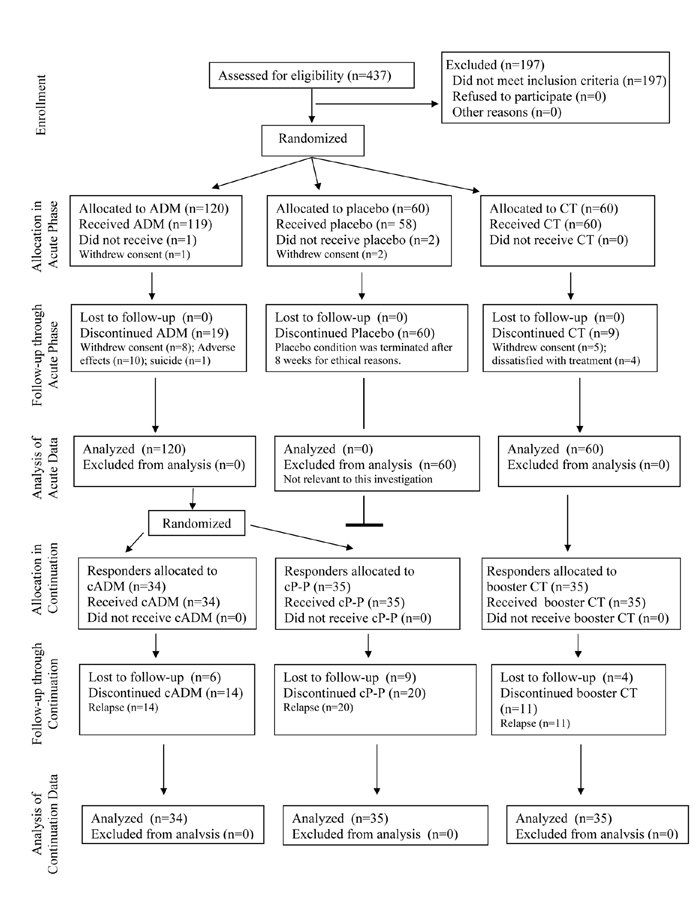
Figure 1.
CONSORT Flow Diagram
Note. ADM = Antidepressant medications; CT = Cognitive Therapy; continuation-medication = Continuation antidepressant medications during the continuation phase; continuation-placebo = Placebo during the continuation phase.
Acknowledgments
Gratitude is expressed to our colleagues for contributing to this research. Jay C. Fournier, M.A., drafted the manuscript and conducted the statistical analyses. Robert J. DeRubeis, PhD, and Steven D. Hollon, PhD, were the principal investigators and oversaw the implementation of cognitive therapy at the respective sites. Jay D. Amsterdam, MD, and Richard C. Shelton, MD, were the co-principal investigators and supervised the implementation of medication treatment. Robert Gallop, PhD provided methodological and statistical expertise. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would also like to give thanks to our colleagues who helped make this research possible. Paula R. Young, PhD, and Margaret L. Lovett, MEd, served as the study coordinators. John P. O’Reardon, MD, Ronald M. Salomon, MD, and the late Martin Szuba, MD, served as study pharmacotherapists (along with Drs. Amsterdam and Shelton). Cory P. Newman, PhD, Karl N. Jannasch, PhD, Frances Shusman, PhD, and Sandra Seidel, MSN, served as the cognitive therapists (along with Drs. DeRubeis and Hollon). Jan Fawcett, MD, provided consultation with regard to the implementation of clinical management pharmacotherapy. Aaron T. Beck, MD, Judith Beck, PhD, Christine Johnson, PhD, and Leslie Sokol, PhD, provided consultation with respect to the implementation of cognitive therapy. Madeline M. Gladis, PhD and Kirsten L. Haman, PhD, oversaw the training of the clinical interviewers, and David Appelbaum, PsyD, Laurel L. Brown, PhD, Richard C. Carson, PhD, Barrie Franklin, PhD, Nana A. Landenberger, PhD, Jessica Londa-Jacobs, PhD, Julie L. Pickholtz, PhD, Pamela Fawcett-Pressman, MEd, Sabine Schmid, MA, Ellen D. Stoddard, PhD, Michael Suminski, PhD and Dorothy Tucker, PhD served as project interviewers. Joyce L. Bell, BA, Brent B. Freeman, BA, Cara C. Grugan, BA, Nathaniel R. Herr, BA, Mary B. Hooper, MS, Miriam Hundert, BSN, Veni Linos, MSc, and Tynya Patton, MA, provided research support. This research was supported by grants MH50129 (R10), MH55875 (R10), MH01697 (K02), and MH01741 (K24) from the National Institute of Mental Health, Bethesda, MD. GlaxoSmithKline provided medications and pill-placebos for the trial.
Supported by grants MH50129 (R10) (Dr DeRubeis), MH55875 (R10) and MH01697 (K02) (Dr Hollon), and MH01741 (K24) (Dr. Shelton) from the National Institute of Mental Health, Bethesda, MD. GlaxoSmithKline provided medications and pill-placebos for the trial.
Footnotes
Declaration of interest: Dr. Shelton has received grant support from Glaxo-SmithKline Pharmaceuticals. No other authors have relevant interests to declare.
Reference List
- 1.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision) American Journal of Psychiatry. 2000;157:49. [PubMed] [Google Scholar]
- 2.Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program: General effectiveness of treatments. Archives of General Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- 3.Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and Medication in the Treatment of Adult and Geriatric Depression: Which Monotherapy or Combined Treatment? Journal of Clinical Psychiatry. 2005;66:455–468. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- 4.Hardy GE, Barkham M, Shapiro DA, et al. Impact of Cluster C personality disorders on outcomes of contrasting brief psychotherapies for depression. Journal of Consulting and Clinical Psychology. 1995;63:997–1004. doi: 10.1037//0022-006x.63.6.997. [DOI] [PubMed] [Google Scholar]
- 5.Kool S, Schoevers R, de Maat S, et al. Efficacy of pharmacotherapy in depressed patients with and without personality disorders: A systematic review and meta-analysis. Journal of Affective Disorders. 2005;88:269–278. doi: 10.1016/j.jad.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. British Journal of Psychiatry. 2006;188:13–20. doi: 10.1192/bjp.188.1.13. [DOI] [PubMed] [Google Scholar]
- 7.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive Therapy vs Medications in the Treatment of Moderate to Severe Depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 8.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of Relapse Following Cognitive Therapy vs Medications in Moderate to Severe Depression. Archives of General Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 9.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR- Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/ PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2001. [Google Scholar]
- 10.Hamilton M. A Rating Scale for Depression. 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-II-R Personality Disorders (SCID-II, Version 1.0) American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- 12.Doyle TJ, Tsuang MT, Lyons M,J. Comorbidity of Depressive Illnesses and Personality Disorders. In: Tohen M, editor. Comorbidity in Affective Disorders. Marcel Dekker, Inc.; New York: 1999. pp. 105–156. [Google Scholar]
- 13.Keller MB, Lavori P, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 14.Kuritz S, Landis J, Koch G. A General overview of Mantel-Haenszel methods: applications and recent developments. Annual Review of Public Health. 1988;9:123–160. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer D, Lemeshow S. Applied Logistic Regression. Wiley; New York: 1989. [Google Scholar]
- 16.Bryk A, Raudenbush S. Hierarchical Linear Modeling: Applications and Data Analysis Methods. Sage Publishing; Newbury Park, CA: 1996. [Google Scholar]
- 17.Goldstein H. Models in Educational and Social Research. Oxford University Press; New York: 1987. [Google Scholar]
- 18.Willett JB. Measuring change: What individual growth modeling buys you. In: Amsel E, Renninger KA, editors. Change and development: Issues of theory, method, and application. The Jean Piaget symposium series. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 1997. pp. 213–243. [Google Scholar]
- 19.Cox D, Oakes D. Analysis of Survival Data. Chapman & Hall; London: 1984. [Google Scholar]
- 20.Klein DF. Preventing hung juries about therapy studies. Journal of Consulting and Clinical Psychology. 1996;64:81–87. doi: 10.1037//0022-006x.64.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Tyrer P, Seivewright N, Ferguson B, et al. The Nottingham study of neurotic disorder: effect of personality status on response to drug treatment, cognitive therapy and self-help over two years. British Journal of Psychiatry. 1993;162:219–226. doi: 10.1192/bjp.162.2.219. [DOI] [PubMed] [Google Scholar]
- 22.Diggle P, Kenward MG. Informative drop-out in longitudinal data analysis. Applied Statistics. 1994;43:49–93. [Google Scholar]
- 23.Gibbons RD, Hedeker DR, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: Application to the NIMH Treatment of Depression Collaborative Research Program dataset. Archives of General Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 24.Rouillon F. Depression comorbid with anxiety or medical illness: The role of paroxetine. International Journal of Psychiatry in Clinical Practice. 2001;5:3–10. doi: 10.1080/136515001300225132. [DOI] [PubMed] [Google Scholar]
- 25.Johnson P, Hurley R, Benkelfat C, et al. Understanding Emotion Regulation in Borderline Personality Disorder: Contributions of Neuroimaging. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:397–402. doi: 10.1176/jnp.15.4.397. [DOI] [PubMed] [Google Scholar]
- 26.New AS, Buchsbaum MS, Hazlett EA, et al. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- 27.Salzman C, Wolfson AN, Schatzberg A, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. Journal of Clinical Psychopharmacology. 1995;15:23–29. doi: 10.1097/00004714-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wolff MC, Leander D. Selective Serotonin Reuptake Inhibitors Decrease Impulsive Behavior as Measured by an Adjusting Delay Procedure in the Pigeon. Neuropsychopharmacology. 2002;27:421–429. doi: 10.1016/S0893-133X(02)00307-X. [DOI] [PubMed] [Google Scholar]