Abstract
Bone marrow mesenchymal stem cells (MSCs) are considered a potential cell source for stem cell-based bone tissue engineering. However, noticeable limitations of insufficient supply and reduction of differentiation potential impact the feasibility of their clinical application. This study investigated the in vitro function of steroids and gender differences on the proliferation and differentiation of rat MSCs. Bone marrow MSCs of age-matched rats were exposed to proliferation and osteogenic differentiation media supplements with various concentrations of 17β-estradiol (E2) and dexamethasone. Cell proliferation was measured by MTS assay; osteogenic markers and steroid-associated growth factors and receptors were evaluated by ELISA and real-time PCR. The results revealed that supplements of E2 and dexamethasone increase MSC proliferation in a biphasic manner. The optimal dose and interaction of steroids required to improve MSC proliferation effectively varied depending on the gender of donors. Supplementation of E2 effectively improves osteogenic differentiation markers including ALP, osteocalcin and calcium levels for MSCs isolated from both male and female donors. The mRNA of TGF-β1 and BMP-7 are also up-regulated. However, effective doses to maximally improve osteogenic potentials and growth factors for MSCs are different between male and female donors. The relationship between steroid receptors, osteogenic markers and cytokines are also varied by genders. The outcomes of the present study strongly indicate that steroids potentially function as an effective modulator to improve the capacity of MSCs in bone regeneration. It provides crucial information for improving and optimizing MSCs for future clinical application of bone regeneration.
Keywords: steroid regulation, mesenchymal stem cells, proliferation, differentiation, gender difference
1. INTRODUCTION
Adult stem cell-based bone tissue engineering has now emerged as a promising alternative approach for clinical treatment of skeleton defects caused by trauma, tumor dissection and congenital deficiency [1]. Bone marrow mesenchymal stem cells (MSCs) are considered a potential cell source for this approach due to its capacity for self-renewal and multiple differentiations [2]. However, the number of bone marrow MSCs that can be feasibly isolated is very small and insufficient for clinical application of bone regeneration [3]. Various path-physiological factors including age, osteoporosis and arthritis have been demonstrated to further reduce the cell number and their capacities of proliferation and differentiation [4–6]. It was reported recently that the MSC number in bone marrow of aged donors is significantly lower than that of young donors. The senescent percentage of MSCs and their duplication time are significantly higher from aged donors [7]. Although in theory the MSC number can be expanded by in vitro cell culture, the manipulation causes remarkable reduction of proliferation and differentiation potential owing to cell senescence [8]. Thus, effective improvement of MSC proliferation and osteogenic differentiation is necessary for clinical application of MSC-based bone regeneration.
Steroid hormones actively participate in bone metabolism and have multifunctional regulation in many types of cells including bone marrow MSCs. For instance, glucocorticoid plays a key role in multiple differentiations of MSCs and up-regulates osteogenic, chondrogenic and adipogenic differentiation potential. In osteoporotic studies, estrogen was found not only to prevent bone resorption but also to increase bone formation by recruiting progenitor cells of osteoblasts [9]. A recent study demonstrated that supplementation of 17β-estradiol (E2) effectively stimulates proliferative capacity of MSCs in humans and mice [7, 10]. E2 also possesses a strong ability to improve the differentiation potential of MSCs including osteogenic and adipogenic differentiation [11]. In addition, estrogen has been discovered recently to inhibit and delay MSC senescence by up-regulation of telomerase activity [12]. These results strongly indicate that steroid hormones can serve as effective stimulators to facilitate MSC capacity, which will benefit bone tissue engineering.
E2 and dexamethasone interact and regulate cell function via estrogen and glucocorticoid receptors [13, 14]. The receptors have been found to distribute differently based on gender in cardiac, brain and fat tissues [15–17]. These differences probably contribute to the sex dimorphism of steroid functions in these tissues. Although estrogen and glucocorticoid regulate MSC proliferation and differentiation, their function and different effects on MSCs derived from different genders of donors are little reported. This new information will be important in the effect to optimize MSCs using steroid regulation for bone tissue engineering. In the present study, we investigated the function of E2 and dexamethasone on proliferation and osteogenic differentiation of rat bone marrow MSCs. The difference of the steroid function and steroid receptors of MSCs from male and female donors is discussed.
2. MATERIALS AND METHODS
2.1. Isolation of rat bone marrow MSCs
Three month-old, male and female F-344 rats (Charles River Lab., Wilmington, MA) were purchased and euthanized by CO2 inhalation. Bone marrow plugs from tibias and femurs were flushed out with steroid-free medium consisting of a phenol red-free Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Madison, WI), 10% charcoal-stripped fetal bovine serum (Gemini Bio-product, West Sacramento, CA) and 1% antibiotic-antimycotic (Sigma, St. Louis, MO). Cells were plated at 5×107 cells/100-mm petri dish and incubated with steroid-free medium at 37°C in 5% CO2. On day 3, the MSCs were isolated by removing unattached cells via medium exchange. The multiple differentiation capacities of isolated cells including adipogenesis, chondrogenesis and osteogenesis were tested in a separated study. The MSCs were incubated up to 80% confluence and then sub-cultured at a density of 106/100-mm dish as passage-1.
2.2. MSC proliferation with E2 and dexamethasone supplements
Passage-1 MSCs of male and female rats were used to investigate the function of E2 and dexamethasone on MSC proliferation. 1,000 passage-1 MSCs were placed into each well of 96-well plates and exposed to steroid-free medium supplemented with different concentrations (10−6 to 10−12M) of E2 and dexamethasone (Sigma, St. Louis, MO). On day 6, the cell proliferation was quantified using a MTS colorimetric assay according to manufacturer’s instructions (CellTiter, Promega Corporation, Madison, WI). MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) produces a water-soluble formazan product in the presence of phenazine methosulfate (PMS). These reductions take place only when mitochondrial reductase enzymes are active, and therefore conversion is often used as a measure of viable cells. The absorbance at 490 nm were measured by a microplate reader (Bio-Rad laboratories, Hercules, CA).
2.3. Osteogenic differentiation of MSCs with steroid treatments
Steroid regulation of osteogenic potential of rat MSCs seeded in monolayers and 3-dimension were investigated. Passage-1 male and female rat MSCs were seeded at a density of 105/100-mm dish. Upon 70% confluence, the medium was exchanged with osteogenic differentiation medium supplemented with different concentrations of E2 (10−6 to 10−12M). The osteogenic differentiation medium consists of LG-DMEM medium supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate. At day 7, cells were collected and analyzed for osteogenic differentiation markers and steroid-associated growth factors and receptors using quantitative real-time PCR. To investigate the osteogenic potentials of MSCs seeded in 3-dimensional scaffolds, we created tissue-engineered constructs by seeding passage-1 MSCs on gelatin sponges as described in our previous studies [18]. The sterile gelatin sponge (Gelfoam@, Pharmacia & Upjohn, Kalamazoo, MI) was trimmed into a cube (4×4×4 mm3) and pre-wetted with DMEM medium. The constructs were prepared by immersing the sponges into a tube containing a prepared MSC suspension of 5×106 cell/ml under a slight vacuum created by a 20 ml syringe. Then the mixture of sponges and cell suspension were incubated at 37°C for two hours. The next day, the constructs were exposed to the osteogenic differentiation medium supplemented with different concentrations of E2 (10−6 to 10−12M). After two and four weeks of differentiation, constructs of each experimental group were collected. The constructs were immersed in 0.2 ml of 1% Triton-X100 and homogenized with sonication. The DNA concentration of cell lysate was measured fluorometrically using Hoechst dye 33258 (Bio-Rad, Hercules, CA). Spectrophotometry with colorimetric kits were used to measure alkaline phosphatase (ALP) (CLINIQA, San Marcos, CA), protein and calcium content (Fisher scientific, Pittsburgh, PA). Rat osteocalcin (OCN) was measured with an ELISA kit according to manufacturer’s instructions (Immunodiagnostic Systems Inc., Fountain Hills, AZ).
2.4. Quantification of MSC gene expressions during differentiation with E2 supplements
Real-time PCR was used to quantify gene expressions of steroid receptors, osteogenic markers and cytokines. Total RNA was extracted from the MSCs using TRIzol reagent (Invitrogen, Carlsbad, CA) and purified using QIAquick PCR Purification Kit (QIAGEN, Valencia, CA) Reverse transcription were carried out after treatment with DNAse I (Sigma–Aldrich, St Louis, MO) using the 1st Strand cDNA Synthesis Kit with random hexamer primers reverse transcription (Invitrogen, Madison, WI). Quantitative real-time PCR was performed using SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA). Real-time PCR amplification was performed on the iCycler iQ detection system, and the data were collected and analyzed using iCycler iQ version 3.0 software (Bio-Rad). Osteogenic differentiation markers (ALP and OCN), osteogenic growth factors (TGF-β1 and BMP-7), and steroid receptors ERα and -β, androgen receptor (AR) and glucocorticoid receptor (GR) were measured and all primes were made commercially (SABiosciences, Frederick, MD)
2.5. Statistical analysis
All data are expressed as the mean ± standard deviation. The data were analyzed by one-way ANOVA to compare treatment and control groups at each time point. The correlations of gene expression among osteogenic potential, steroid-associated growth factors and receptors were analyzed by SSPS. p values less than 0.05 were considered as significant.
3. RESULTS
3.1. MSC proliferation with steroid hormone supplements
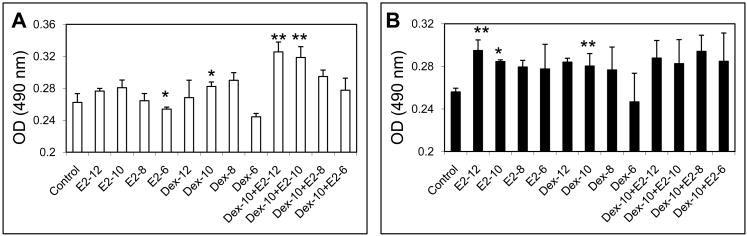
Supplement of E2 and dexamethasone significantly increase cell proliferation of male and female rat MSCs. However, effective doses and combinations of E2 and dexamethasone are varied according to the gender of donors. MTS assay revealed that MSC proliferation of female rats significantly increases after supplements of E2 at concentrations of 10−10 and 10−12M and dexamethasone of 10−10 M. However, no significant effect was observed in combining treatment with E2 and dexamethasone (Fig. 1A). For the male rat MSCs, 10−10M of dexamethasone significantly increases MSC proliferation while a higher concentration (10−6M) inhibits the proliferation. No statistical increase of cell proliferation is observed after E2 supplements. However, combinational supplements of E2 (10−10 and 10−12M) and dexamethasone (10−10M) significantly improve cell proliferation (Fig. 1B).
Figure 1.
The effect of E2 and dexamethasone on the proliferation of male (A) and female (B) rat MSCs. N=5, *: p<0.05, **: p<0.01.
3.2. Osteogenic potential of MSCs with steroid treatments
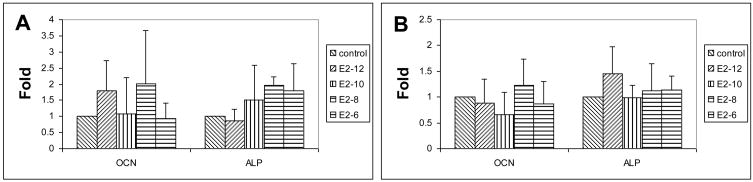
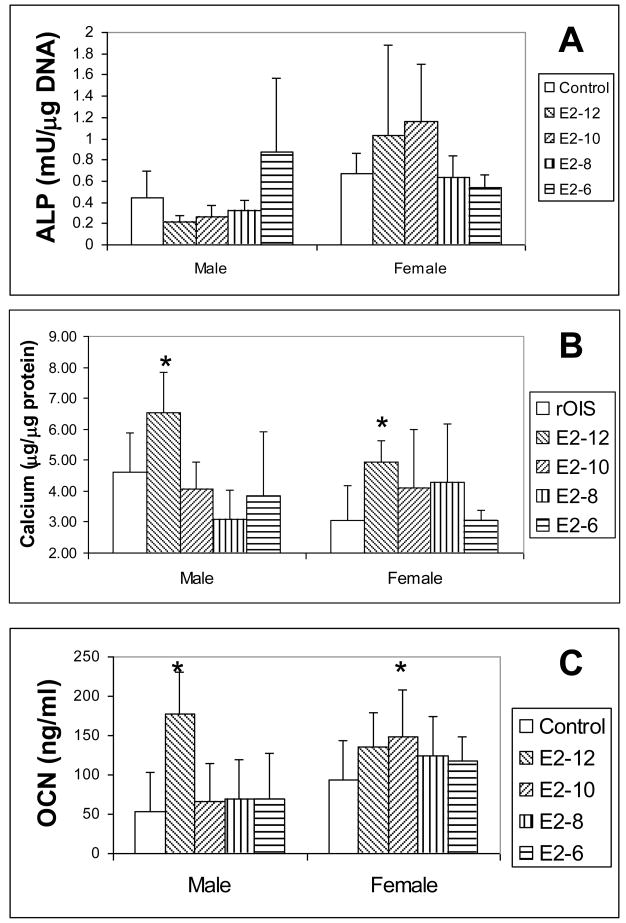
E2 supplements effectively improve the osteogenic potential of male and female rat MSCs. After one week of osteogenic differentiation with E2 supplement, mRNA of ALP and OCN are up-regulated in both male and female rat MSCs (Fig. 2). A peak expression of OCN occurs at 10−8M of E2 at both male and female rat MSCs while the peak of ALP occur at different concentration of E2. The concentration of E2 at male rat MSCs (10−8M) is higher than that in female MSCs (10−10M). A similar feature was also found in osteogenic potential of MSCs seeded in gelatin sponges. The concentration of E2 of peak ALP activity after 2 weeks in male rat MSCs (10−6M) is higher than that in female’s (10−10M) (Fig. 3A). After 4 weeks of differentiation, E2 supplement significantly improves the calcium content and OCN of both male and female rat MSCs (Fig. 3B and C). However, the effective E2 concentration required to significantly increase OCN for male (10−12 M) is lower than that for females (Fig. 3C).
Figure 2.
Quantitative mRNA of OCN and ALP of MSCs from male (A) and female (B) rats one week after osteogenic differentiation with E2 supplements.
Figure 3.
Osteogenic differentiation of MSCs seeded on gelatin sponges. A: ALP activity of MSCs 2 weeks after differentiation; B and C: Calcium contents (B) and OCN (C) of MSCs 4 weeks after differentiation with E2 supplements. N=5; *: p<0.05.
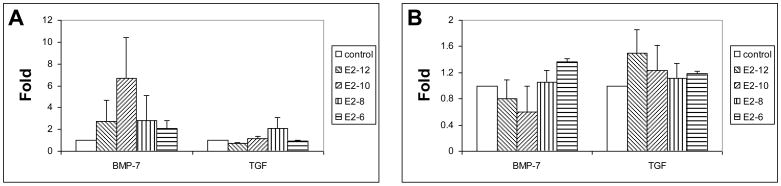
The osteogenic growth factors of BMP-7 and TGF-β1 are up regulated with E2 supplement during differentiation. The maximal up-regulation of BMP-7 and TGF-b for males’ MSCs are 6.7±3.72 and 2.05±1.06 folds, and for females are 1.36±0.06 and 1.5±0.36 fold. The E2 concentrations for maximal up-regulation are different in male and female rat MSCs (Fig. 4). A higher E2 concentration for stimulating a peak of BMP-7 mRNA is required for female rat MSC (10−6M) than that required for males’ (10−10M). In contrast, E2 concentration (10−12 M) for maximum up regulation of TGF-β1 for female rat MSCs is lower than that for males (10−8M).
Figure 4.
Quantitative mRNA of BMP-7 and TGF-β1 of MSCs from male (A) and female (B) rats one week after osteogenic differentiation with E2 supplements.
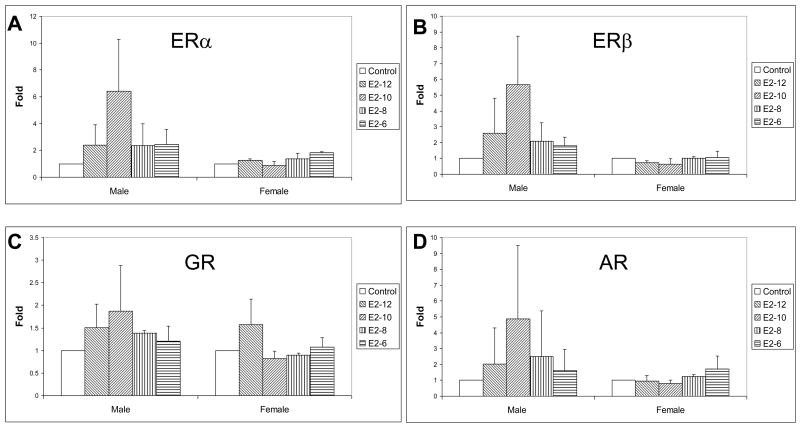
3.3. MSC steroid receptors during differentiation with E2 supplement
E2 supplementation up-regulates ERα, β, GR and AR of MSCs during osteogenic differentiation. However, the regulation varied depending on the gender of MSC donors (Fig. 5). For male rat MSCs, all steroid receptors are up regulated by the entire range of E2 supplements used with the peak occurring with 10−8M dose. The maximum improvement of ERα, β, GR and AR are 6.4±3.89, 5.65±3.07, 1.86±1.01 and 4.88±4.59 folds, respectively. For female rat MSCs, the maximal up-regulation of ERα (1.82±0.1 folds) and AR (1.69±0.82 folds) after E2 supplement are at a concentration of 10−6M while GR (1.57±0.56 folds) at the 10−12M of E2. No significant up-regulation of ERβ expression is observed after E2 supplement.
Figure 5.
Quantitative mRNA of steroid receptors of MSCs one week after osteogenic differentiation with E2 supplements.
We further correlated steroid receptors, osteogenic markers and growth factors. In both male and female rat MSCs after E2 supplement during osteogenic differentiation, steroid receptors ERα, ERβ, GR, and AR highly correlate to BMP-7. ERα correlate ERβ, GR, and AR; while ERβ correlate to AR (Table 1). TGFβ correlates to osteogenic markers of ALP. However, a gender difference was found in the correlations between steroid receptors and osteogenic markers. GR correlates to ERβ and AR in male rat MSCs while ERβ correlate to OCN in females.
Table 1.
Correlations of osteogenic markers, steroid receptors and osteogenic cytokines of MSCs one week after osteogenic differentiation with E2 supplements
| Male | ERα | ERβ | GR | AR | TGF | BMP-7 | ALP | OCN |
|---|---|---|---|---|---|---|---|---|
| ERα | 0.972** | 0.901** | 0.948** | 0.986** | ||||
| ERβ | 0.972** | 0.910** | 0.952** | 0.983** | ||||
| GR | 0.901** | 0.910** | 0.807** | 0.886** | ||||
| AR | 0.948** | 0.952** | 0.807** | 0.972** | ||||
| TGF | 0.689* | |||||||
| BMP-7 | 0.986** | 0.983** | 0.886** | 0.972** | ||||
| ALP | 0.689* | |||||||
| OCN | ||||||||
| Female | ERα | ERβ | GR | AR | TGF | BMP-7 | ALP | OCN |
|
| ||||||||
| ERα | 0.687* | 0.835** | 0.747* | 0.914** | ||||
| ERβ | 0.687* | 0.823** | 0.719* | 0.882** | ||||
| GR | 0.825** | 0.79** | ||||||
| AR | 0.747* | 0.823** | 0.761* | |||||
| TGF | 0.654* | |||||||
| BMP-7 | 0.914** | 0.719* | 0.79** | 0.761* | ||||
| ALP | 0.654* | |||||||
| OCN | 0.882** | |||||||
p<0.05
p<0.01
4. DISCUSSION
The obstacle limiting bone marrow MSCs as a promising cell source for bone tissue engineering is limited cell supply and reduced differentiation potential. Effective improvement of MSC proliferation and differentiation play a critical role in determining the successful clinical application of MSC-based bone regeneration. Steroids have recently been demonstrated to increase the proliferation and differentiation of MSCs, and delay the senescence. The present study revealed that E2 and dexamethasone interactively increase MSC proliferation and osteogenic differentiation. Supplementation of E2 significantly improves synthesis of osteogenic growth factors during osteogenic differentiation. These data strongly suggest that steroids potentially function as an effective modulator to improve MSC capacity of bone regeneration. Furthermore, this study demonstrated for the first time the gender difference of steroid regulation of bone marrow MSCs. The optimal doses and interaction of steroids to improve proliferation and osteogenic potential of MSCs effectively varied depending on the gender of the MSC donors. The relationships between steroid receptors, osteogenic markers and cytokines are also gender-dependent. These results provide crucial information for the future improvement and optimization of MSC capacity for successful bone regeneration; although, the mechanism needs to be further investigated.
Gender differences of stem cells have been noted recently in the adult stem cell research literature [19–23]. Deasy found that muscle derived stem cells (MDSCs) isolated from a female mouse have a greater skeletal muscle regeneration efficiency than MDSCs isolated from a male mouse [20]. The MDSCs isolated from a male mouse have stronger osteogenic potential and bone regeneration than MDSCs isolated from a female mouse [21]. Human adipose-derived stem cells isolated from males were also found to be more osteogenic than those isolated from females [22]. Although the mechanism was little discussed, one of the possible factors is the gender difference of steroid receptors that play critical roles in stem cell proliferation and differentiation. It has been demonstrated that GRs have regional and gender differences in human preadipocytes [15]. GR and ERs have been also found to have gender differences in the distribution and regulation in the cardiac muscles and brain [16, 17]. Effects of the sex steroid receptors to regulate skeletal metabolism have been demonstrated to be varied by sex [23]. In the present study, estradiol and dexamethasone exhibit strong mitogenic effects on MSCs. The effect of steroids is biphasic and the steroids lose the function or even inhibit cell proliferation at a high concentration. Estrogen was found to have more mitogenic functions on female rat MSCs while combinations of estrogen and dexamethasone have more mitogenic function on male rat MSCs. Estrogen and dexamethasone at low concentrations has a synergetic effect on male rat MSC proliferation. Similarly, supplementation of estradiol effectively improves osteogenic differentiation of MSCs in the presence of dexamethasone; however, the dose to up regulate osteogenic markers maximally is gender different. A lower concentration of E2 up regulates a peak ALP activity, an early marker of osteogenic differentiation in female MSCs than that in males. However, the peak amount of OCN, a mature osteoblastic marker occurs at a lower concentration of E2 in male MSCs as compared to that of female MSCs. The E2 concentrations to produce peak OCN amounts in both males and females are consistent with that of BMP-7 expression. The latter is highly correlated to steroid receptors in male and female rat MSCs. Thus, we believe that the gender difference of MSCs during osteogenic differentiation with E2 supplementation is probably caused by the variation of steroid receptors. Relationships between multiple steroid receptors in the present study also showed the differences between male and female MSCs. This also indicates that the differences in steroid receptors may be the cause of sex dimorphism in osteogenic differentiation.
Steroid hormones interact in cell and tissue metabolism. Glucocorticoid and estrogen have been demonstrated to act on their receptors interactively and regulate bone marrow cells. Estradiol has been reported to increase ERα during osteogenic differentiation but not ERβ in female mouse MSCs. In the present study we found that E2 supplements not only up regulate ERα but also GR and AR of MSCs of both male and female donors. E2 also up-regulate ERβ of MSCs of male donors while less function on female MSCs. The variations of ERα highly correlates with ERβ, GR and AR levels in both male and female MSCs. ERβ correlates with GR and AR in male MSCs and does not correlate with GR in female MSCs. This indicates that ERβ may play a different role in osteogenic differentiation of male and female MSCs treated with E2 supplements.
Estrogen has been reported to increase osteogenic differentiation of bone marrow cells via up regulation of numbers of osteogenic growth factors, including BMP2, 6 and TGF-β1 [24–26]. The present study demonstrated that E2 supplementation effectively up-regulates TGF-β1 in both male and female MSCs. Up-regulated TGFβ1 correlates highly with the ALP of MSCs. Furthermore, it has been explored for the first time that E2 effectively up-regulate BMP-7 during osteogenic differentiation of MSCs. BMP-7 has been extensively considered as an osteogenic protein for bone regeneration [27]. The function of BMP-7 including bone formation and cancer cell proliferation is estrogen-dependent [28, 29]. In the present study, mRNA of BMP-7 was up-regulated by E2 supplement and highly correlates to the steroids receptors in both male and female rat MSCs during osteogenic differentiation. This indicates that one of the mechanisms of E2 to increase osteogenic differentiation is possibly via up-regulation of BMP-7.
Although currently used osteogenic markers including ALP and OCN certainly reflect the osteogenic potential of MSCs, they are not reliable predictors of the bone forming capacity of MSCs. Thus, quantitative measurement of bone formation of MSCs in vitro and in vivo is needed to confirm further the function of steroid regulations of MSCs. The gender differences observed regarding MSC ability to proliferation and differentiation and the acting mechanism also need to be further investigated on human bone marrow MSCs. This study preliminarily revealed the correlations between osteogenic- and steroid receptor-associated gene expressions during osteogenic differentiation. However, future studies using gene knock-out technique and antagonists are necessary to further demonstrate the mechanism of steroids on MSC proliferation and differentiation, and to clarify the correlation of gene expressions and receptor dependency of steroid hormones.
Acknowledgments
We are indebted to Professor Carla Evans for her constructive comments and correction of the manuscript. This research was supported by NIH/NIDCR (R03DE017715) and the Department of Orthodontics at UIC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Triffitt JT. Osteogenic stem cells and orthopedic engineering: summary and update. J Biomed Mater Res. 2002;63(4):384–9. doi: 10.1002/jbm.10260. [DOI] [PubMed] [Google Scholar]
- 2.Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2008 Sep 15; doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- 3.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11(5–6):87–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 4.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 5.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75(3):414–23. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–43. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205(2):194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 9.Compston JE. The risks and benefits of HRT. J Musculoskelet Neuronal Interact. 2004;4(2):187–90. [PubMed] [Google Scholar]
- 10.DiSilvio L, Jameson J, Gamie Z, Giannoudis PV, Tsiridis E. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs) Injury. 2006;37(Suppl 3):S33–42. doi: 10.1016/j.injury.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hong L, Colpan A, Peptan IA. Modulations of 17-beta Estradiol on Osteogenic and Adipogenic Differentiations of Human Mesenchymal Stem Cells. Tissue Eng. 2006;12(10):2747–53. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 12.Cha Y, Kwon SJ, Seol W, Park KS. Estrogen Receptor-alpha Mediates the Effects of Estradiol on Telomerase Activity in Human Mesenchymal Stem Cells. Mol Cells. 2008;26(5) [PubMed] [Google Scholar]
- 13.Cuzzocrea S, Bruscoli S, Crisafulli C, Mazzon E, Agostini M, Muià C, Esposito E, Di Virgilio R, Meli R, Vegeto E, Maggi A, Riccardi C. Estrogen receptor antagonist fulvestrant (ICI 182,780) inhibits the anti-inflammatory effect of glucocorticoids. Mol Pharmacol. 2007;71(1):132–44. doi: 10.1124/mol.106.029629. [DOI] [PubMed] [Google Scholar]
- 14.Sukhu B, Rotenberg B, Binkert C, Kohno H, Zohar R, McCulloch CA, Tenenbaum HC. Tamoxifen attenuates glucocorticoid actions on bone formation in vitro. Endocrinology. 1997;138(8):3269–75. doi: 10.1210/endo.138.8.5340. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14(27):2918–24. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]
- 16.Turner BB, Moses LF. Sex differences in the distribution and regulation of glucocorticoid receptors in cardiac tissues of rats. J Mol Cell Cardiol. 1986;18(3):331–3. doi: 10.1016/s0022-2828(86)80415-1. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28(5):997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 18.Hong L, Peptan IA, Xu H, Magin RL. Nondestructive evaluation of osteogenic differentiation in tissue-engineered constructs. J Orthop Res. 2006;24(5):889–97. doi: 10.1002/jor.20140. [DOI] [PubMed] [Google Scholar]
- 19.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142(2):215–21. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177(1):73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corsi KA, Pollett JB, Phillippi JA, Usas A, Li G, Huard J. Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res. 2007;22(10):1592–602. doi: 10.1359/jbmr.070702. [DOI] [PubMed] [Google Scholar]
- 22.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60(3):306–22. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 23.Tözüm TF, Oppenlander ME, Koh-Paige AJ, Robins DM, McCauley LK. Effects of sex steroid receptor specificity in the regulation of skeletal metabolism. Calcif Tissue Int. 2004;75(1):60–70. doi: 10.1007/s00223-004-0119-8. [DOI] [PubMed] [Google Scholar]
- 24.Qu Q, Perälä-Heape M, Kapanen A, Dahllund J, Salo J, Väänänen HK, Härkönen P. Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone. 1998;22(3):201–9. doi: 10.1016/s8756-3282(97)00276-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S, Turgeman G, Harris SE, Leitman DC, Komm BS, Bodine PV, Gazit D. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Mol Endocrinol. 2003;17(1):56–66. doi: 10.1210/me.2002-0210. [DOI] [PubMed] [Google Scholar]
- 26.Plant A, Tobias JH. Increased bone morphogenetic protein-6 expression in mouse long bones after estrogen administration. J Bone Miner Res. 2002;17(5):782–90. doi: 10.1359/jbmr.2002.17.5.782. [DOI] [PubMed] [Google Scholar]
- 27.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31(6):721–7. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, Theerajunyaporn T, Chen JL, Reddi AH, Martin RB. Estrogen-dependent actions of bone morphogenetic protein-7 on spine fusion in rats. Spine. 2005;30(15):1706–11. doi: 10.1097/01.brs.0000172230.01655.55. [DOI] [PubMed] [Google Scholar]
- 29.Schwalbe M, Sänger J, Eggers R, Naumann A, Schmidt A, Höffken K, Clement JH. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol. 2003;23(1):89–95. [PubMed] [Google Scholar]