Abstract
It has long been a major challenge to achieve synthetic control over size and monodispersity of gold thiolate nanoclusters. Among the reported Aun thiolate clusters, Au38 has been shown to be particularly stable, but was only obtained as a minor product in previous syntheses. In this work, we report a bulk solution synthetic method that permits large scale, facile synthesis of truly monodisperse Au38 nanoclusters. This new method explores a two-phase ligand exchange process utilizing glutathione-capped Aun clusters as the starting material. The ligand exchange process with neat dodecanethiols causes gold core etching and secondary growth of clusters, and eventually leads to monodisperse Au38 clusters in high purity, which eliminates nontrivial postsynthetic separation steps. This method can be readily scaled up to synthesize Au38(SC12H25)24 in large quantities, and thus makes the approach and Au38 nanoclusters of broad utility.
Metal nanoclusters that are composed of ~10 to hundreds of atoms bridge the gap of small organometallic compounds and nanocrystals.1 Among precious metals, gold nanoclusters capped by thiolate ligands have attracts wide research interests due to their extraordinary chemical stability and interesting electronic and optical properties fundamentally different than their larger counterparts—gold nanocrystals. Unlike gold nanocrystals whose electronic properties resemble the bulk state, gold clusters with core diameter less than 2 nm exhibit quantized electronic states due to quantum confinement effect of electrons. These ultrasmall nanoparticles hold promise for a number of applications such as catalysis2–4, biological labeling5,6 and as building block for nanoscale devices.7
With respect to the chemical synthesis of Aun thiolate clusters (n: the number of atoms), Whetten and coworkers previously synthesized glutathione (G-SH) stabilized gold clusters, Aun(SG)m, and separated the mixture by polyacrylamide gel electrophoresis (PAGE).8 The most abundant cluster species separated was determined to be Au28(SG)16;8,9 note that it has recently been corrected as Au25(SG)18.10 Tsukuda and coworkers performed careful isolation and electrospray ionization mass spectrometry (ESI-MS) analysis of isolated Au:SG clusters, and identified a range of Aun cluster species from Au10 to Au39.10 Thus far, Au:SR (where, R=the tail of thiolate) clusters with core masses of 5 kDa (25 atoms), 8 kDa (38 atoms), 14 kDa (~75 atoms), 22 kDa (~101 atoms) and 29 kDa (~140 atoms) have been reported.11–13 Thiol etching experiments demonstrate that Au:SR clusters with core masses of 5 kDa (Au25), 8 kDa (Au38) and 29 kDa are extraordinarily stable.12–16 For Au25(SR)18 clusters, improved syntheses have been reported in recent years.17–19 And, the single crystal structure of [Au25(SCH2CH2Ph)18]−[TOA]+ has been independently determined by Murray20 and Jin21a groups, including the crystal structure [Au25(SCH2CH2Ph)18]0 clusters.21b Both the anionic and neutral of charge neutral Au25(SCH2CH2Ph)18 clusters consist of an icosahedral Au13 core and an exterior shell composed of six orthogonal Au2(SCH2CH2Ph)3 motifs.21 This structure was also theoretically described by Hakkinen et al.22 Among the extraordinarily stable 5 kDa (Au25), 8 kDa (Au38) and 29 kDa species, the 29 kDa Au:SR clusters (~1.6 nm core size, where R is C4, C6, C12, C18, or benzyl) were found to be predominantly formed in synthesis.17,23–25 Recently, Tsukuda et al. improved the synthesis and formulated the composition of the 29 kDa species as Au144(SR)59 on the basis of detailed ESI-MS analysis.26 The 8 kDa species was, however, only be obtained in very small quantities as a minor product following column chromatography separation11,12,26 or a thiol etching method.13,14,16 Nevertheless, the exact composition of the 8 kDa species has been determined to be Au38(SR)24 with ESI-MS.11–13
Despite the significant amount of synthetic work that has been done on size and monodispersity control of gold thiolate clusters, most reported methods suffer from the production of a mixture of different sized clusters (except the case of Au25(SR)18 clusters), and the clusters of a desired size are often in low yield and small quantities. Thus, to develop bulk solution synthetic methods that offer precise size control of gold thiolate clusters at the atomic level is of critical importance if the full potential of this new type of nanomaterial is to be realized.
In this work, we report a bulk solution synthetic method that permits facile, large scale synthesis of truly monodisperse Au38(SC12H25)24 nanoclusters. This new method explores a two-phase ligand exchange process in which glutathione-capped Aun clusters (a mixture) are utilized as the starting material. Neat dodecanethiol causes gold core etching of the starting material and secondary growth of clusters, ultimately resulting in monodisperse Au38 clusters in high purity.
In a typical experiment, the two-phase synthesis of Au38 thiolate clusters involves two major steps, i) synthesis of Aun(SG)m clusters and ii) two-phase ligand exchange induced growth of Au38 clusters from the Aun(SG)m mixture (see Supporting Information for experimental details). Briefly, the Au:SG clusters were synthesized according to a well established literature method.8–10, 28 The aqueous Au:SG clusters were then subject to ligand exchange using neat 1-dodcanethiol (C12-SH) in a two phase system, Figure 1. According to the previous work by Tsukuda et al.,10 the as-prepared Au:SG clusters contain a mixture of Aun clusters ranging from Au10 to Au39. It has also been found that in the presence of excess G-SH and at 55 °C, Aun clusters with n < 25 were oxidized to Au(I)-SG complexes while Aun clusters with n ≥ 25 were converted to Au25(SG)18 clusters.15 In our work, we use C12-SH (insoluble in water, i.e., a two phase system) to effect core etching of Au:SG clusters during the course of the two-phase ligand exchange process, and finally attain highly pure Au38(SC12H25)24 clusters. It is worth noting that Shichibu et al. previously explored a ligand-exhange approach to the synthesis of Au25(SG)18 clusters from phosphine-capped Au11 clusters.27
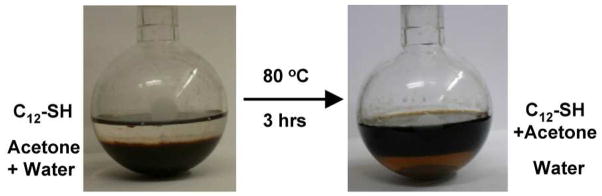
Figure 1.
Phase transfer of gold-glutathione clusters from water to dodecanethiol and further conversion to truly monodisperse Au38(SC12H25)24 clusters.
A key to our new synthetic approach is to effect an efficient phase transfer of Au:SG clusters from the aqueous solution to the C12-SH phase (neat C12-SH). We found that the Au:SG clusters can not be directly transferred into the C12-SH phase due to a surface tension issue, hence, acetone was added to the water/C12-SH two phase system to reduce the surface tension29 and prompt phase transfer of Au:SG clusters from water to the C12-SH phase. The ratio of water to acetone was found to influence the phase transfer efficiency. The optimal water to C12-SH ratio was determined to be 1.0–1.5. More acetone results in precipitation of Au:SG clusters (black precipitate forms at the water/C12-SH interface, while less acetone slows down the phase transfer rate. Figure 1 shows the phase transfer of Au:SG clusters from water to C12-SH. The water phase is initially at the bottom of the flask and the C12-SH phase forms the upper layer of the mixture. Prior to the phase transfer reactions, gold clusters are solely protected by G-SH, which makes them only soluble in water. After the solution was vigorously stirred for ~3 hours at 80 °C, gold clusters were almost completely transferred to the C12-SH phase (Figure 1, right). During this process, gold cluster core etching by C12-SH and secondary growth of clusters are expected to occur, which ultimately results in monodisperse Au38(SC12H25)24 clusters (vide infra).
The Au clusters in the C12-SH phase were isolated after the two phase reaction process was completed. The excess C12-SH was removed by washing the solution with methanol for several times. To isolate Au38 clusters, toluene was used to extract the Au38 clusters (see supporting Figure S1 for the absorption spectrum of the extracted clusters). The residue is poorly soluble in typical organic solvents (e.g. toluene or CH2Cl2, etc.), which is possibly Au(I)-SC12 polymers since Au(I)-SC12 polymer tends to form in thiol etching processes14 and has a poor solubility in organic solvents. To confirm that, we characterized the residue by thermal gravimetric analysis (TGA) and found that the weight loss is 50.3% (Figure S2), consistent with the calculated organic content (50.5%) of Au(I)-SC12 polymer.
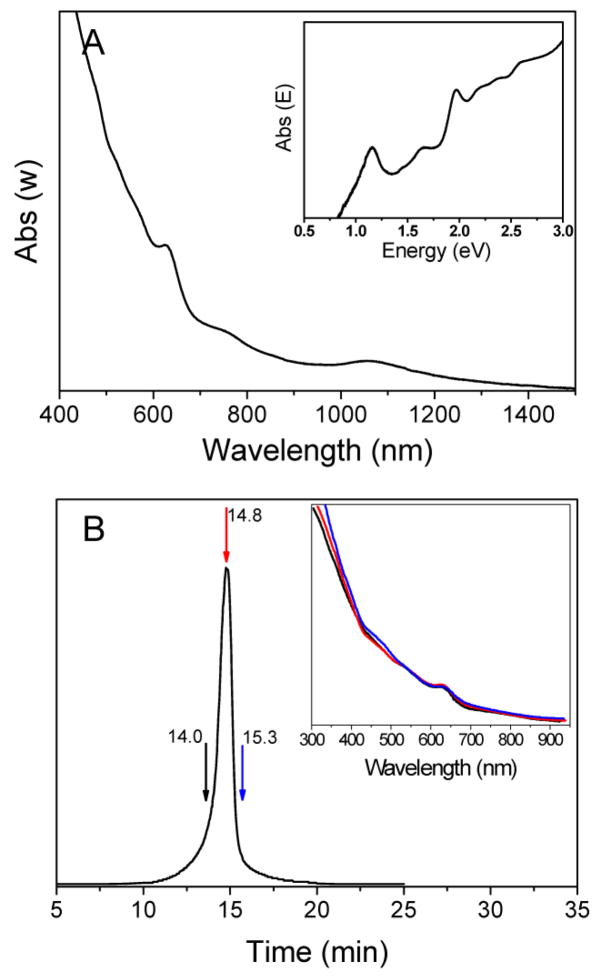
To further increase the purity of Au38 clusters, a mixed toluene/acetone (1:5 v:v) solvent was used to extract Au38 clusters from the crude product. The UV-vis spectrum of as-isolated Au38 clusters shows a step-wise, multiple absorption band spectrum (Figure 2A), which is typical of gold thiolate clusters. The spectrum is almost superimposable with previously reported Au38(SR)24 optical spectrum.11–13 The optical band edge energy is ~ 0.9 eV, and there is a prominent peak centered at 625 nm (2.0 eV). Other distinct peaks are at 1060 nm (1.17 eV), 755 nm (1.64 eV), and less intense ones at 2.2, 2.4 and 2.6 eV (Figure 2A and inset).
Figure 2.
(A) The UV-vis spectrum of Au38 clusters. The inset shows the absorbance (abs (E)) vs photo energy (eV). The wavelength dependent absorbance is converted to energy dependent absorbance according to the equation: Abs(E) ∝ Abs(w) × w2. (B) Typical chromatogram of Au38 sample detected by DAD at 630 nm. Inset: UV-vis spectra are obtained by DAD at 14.0 min (black line), 14.8 min (red line), and 15.3 min (blue line).
Size exclusion chromatography was used to further confirm the purity of the as-prepared Au38 clusters. A diode array detector (DAD) in situ monitors the UV-Vis spectra of eluted Au clusters. Figure 2B shows one single symmetric peak; the inset shows the spectra (190–950nm) corresponding to the eluted components at 14.0 min (left side of the peak), 14.8 min (the peak position) and 15.3 min (right side of the peak), respectively. The three spectra are indeed superimposable, which confirms high purity of the as-prepared Au38 clusters.
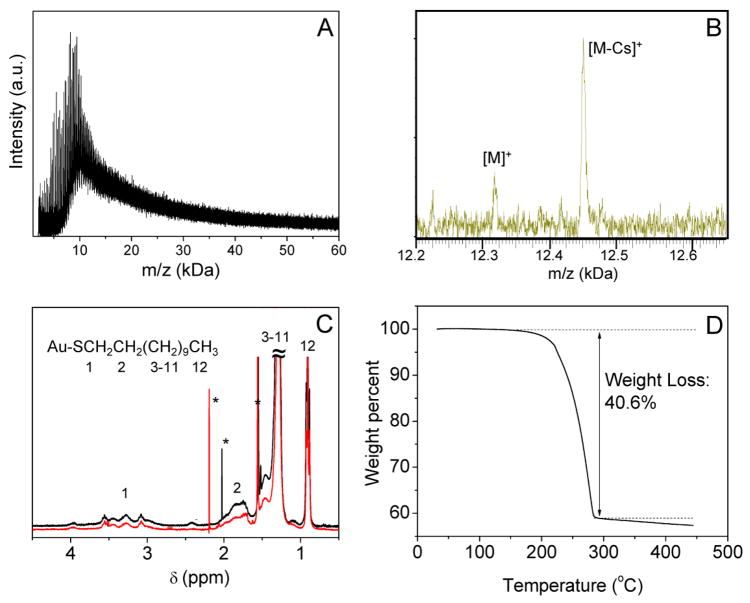
The as-prepared Au38 clusters were also analyzed by laser desorption ionization (LDI) mass spectrometry. In previous work, the formula of Au38 clusters has been determined to be Au38(SR)24.13,30 The LDI mass spectrum (negative mode) of the Au38 clusters is shown in Figure 3A. A broad peak centered at ~8.2 kDa was observed. The 8.2 kDa peak mass corresponds to [Au38S24]−. The broadness of the mass peak is due to fragmentation of the [Au38S24] − ions as well as a series of recombination processes of the fragments in gas phase. Unlike the previous work in which the Au38 clusters were found to always coexist with the Au144 clusters.13 Herein, our new synthetic approach does not involve or generate Au144 clusters, which greatly improves the purity of Au38 clusters.
Figure 3.
(A) LDI mass spectrum and (B) ESI mass spectrum of Au38(SR)24 clusters, (C) NMR of Au38(SR)24 prepared from ligand exchange (black line) and Au38(SC12)24 prepared by literature procedure (red line).13 The asterisks indicate the peaks from residual solvents. (D) TGA of Au38(SR)24 clusters.
Since our synthesis of Au38 clusters involve two types of thiol ligands (G-SH and C12-SH), it is important to find out whether the final Au38 clusters possess a mixed ligand shell or sole dodecanethiolates. Given that the Au38 clusters are only soluble in organic phase (toluene, CH2Cl2, etc.), the ligand shell of as-prepared Au38 clusters may be only dodecanethiolates. To determine the exact ligand composition, we have performed electrospray mass spectrometry (ESI-MS), nuclear magnetic resonance (NMR), and thermogravimetric analysis (TGA).
Figure 3B shows the ESI-MS result of the Au38 clusters. The peak at m/z 12,451 corresponds to the adducted species Au38(SR)24Cs+. After subtracting Cs+, the m/z 12,318 indeed matches the formula Au38(SC12H25)24. This is further confirmed by observing the intact cluster ion signal at m/z 12,318 (Figure 3B). These results show that the ligand exchange process between C12-SH and G-SH on the Au cluster surface is thorough, which can be attributed to three reasons. First of all, a large excess of C12SH was used (the ratio of C12SH/GSH ~ 45). Secondly, the high temperature (80 °C) accelerates the ligand exchange process and tends to drive the process to completeness. Thirdly, the highly hydrophilic GSH ligand would be difficult to enter the C12SH phase where Au38 clusters are formed. The pure ligand composition of the Au38 clusters was also confirmed by NMR analysis. We prepared and isolated Au38(SC12H25)24 clusters following a literature procedure,13 albeit the yield is very low. The 1H NMR spectra of these two types of Au38 clusters are indeed almost identical (Figure 3C), which confirms that no GSH ligands should exist in the final Au38 clusters prepared by our two-phase exchange approach. In both NMR spectra, significantly broadened peaks (compared to free ligands) were observed, similar to previous report.23 A set of broad peaks in the 2.8–4.1 ppm range are tentatively assigned to the H1 position (Figure 3C inset). The peaks in the 1.6–2.1 and 1.1–1.6 ppm range are assigned to H2 and H3-11, respectively. Finally, the triplet at ~0.9 ppm is assigned to the C12 position. TGA analysis also indicates that the Au38 cluster composition should be Au38(SC12H25)24, Figure 3D; the weight loss of Au38(SC12H25)24 clusters prepared by the ligand exchange approach is 40.6%, which is very close to the theoretical value (39.2%).
It is worthy of comparing our two-phase exchange approach with previous work;12–14 the latter has demonstrated that Au38 clusters were formed during the etching process of large Au clusters (14 kDa)14 or a mixture of 14 kDa, 22 kDa and 29 kDa Au clusters.12,13 The 22 kDa and 29 kDa Au clusters were found to coexist with Au38 clusters even after reaction with neat C12-SH for 24 hrs. To obtained pure Au38 clusters, Tsukuda et al. used recycling size exclusion chromatography12,26 or a complicated solvent extraction method13 to isolate 8 kDa, 22 kDa, 29 kDa Au clusters. This time-consuming procedure requires significant effort to obtain pure Au38 in bulk quantities. In our work, we use an Aun(SG)m mixture (n=10–39) as the starting material to prepare Au38 by ligand exchange with neat C12-SH. Analogous to the reaction between excess GSH and small Au:SG clusters,15 those small, relatively unstable Au:SG clusters would decompose to form Au(I)-SC12 complexes and only the thermodynamically stable Au38 clusters survive the rather harsh thiol etching reaction at 80 °C. It is interesting that we did not observe Au25 cluster formation in the exchange reaction with neat thiols at high temperature (80 °C), albeit Au25 clusters are very stable under mild environment due to their highly symmetric and stable structure.21 A similar observation (i.e. not observing Au25 cluster formation) has been noticed by Quinn et al.17 In our procedure, the Au38 clusters are the sole cluster species after ligand exchange with neat C12-SH. The extraordinary stability of Au38(SR)24 clusters at elevated temperatures (e.g. 80 °C) should account for the favorable growth of highly pure Au38 clusters from the Aun(SG)m mixture in the two phase exchange process.
The above results exclusively demonstrate that the Au38 clusters prepared by the ligand exchange process are Au38(SC12H25)24 in high purity, which is of critical importance for practical applications such as catalysis and biological labeling. This method allows easy scale-up for synthesizing Au38(SC12H25)24 in large quantities, which makes this synthetic approach and Au38 clusters of broad utility. The crystal structure of Au38(SR)24 cluster is still unknown, but several groups have done theoretical calculations30–34 and predict that Au38(SR)24 has 22 to 26 Au atoms as core and mixed Au(SR)2 and Au2(SR)3 motifs as protecting groups.13,33,34 This prediction is based upon some empirical structural rules learned from the single crystal structures of Au102(p-MBA)44 (p-MBA= p-mercaptobenzoic acid)35 and [Au25(SCH2CH2Ph)18]−[TOA]+ 20, 21 reported recently.
In summary, we have developed a facile method for synthesizing Au38(SC12H25)24 clusters in high purity and yield via ligand exchange reactions of Au:SG clusters with neat C12SH in a two phase reaction system. A key to this approach is the use of acetone to effect phase transfer of Au:SG clusters from the water phase to the neat C12SH phase. Surprisingly, the ligand shell of Au38 clusters was found to be sole dodecanethiolates, rather than a mixed ligand shell of dodecanethiolates and glutathionates. The exact formula of the as-prepared Au38 clusters is determined to be Au38(SC12H25)24 based upon ESI-MS, NMR, and TGA analyses. The crystal structure of Au38(SR)24 cluster is yet to be unraveled.
Supplementary Material
Acknowledgments
We acknowledge CMU, AFOSR (9550-07-1-0245), and PITA for supporting this work, and NIH (1S10RR022393-01) for acquisition of the Q-TOF mass spectrometer.
Footnotes
Supporting Information Available: Detailed information about the synthesis and characterization of Au38(SC12H25)24 and supporting figures S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mednikov EG, Dahl LF. J Am Chem Soc. 2008;130:14813–14821. doi: 10.1021/ja805679j. [DOI] [PubMed] [Google Scholar]
- 2.Turner M, Golovko VB, Vaughan OPH, Abdulkin P, Berenguer-Murcia A, Tikhov MS, Johnson BFG, Lambert RM. Nature. 2008;454:981–984. doi: 10.1038/nature07194. [DOI] [PubMed] [Google Scholar]
- 3.Herzing AA, Kiely CJ, Carley AF, Landon P, Hutchings GJ. Science. 2008;321:1331–1335. doi: 10.1126/science.1159639. [DOI] [PubMed] [Google Scholar]
- 4.Tsunoyama H, Sakurai H, Ichikuni N, Negishi Y, Tsukuda T. Langmuir. 2004;20:11293–11296. doi: 10.1021/la0478189. [DOI] [PubMed] [Google Scholar]
- 5.Hainfeld JF, Liu WQ, Barcena M. J Struct Biol. 1999;127:120–134. doi: 10.1006/jsbi.1999.4146. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie CM, Johnsen KR, Kiser JR, Antoku Y, Dickson RM, Petty JT. J Phys Chem C. 2007;111:175–181. doi: 10.1021/jp0648487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu ST, Maoz R, Schmid G, Sagiv J. Nano Lett. 2002;2:1055–1060. [Google Scholar]
- 8.Schaaff TG, Knight G, Shafigullin MN, Borkman RF, Whetten RL. J Phys Chem B. 1998;102:10643–10646. [Google Scholar]
- 9.Schaaff TG, Whetten RL. J Phys Chem B. 2000;104:2630–2641. [Google Scholar]
- 10.(a) Negishi Y, Takasugi Y, Sato S, Yao H, Kimura K, Tsukuda T. J Am Chem Soc. 2004;126:6518–6519. doi: 10.1021/ja0483589. [DOI] [PubMed] [Google Scholar]; (b) Negishi Y, Nobusada K, Tsukuda T. J Am Chem Soc. 2005;127:5261–5270. doi: 10.1021/ja042218h. [DOI] [PubMed] [Google Scholar]
- 11.Schaaff TG, Shafigullin MN, Khoury JT, Vezmar I, Whetten RL, Cullen WG, First PN, GutierrezWing C, Ascensio J, JoseYacaman MJ. J Phys Chem B. 1997;101:7885–7891. [Google Scholar]
- 12.Tsunoyama H, Nickut P, Negishi Y, Al-Shamery K, Matsumoto Y, Tsukuda T. J Phys Chem C. 2007;111:4153–4158. [Google Scholar]
- 13.Chaki NK, Negishi Y, Tsunoyama H, Shichibu Y, Tsukuda T. J Am Chem Soc. 2008;130:8608–8610. doi: 10.1021/ja8005379. [DOI] [PubMed] [Google Scholar]
- 14.Schaaff TG, Whetten RL. J Phys Chem B. 1999;103:9394–9396. [Google Scholar]
- 15.Shichibu Y, Negishi Y, Tsunoyama H, Kanehara M, Teranishi T, Tsukuda T. Small. 2007;3:835–839. doi: 10.1002/smll.200600611. [DOI] [PubMed] [Google Scholar]
- 16.Toikkanen O, Ruiz V, Ronholm G, Kalkkinen N, Liljeroth P, Quinn BM. J Am Chem Soc. 2008;130:11049–11055. doi: 10.1021/ja802317t. [DOI] [PubMed] [Google Scholar]
- 17.Donkers RL, Lee D, Murray RW. Langmuir. 2004;20:1945–1952. doi: 10.1021/la0497494. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Lema K, Ukaigwe M, Lee D. Langmuir. 2007;23:7853–7858. doi: 10.1021/la700753u. [DOI] [PubMed] [Google Scholar]
- 19.Zhu MZ, Lanni E, Garg N, Bier ME, Jin R. J Am Chem Soc. 2008;130:1138–1139. doi: 10.1021/ja0782448. [DOI] [PubMed] [Google Scholar]
- 20.Heaven MW, Dass A, White PS, Holt KM, Murray RW. J Am Chem Soc. 2008;130:3754. doi: 10.1021/ja800561b. [DOI] [PubMed] [Google Scholar]
- 21.(a) Zhu MZ, Aikens CM, Hollander FJ, Schatz GC, Jin R. J Am Chem Soc. 2008;130:5883–5885. doi: 10.1021/ja801173r. [DOI] [PubMed] [Google Scholar]; (b) Zhu M, Eckenhoff WT, Pintauer T, Jin R. J Phys Chem C. 2008;112:14221–14224. [Google Scholar]
- 22.Akola J, Walter M, Whetten RL, Hakkinen H, Gronbeck H. J Am Chem Soc. 2008;130:3756–3757. doi: 10.1021/ja800594p. [DOI] [PubMed] [Google Scholar]
- 23.Schaaff TG, Shafigullin MN, Khoury JT, Vezmar I, Whetten RL. J Phys Chem B. 2001;105:8785–8796. [Google Scholar]
- 24.Hicks JF, Miles DT, Murray RW. J Am Chem Soc. 2002;124:13322–13328. doi: 10.1021/ja027724q. [DOI] [PubMed] [Google Scholar]
- 25.Ingram RS, Hostetler MJ, Murray RW, Schaaff TG, Khoury JT, Whetten RL, Bigioni TP, Guthrie DK, First PN. J Am Chem Soc. 1997;119:9279–9280. [Google Scholar]
- 26.Tsunoyama H, Negishi Y, Tsukuda T. J Am Chem Soc. 2006;128:6036–6037. doi: 10.1021/ja061659t. [DOI] [PubMed] [Google Scholar]
- 27.Shichibu Y, Negishi Y, Tsukuda T, Teranishi T. J Am Chem Soc. 2005;127:13464–13465. doi: 10.1021/ja053915s. [DOI] [PubMed] [Google Scholar]
- 28.Shibu ES, Muhammed MAH, Tsukuda T, Pradeep T. J Phys Chem C. 2008;112:12168–12176. [Google Scholar]
- 29.Gaponik N, Talapin DV, Rogach AL, Eychmuller A, Weller H. Nano Lett. 2002;2:803–806. [Google Scholar]
- 30.Hakkinen H, Barnett RN, Landman U. Phys Rev Lett. 1999;82:3264–3267. [Google Scholar]
- 31.Hakkinen H, Walter M, Gronbeck H. J Phys Chem B. 2006;110:9927–9931. doi: 10.1021/jp0619787. [DOI] [PubMed] [Google Scholar]
- 32.Jiang DE, Tiago ML, Luo W, Dai S. J Am Chem Soc. 2008;130:2777–2779. doi: 10.1021/ja710991n. [DOI] [PubMed] [Google Scholar]
- 33.Jiang DE, Luo W, Tiago ML, Dai S. J Phys Chem C. 2008;112:13905–13910. [Google Scholar]
- 34.Pei Y, Gao Y, Zeng XC. J Am Chem Soc. 2008;130:7830–7832. doi: 10.1021/ja802975b. [DOI] [PubMed] [Google Scholar]
- 35.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Science. 2007;318:430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.