Abstract
Polarity is achieved partly through the localized assembly of the cytoskeleton. During growth in budding yeast, the bud cortex and neck localized formins Bni1p and Bnr1p nucleate and assemble actin cables that extend along the bud-mother axis, providing tracks for secretory vesicle delivery. Localized formins are believed to determine the location and polarity of cables, hence growth. However, yeast expressing the nonlocalized actin nucleating/assembly formin homology (FH) 1-FH2 domains of Bnr1p or Bni1p as the sole formin grow well. Although cables are significantly disorganized, analysis of directed transport of secretory vesicles is still biased toward the bud, reflecting a bias in correctly oriented cables, thereby permitting polarized growth. Myosin II, localized at the bud neck, contributes to polarized growth as a mutant unable to interact with F-actin further compromises growth in cells with an unlocalized formin but not with a localized formin. Our results show that multiple mechanisms contribute to cable orientation and polarized growth, with localized formins and myosin II being two major contributors.
INTRODUCTION
The eukaryotic cell cytoskeleton, especially microfilaments and microtubules, provides the framework that determines cell shape and the organization of organelles, and their overall polarity (Nelson, 2003). Budding yeast provide a simple system for the study of microfilaments, because they are solely responsible for polarity of cell growth (Pruyne et al., 2004b). Three distinct microfilament organizations, cortical patches, cables, and the contractile ring, also provide clearly defined functions. Cortical patches are dynamic structures that drive endocytic vesicles into the cell (Kaksonen et al., 2003; Huckaba et al., 2004; Kaksonen et al., 2005). Actin cables, which extend from a growing bud and the bud neck into the mother cell during cell growth, are used as tracks to transport secretory vesicles for polarized growth, as well as for the segregation of most organelles (Pruyne et al., 2004b). Finally, the contractile ring forms at cytokinesis to pinch the cell into two (Bi et al., 1998; Lippincott and Li, 1998). To perform these diverse functions, locally activated factors nucleate the assembly of actin filaments. Actin assembly at cortical patches is mediated by the activated Arp2/3 (Mullins et al., 1998; Kaksonen et al., 2006), and formins nucleate actin filaments in cables and contractile ring (Evangelista et al., 2002; Pruyne et al., 2002, 2004a; Sagot et al., 2002a,b; Tolliday et al., 2002).
Both nucleators, the Arp2/3 complex and the formins, are well conserved among eukaryotes, and both have been shown to direct the assembly of different actin structures in various organisms (Goley and Welch, 2006; Goode and Eck, 2007). It is generally believed that the location of the actin nucleators determines the location of the actin structures assembled. In budding yeast, the current model suggests that upstream factors localize and activate formins, which then assemble actin filaments with their barbed ends associated with the localized formins (Pruyne et al., 2002). In this way, it is believed that the location of active formin proteins determines the location and polarity of actin cables (Pruyne et al., 2004a). Because the polarized actin cables provide the tracks for the delivery of secretory cargo by the myosin-V whose heavy chain is encoded by MYO2, Myo2p and the formins are essential proteins in yeast (Pruyne et al., 2004a).
Yeast's two formins, Bni1p and Bnr1p, have distinct localizations. Bni1p is found at the site of bud emergence, at the tip of small buds, at the cortex of larger buds, and then redistributes to the bud neck during septum formation (Fujiwara et al., 1998; Ozaki-Kuroda et al., 2001; Pruyne et al., 2004a; Buttery et al., 2007). Bnr1p is delocalized before bud emergence, and then becomes associated with the bud neck during the rest of the cell cycle until cytokinesis (Kikyo et al., 1999; Pruyne et al., 2004a; Buttery et al., 2007). Bni1p and Bnr1p exhibit different dynamics. It is proposed that after nucleation Bni1p detaches from the cortex and stays attached to the filament barbed ends, whereas Bnr1p stays at the localization foci and detaches from the actin filaments (Buttery et al., 2007). In both cases, actin cables are composed of bundles of short filaments (Karpova et al., 1998; Yang and Pon, 2002), stabilized by tropomyosins and bundling factors (Moseley and Goode, 2006).
Like all members of the formin family, Bni1p and Bnr1p are modular proteins. Common to all formins are two adjacent domains known as formin homology domain (FH) 1, which is rich in proline and recruits profilin-actin, and FH2, which provides the actin nucleating and processive capping activities (Goode and Eck, 2007). Formins have sequences that direct their appropriate localization, which have been mapped to the first 500 residues for Bnr1p (Kikyo et al., 1999), although the molecular mechanism of this localization is unclear.
To explore the functional domains of Bnr1p further, we set out to identify the minimal region of Bnr1p that can provide viability in a strain deleted for both BNR1 and BNI1. An earlier study indicated that overexpression of the FH1-FH2 region of Bni1p was sufficient for some limited growth but did not characterize this observation further (Sagot et al., 2002a). Here, we show that low level expression of just the FH1-FH2 region of Bnr1p or Bni1p supports growth comparable to full-length localized Bnr1p. Remarkably, although the FH1-FH2 domain is delocalized, cables are still partially oriented toward the bud neck. Our findings implicate the existence of additional mechanisms to orient actin cables toward the bud neck for polarized growth, and we show that part of this orientation is provided by bud neck localized myosin II.
MATERIALS AND METHODS
Construction of Yeast Strains
Yeast strains and plasmids used in this study are described in Tables 1 and 2.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| ABY1599 | MATa his3Δ1 leu2Δ0 ura3Δ0 bni1Δ::KanR bnr1Δ::NatR pRS316-BNI1 | Lab collection |
| ABY1867 | MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/MET15 lys2Δ0/LYS2 bni1Δ::KanR/bni1Δ::KanR | Evangelista et al. (2002) |
| ABY2804 | MATa his3 leu2 ura3 trp1 bni1Δ::KanR bnr1Δ::BNR1::HIS3 myo1Δ::HIS3 YCp50-MYO1 | This study |
| ABY2806 | MATα his3 leu2 ura3 trp1 met15 lys2 bni1Δ::KanR bnr1Δ::BNR1 FH1-FH2-COOH::HIS3 myo1Δ::HIS3 YCp50-MYO1 | This study |
| ABY2820 | MATa his3 leu2 ura3 trp1 bni1Δ::KanR bnr1Δ::BNR1 FH1-FH2-COOH::HIS3 myo1Δ::HIS3 YCp50-MYO1 | This study |
| ABY2821 | MATa/α his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 met15/MET15 lys2/LYS2 bni1Δ::KanR/bni1Δ::KanR bnr1Δ::BNR1 FH1-FH2-COOH::HIS3/bnr1Δ::BNR1 FH1-FH2-COOH::HIS3 myo1Δ::HIS3/myo1Δ::HIS3 YCp50-MYO1 | This study |
| YEF2057 | MATα his3 leu2 ura3 trp1 myo1Δ::HIS3 YCp50-MYO1 | E. Bi |
Table 2.
Plasmids used in this study
| Plasmid | Backbone | Selectable marker | Features | Source |
|---|---|---|---|---|
| pGFP-MYO1 | pRS314 | TRP1 | GFP-MYO1 under MYO1 promoter | Lord et al. (2005) |
| pGFP-MYO1T | pRS314 | TRP1 | GFP-MYO1 tail under MYO1 promoter | Lord et al. (2005) |
| pLG212 | pRS313 | HIS3 | BNR1 under BNR1 promoter | This study |
| pLG219 | pRS313 | HIS3 | BNR1 (2236–3864) under BNR1 promoter | This study |
| pLG220 | pRS313 | HIS3 | BNR1 (2236–4128) under BNR1 promoter | This study |
| pLG232 | pRS313 | HIS3 | BNR1 under GAL1–10 promoter | This study |
| pLG240 | pRS313 | HIS3 | BNR1 (2236–4128) under GAL1–10 promoter | This study |
| pLG335 | pRS313 | HIS3 | BNI1 (3679–5298) under BNI1 promoter | This Study |
| pRC651 | pRS313 | LEU2 | GFP-tagged SEC4 | Lab collection |
To test the synthetic effect of bni1Δ BNR1 FH1-FH2-COOH and headless MYO1, strains with BNR1 FH1-FH2-COOH or full-length BNR1 (as control) integrated into the chromosome were first generated. Linear DNA composed of BNR1 promoter (with a HIS3 marker inserted at the Bst1107I site)–BNR1 coding sequence (BNR1 FH1-FH2-COOH or BNR1)–BNR1 3′ untranslated region were used to replace the BNR1 locus in ABY1867 (bni1Δ/bni1Δ). The resulting strains were sporulated and haploids with bni1Δ and the integrated BNR1 sequences were selected and then crossed to YEF2057 (myo1Δ, YCp50-MYO1). The resulting strains were then sporulated to obtain ABY2804 (bni1Δ, BNR1, myo1Δ, YCp50-MYO1) and ABY2806 and ABY2820 (bni1Δ, BNR1 FH1-FH2-COOH, myo1Δ, YCp50-MYO1). ABY2806 and ABY2820 were mated to obtain ABY 2821 (bni1Δ/bni1Δ, BNR1 FH1-FH2-COOH/BNR1 FH1-FH2-COOH, myo1Δ/myo1Δ, YCp50-MYO1).
Rescue of bni1Δ bnr1Δ Lethality
ABY1599 was transformed with pLG212, pLG219, pLG220, pLG232, pLG240, or pLG335 and plated on SC-Ura, His. Transformants were streaked on SC-His and then SC-His + 5-fluoroorotic acid (5FOA) twice to select for the removal of pRS316-BNI1 plasmid from ABY1599.
Immunoblotting
Rabbit polyclonal antiserum was raised and affinity purified against recombinant GST-Bnr1p FH1-FH2 (residues 757-1336) (Pruyne et al., 2004a). Immunoblotting used Bnr1p FH1-FH2-COOH antibody (0.72 μg/ml) and β-tubulin antibody.
Determination of Internal and External Bgl2p Levels
Spheroplasts were prepared by killing yeast cells with 40 mM NaF and 10 mM NaN3 and incubating at 37°C in digestion buffer (1.2 M sorbitol, 0.1 M KPi, pH 7.4, 25 mM β-mercaptoethanol, 40 mg/ml Zymolyase (Zymo Research, Orange, CA), 40 mM NaF, and 10 mM NaN3) for 1 h. Internal and external proteins were separated by centrifugation. Immunoblotting was performed with Bgl2p antiserum (Mrsa et al., 1993; 1:10,000) and tropomyosin antiserum (Pruyne et al., 1998; 1:2000) as internal control.
Microscopy
Immunofluorescence microscopy with affinity-purified antibodies to actin, Tpm1p and Myo2p was performed as described previously (Pruyne et al., 1998). To stain for Bnr1p with undiluted affinity purified Bnr1p FH1-FH2-COOH antibody, the procedure for Bni1p staining was used (Pruyne et al., 2004a) except that the fixation time with formaldehyde was 15 min. Micrographs were acquired with a Nikon Eclipse TE-2000U microscope on a confocal imaging system (UltraView LCI; PerkinElmer Life and Analytical Sciences, Boston, MA) by using a Nikon 100× 1.4 numerical aperture (NA) lens and digital camera (C4742-95-12ERG; Hamamatsu, Bridgewater, NJ) except differential interference contrast (DIC) pictures (Figures 2A and 5A) and immunofluorescence pictures showing Bnr1p localization (Figure 1C), which were acquired with an Axiovert 100TV microscope (Carl Zeiss, Thornwood, NY) by using a Fluor 100× NA 1.30 oil lens and digital camera (C4742-95-12ERG; Hamamatsu).
Figure 2.
Cells expressing the FH1-FH2 domains of Bnr1p or Bni1p remain polarized. (A) Morphology and actin cytoskeleton of bni1Δ bnr1Δ cells expressing different formin constructs. DIC images of cells (top row), cells stained with antibodies to actin (middle row) and tropomyosin (bottom row). Arrows indicate cables oriented to the bud neck. Arrowheads indicate actin bars. Bar, 2 μm. (B) Quantitation of the polarization of the actin cytoskeleton in small- and medium-budded cells. n ≥100 for each of at least three experiments. (C) Growth is directed to the bud in bni1Δ bnr1Δ cells expressing just FH1-FH2 formin domains. Cell walls were labeled with FITC-ConA, washed, and then incubated for 2.5 h before imaging.
Figure 5.
Myosin II contributes to cell growth in the absence of localized formins. (A) In the absence of a localized formin, the actin-binding domain of Myo1p is needed to maintain normal growth. bni1Δ myo1Δ cells with either BNR1, or BNR1 FH1-FH2-COOH, and containing a URA3-MYO1 plasmid (YCp50-MYO1), were transformed with either pGFP-MYO1 or pGFP-MYO1T (both containing a TRP1 marker) and spotted on SC-Trp and SC-Trp + 5FOA (to select for loss of the YCp50-MYO1 plasmid) for 2 d at 26°C. (B) Quantitation of the localization of Myo2p in small- and medium-budded cells. n ≥100 for each of at least three experiments. (C) Number of GFP-Sec4p particles per mother cell. Showing mean ± SEM, n ≥100. *p < 0.0001 (calculated with Quickcalcs online calculators for scientists; GraphPad Software, San Diego, CA).
Figure 1.
The FH1-FH2 domains of Bnr1p or Bni1p can provide the essential formin function. (A) Domain organization of Bnr1p and the expression constructs used in this study. GBD, GTPase-binding domain; DID, diaphanous inhibitory domain; DAD, diaphanous autoregulatory domain. Domain boundaries are based on homology and structure of mDia1 (Goode and Eck, 2007) and published domain organizations (Evangelista et al., 2002). (B) Growth curves of bni1Δ bnr1Δ cells expressing Bni1p, Bnr1p, or Bnr1p FH1-FH2-COOH. Overnight cultures were diluted and then incubated at room temperature. Samples were taken every 2 h, and the OD600 was determined and normalized. (C) Rescue of bni1Δ bnr1Δ cells by different formin constructs. Equivalent 10-fold dilutions of bni1Δ bnr1Δ cells expressing Bni1p, Bnr1p, Bnr1p FH1-FH2-COOH, Bnr1p FH1-FH2, or Bni1p FH1-FH2 were spotted on SC plates and incubated for 2 d at 26°C. (D) bni1Δ bnr1Δ cells expressing Bni1p from the BNI1 promoter, or Bnr1p or Bnr1p FH1-FH2-COOH from the BNR1 promoter (top row) or from the GAL1-10 promoter after 2.5-h galactose induction (bottom row) and stained with an antibody to Bnr1p FH1-FH2. Bar, 2 μm. (E) Total lysates of strains expressing formins from the endogenous promoters and blotted with antibodies to Bnr1p FH1-FH2-COOH and β-tubulin.
Fluorescein isothiocyanate (FITC)- and tetramethylrhodamine B isothiocyanate (TRITC)-concanavalin A (ConA) experiments were performed as described previously (Matheos et al., 2004), and images were acquired with the confocal imaging system described above.
Observation of Green Fluorescent Protein (GFP)-Sec4p–associated Particles
Movies of at least 31 small- to medium-budded cells from each strain were acquired on the confocal imaging system described above. Candidate cells showing a bright GFP signal were picked randomly and imaged in a single confocal plane at five frames per second for 30 s. To calculate the percentage of particles undergoing directed movement, the number of GFP-Sec4p puncta was counted in 5-s intervals for each movie as long as the GFP particles were still clearly visible. A moving particle was counted as showing “directed movement” if it moves along a single line or curve for at least 2 s or at least one third of the mother cell length. To measure the velocity of moving particles, movies were imported into ImageJ (National Institutes of Health, Bethesda, MD), and the distance a particle moved over time was measured. The data set were imported into Excel (Microsoft, Redmond, WA) for analysis.
RESULTS
Nonlocalized Formin Fragments Provide Viability in Yeast
Cells deleted for the two formin genes, BNI1 and BNR1, are inviable (Kamei et al., 1998; Vallen et al., 2000; Ozaki-Kuroda et al., 2001). Introduction of either BNI1 or BNR1 on a low copy plasmid restores viability, thereby providing an assay to determine the functionally essential parts of the proteins. Removal of the entire N-terminal domain of Bnr1p to express a construct that includes the FH1 domain to the C terminus (Figure 1A) allowed bni1Δ bnr1Δ cells to grow at a rate indistinguishable from cells expressing full-length Bnr1p, albeit slightly slower than those expressing full-length Bni1p (Figure 1, B and C). Moreover, expression of Bnr1p FH1-FH2-COOH could also rescue bni1Δ bnr1Δ under conditions of high osmotic stress or at low or high temperatures (Supplemental Figure S1).
We next examined whether expressed Bnr1p FH1-FH2-COOH might be localized in bni1Δ bnr1Δ cells. We generated an antibody to the FH1-FH2 region of Bnr1p that does not detectably cross-react with Bni1p and used it to localize Bnr1p FH1-FH2 in bni1Δ bnr1Δ cells expressing either Bni1p from the BNI1 promoter, or Bnr1p or Bnr1p FH1-FH2-COOH from the BNR1 promoter (Figure 1D). As expected, no staining was seen in cells expressing Bni1p, and in cells expressing Bnr1p, staining was seen at the bud neck. No specific localization could be seen for cells expressing Bnr1p FH1-FH2-COOH, although it was expressed as shown by immunoblotting (Figure 1E). When the Bnr1p constructs were overexpressed from the GAL1-10 promoter (Figure 1D), cytoplasmic Bnr1p FH1-FH2-COOH was evident but no bud neck localization was seen, whereas Bnr1p was appropriately localized. Next, we wanted to exclude the possibility that a small, yet sufficient amount of Bnr1p FH1-FH2-COOH might nevertheless be localized to the bud neck. The Bnr1p FH1-FH2-COOH region has been reported to bind two bud neck-associated proteins, Bud6p (Kikyo et al., 1999) and Hof1p (Kamei et al., 1998), which might contribute to its localization. It is unlikely that Bnr1p localizes through Hof1p as it only localizes to the bud neck around G2/M (Vallen et al., 2000), which is after Bnr1p (Pruyne et al., 2004a). We then found that expression of Bnr1p FH-FH2 lacking the C-terminal Bud6p binding site (Moseley and Goode, 2005) also supported good growth (Figure 1C). Unlike Bnr1p, Bni1p is localized to the bud tip and bud cortex during bud growth, and only later in the cell cycle relocalizes to the bud neck (Pruyne et al., 2004a). Bni1p FH1-FH2 lacking the Bud6p binding site could also support good growth (Figure 1, A and C). We conclude that yeast can grow without a localized formin.
Cells Expressing Only a Nonlocalized Formin Maintain a Partially Polarized Actin Cytoskeleton
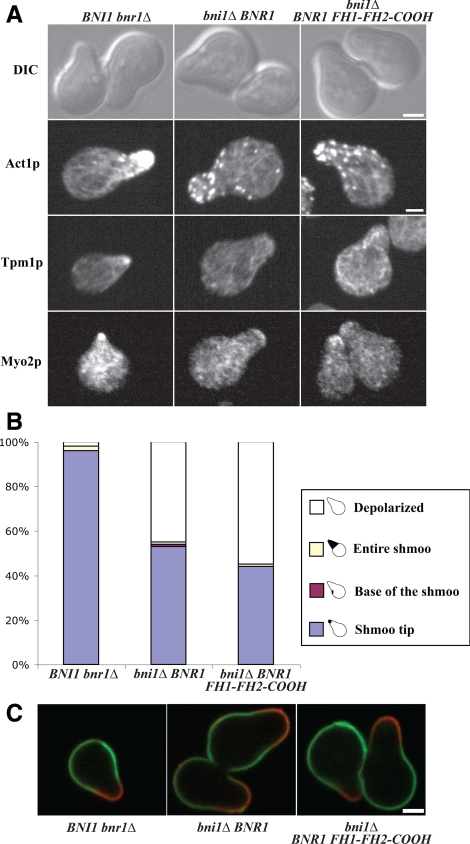
We next examined the morphology and actin cytoskeleton (Figure 2). Cells expressing Bni1p had the normal oval shape, whereas Bnr1p-expressing cells were a bit rounder and had wider necks (Figure 2A), as described previously (Ozaki-Kuroda et al., 2001). Cells expressing just Bnr1p FH1-FH2-COOH were indistinguishable from those expressing Bnr1p (Figure 2A). However, the actin cytoskeleton, visualized with antibodies to either actin or the cable-specific protein tropomyosin, were significantly different. Cells expressing just Bni1p had a polarized cytoskeleton with actin patches and cables in the bud, with a few cables extending into the mother cell. Cells expressing just Bnr1p had abundant cables that arise from the bud neck (Pruyne et al., 2004a). Cells expressing Bnr1p FH1-FH2-COOH had many randomly oriented cables that were difficult to image, although in favorable cases some seem to be directed toward the bud neck (Figure 2A, arrow). The actin cytoskeleton in cells expressing Bni1p FH1-FH2 looked very similar to those expressing only Bnr1p FH1-FH2-COOH, except that these cells often exhibited actin bars (Figure 2A, arrowhead). To explore whether the lack of oriented cables affected overall cell polarity, we quantitated the percentage of polarized cells. Because actin patches are polarized toward growth sites in a cable-dependent manner (Pruyne et al., 1998), and they are more easily detected than cables (Adams and Pringle, 1984), we classified small- to medium-budded cells, which normally exhibit the highest degree of polarization, as polarized (polarized cables or patches), or depolarized (no visible cables and patches are randomly distributed). Where cells expressing Bni1p were highly polarized, cells expressing either Bnr1p, Bnr1p FH1-FH2-COOH or Bni1p FH1-FH2 were slightly less, but equivalently, polarized (Figure 2B). Consistent with this, new cell wall growth was polarized in all four strains (Figure 2C).
Cells Expressing Only a Nonlocalized Formin Still Polarizes Myo2p and Secretory Vesicles to the Bud Neck
Actin cables serve as oriented tracks for the barbed-end transport of secretory vesicles by the motor Myo2p (Pruyne et al., 2004b). In wild-type cells, Myo2p is enriched at the tip of small budded cells, and as a crescent in medium-budded cells (Pruyne et al., 1998; Schott et al., 1999). A similar localization was seen in cells expressing just Bni1p, but in cells expressing only Bnr1p, Myo2p was found enriched at the neck throughout the cell cycle (Figure 3, A and B), as reported previously (Pruyne et al., 2004a). The localization of Myo2p in small- to medium-budded cells thus provides a readout for the polarity and average location of the plus ends of actin cables. Myo2p shows a preference for polarization toward the bud neck in cells expressing either Bnr1p FH1-FH2-COOH or Bni1p FH1-FH2 (Figure 3, A and B). Importantly, because Bni1p does not localize to the bud neck in small- and medium-budded cells, yet Myo2p localized to the bud neck in cells expressing just Bni1p FH1-FH2, cables randomly nucleated by Bni1p FH1-FH2 must become partially oriented to the bud neck.
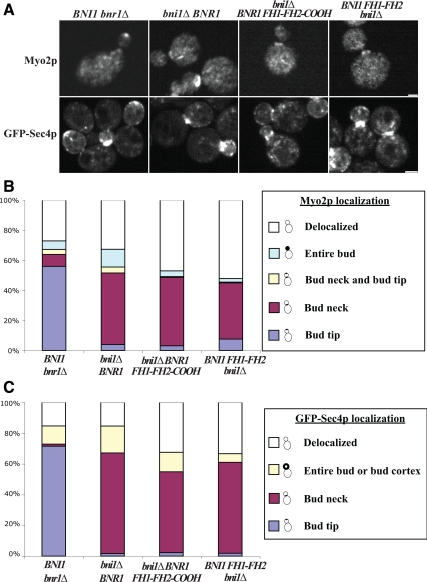
Figure 3.
Cells expressing Bnr1p FH1-FH2-COOH or Bni1p FH1-FH2 polarize Myo2p and secretory vesicles to the bud neck. (A) Localization of Myo2p (fixed cells) and GFP-Sec4p (live cells) in bni1Δ bnr1Δ cells expressing formin constructs as indicated. Bar, 2 μm. (B and C) Quantitation of the localization of Myo2p and GFP-Sec4p in small- and medium-budded cells. n ≥100 for each of at least three experiments.
An essential cargo of Myo2p is post-Golgi secretory vesicles that can be visualized in cells expressing GFP-Sec4p (Schott et al., 2002). Small- to medium-budded cells had a distribution of GFP-Sec4p that corresponds to the distribution of Myo2p, namely, at the bud cortex in Bni1p-expressing cells and at the bud neck in Bnr1p-, Bnr1p FH1-FH2-COOH–, or Bni1p FH1-FH2–expressing cells (Figure 3, A and C), further confirming that actin cables are oriented toward the bud neck in these latter three strains.
The directed movement of secretory vesicles, marked by GFP-Sec4p, provides a direct measure of cable polarity (Schott et al., 2002). Cells expressing Bnr1p FH1-FH2-COOH have more GFP-Sec4p puncta (19 ± 7 [mean ± SD], n = 44) per mother cell than those expressing Bni1p or Bnr1p (10 ± 3 [n = 35] and 8 ± 4 [n = 31], respectively; p < 0.0001). In all cells, most puncta seemed to be moving randomly. However, directed movements at similar rates (0.9–3.3 μm/s) were observed in every strain, although patterns and chances of such directed movement were different. The frequency of particles undergoing directed movement was similar, 12.8% (n = 428) for Bni1p- and 14.3% (n = 210) for Bnr1p-expressing cells. The majority of these were toward the bud (98 and 87%, respectively) as defined by their extrapolated trajectory crossing the bud neck. By contrast in Bnr1p FH1-FH2-COOH–expressing cells, 5.6% (n = 1268) of the puncta showed directed movement, probably because the random cables are short and the movements along them were not observed. 52% (37 out of 71) of the directed movements were toward the bud neck. However, most of the randomly oriented events were in the lower half of the mother cell distal. When only directed movements in the half of the mother cell adjacent to the bud were considered (accounting for 51 of the events), the majority, (34/51, or 67%) were toward the neck (Supplemental Video 1). Thus, there is a local bias for actin cable polarization toward the bud neck in cells expressing Bnr1p FH1-FH2-COOH. However, the greater number of secretory vesicles in the mother cell suggests that it takes longer for a randomly moving secretory vesicle to land on an appropriately-oriented cable to be transported.
Cells Expressing Only a Nonlocalized Formin Are Capable of Shmooing
Another form of polarized growth occurs during yeast mating, where haploid cells shmoo in response to the appropriate mating pheromone (Pruyne and Bretscher, 2000). We found that 40 ± 8, 27 ± 5, and 33 ± 1% (mean ± SD; 2 experiments) of the cells expressing only Bni1p, Bnr1p, or Bnr1p FH1-FH2-COOH, respectively, formed a shmoo 2.5 h after α-factor treatment. Cells expressing Bni1p shmooed similarly to wild-type cells with Myo2p enriched at the tip, whereas Bnr1p and Bnr1p FH1-FH2-COOH cells both had rounder shmoos, the actin cytoskeleton was not as polarized, and Myo2p was not as highly enriched at the tip (Figure 4, A and B). Labeling of the old cell wall with FITC-ConA and new cell wall growth by TRITC-ConA after α-factor treatment confirmed that the shmoo was the site of polarized growth in all three strains (Figure 4C).
Figure 4.
Role of formin proteins during mating factor induced shmooing. (A) bni1Δ bnr1Δ MAT a cells expressing either Bni1p, Bnr1p, or Bnr1p FH1-FH2-COOH were treated with 10 μM α-factor for 2.5 h and then visualized by DIC, or stained for actin, tropomyosin, or Myo2p. Bar, 2 μm. (B) Quantitation of the localization patterns of Myo2p. n ≥100 for each of at least three experiments. (C) Cells were labeled for old cell wall with FITC-ConA (green), washed, and treated with α factor for 2.5 h, and new cell wall stained with TRITC-ConA (red).
Myosin II Contributes to Cable Organization at the Bud Neck
Our results have shown that eliminating the ability of a formin to be recruited to localized sites has little effect on overall cell growth. Because the presence of actin cables nucleated by formins is essential to polarize growth (Pruyne et al., 1998; Evangelista et al., 2002; Sagot et al., 2002a); yet, cells with cables nucleated by a delocalized formin grow and have partially oriented cables, some heretofore unappreciated mechanism for actin cable polarization must exist.
One candidate is the neck-localized myosin II, whose heavy chain is encoded by MYO1, involved in cytokinesis (Bi et al., 1998; Lippincott and Li, 1998). Although Myo1p is only needed for actomyosin ring contraction during cytokinesis, it localizes to the bud neck early in the cell cycle (Bi et al., 1998; Lippincott and Li, 1998). Moreover, actin cables are less robust and less organized in cells deleted for MYO1 (Huckaba et al., 2006). Therefore, Myo1p might localize to the bud neck early in the cell cycle allowing its actin-binding head domain to contribute to actin cable orientation. To examine this possibility and to avoid the complications of a cytokinesis defect in myo1Δ cells, we expressed a headless Myo1p construct, but which is nevertheless fully functional in cytokinesis (Lord et al., 2005). In cells expressing full length Bnr1p, the growth rate was no different between those expressing full-length Myo1p or headless Myo1p. However, when only Bnr1p FH1-FH2-COOH is present, cells expressing headless Myo1p grew much slower than those expressing full-length Myo1p (Figure 5A). This growth defect is not due to a problem in cytokinesis as there is no increase in unseparated cells (Supplemental Figure S2). Cells expressing Bnr1p FH1-FH2-COOH and headless Myo1p are sick and it is difficult to quantitate the state of the actin cytoskeleton in these cells. We therefore examined the localization of Myo2p, as an indicator of how well the actin cytoskeleton is polarized. We found that when Bnr1p FH1-FH2-COOH is the only form of formin, cells expressing headless Myo1p did not concentrate Myo2p as well as those expressing full-length Myo1p, although the difference was subtle (p < 0.22; Figure 5B). Also, they seemed to have slightly more GFP-Sec4 particles than those expressing Bnr1p FH1-FH2-COOH and full-length Myo1p (Figure 5C; p < 0.0001), perhaps reflecting a decreased ability in transporting secretory vesicles to the growth site. Because of the high density of GFP-containing particles, it was difficult to identify particles with direct movement by using our standard protocol. We therefore made two changes. First, we found that diploid cells provided better resolution of the particles. Second, using shorter exposure times allowed us to capture just the relatively bright particles. With this limitation, we found that when only directed movements in the half of the mother cell adjacent to the bud were considered, less (39/68, or 57%) were toward the neck in cells expressing only Bnr1p FH1-FH2-COOH and headless Myo1p than those expressing only Bnr1p FH1-FH2-COOH and full-length Myo1p (45/59, or 76%). These results imply that bud neck-localized Myo1p can contribute to actin cable polarity through the actin-binding head domain.
DISCUSSION
In this study, we have shown that cells expressing either Bnr1p or nonlocalized Bnr1p FH1-FH2-COOH as the only form of formin grow at the same rate. This is surprising because the current model of cell polarity in yeast suggests that signals at specific localized sites on the cell cortex recruit and activate formins to assemble polarized actin cables to provide tracks for Myo2p to transport secretory vesicles that support growth (Pruyne et al., 2004b). However, we now show that eliminating the ability of the formin to be recruited to localized sites has very little effect on overall cell growth. In part, this lack of a growth defect may reflect the ability of wild-type yeast to polarize its secretory system more efficiently than is absolutely necessary. The internal pool of the secretory cargo Bgl2p was similar in cells expressing just Bni1p, Bnr1p, or Bnr1p FH1-FH2-COOH (Supplemental Figure S3), indicating no significant block in overall secretion.
Furthermore, we find that although Bnr1p FH1-FH2-COOH is not localized, cells expressing it as the only form of formin still concentrate Myo2p and secretory vesicles at the bud neck. More importantly, we find that in these cells, direct movements of the secretory vesicles are biased toward the bud neck, reflecting a local bias for actin cable polarization toward the bud neck. Consistent with this, we find that although these cells have many randomly oriented actin cables, some still seem to be oriented toward the bud neck.
What might be the basis for this cable polarization? Additional factors localized at the bud neck might activate Bnr1p FH1-FH2-COOH selectively, although such a mechanism might be expected to partially enrich Bnr1p FH1-FH2-COOH at the neck, which is not seen. The fact that Bni1p FH1-FH2, which should not be associated with the bud neck in small to medium buds, can polarize Myo2p and GFP-Sec4p to the bud neck, implies that the observed growth is not because of enrichment of the FH1-FH2 domain at the bud neck. Alternatively, the bud neck might have a factor, besides the formins, that can orient actin cables.
Our results have shown that Myo1p, a class II myosin, localized at the bud neck, may contribute to actin cable organization to the bud neck. To date, the major identified function of Myo1p in yeast is contractile ring contraction during cytokinesis (Bi et al., 1998; Lippincott and Li, 1998). However, Myo1p is localized to the bud neck very early in the cell cycle when the actomyosin ring is not yet assembled (Bi et al., 1998; Lippincott and Li, 1998). Moreover, the F-actin binding motor domain of Myo1p is not needed for its role in cytokinesis (Lord et al., 2005). We find that in cells expressing the nonlocalized Bnr1p FH1-FH2-COOH as the only form of formin, the F-actin binding head domain of Myo1p is needed to maintain a normal growth rate. Moreover, direct movements of secretory vesicles are less oriented toward the neck in cells expressing only Bnr1p FH1-FH2-COOH and headless Myo1p than those expressing only Bnr1p FH1-FH2-COOH and full-length Myo1p, indicating the cables are less oriented toward the bud neck in the former.
It is not clear yet how Myo1p can organize polarized actin filaments. There is evidence from other systems suggesting that myosin II can contribute to actin filament organization. In sea urchin coelomocytes, a “push-pull” model has been proposed where myosin II forms a ring at the perinuclear region to pull the radial actin bundles for actin-based retrograde flow (Henson et al., 1999). In migrating fibroblasts, myosin II seeds the formation of graded polarity bundles, probably by pulling the actin filaments away from the membrane (Anderson et al., 2008). A likely explanation for our observations in yeast is that Myo1p localized at the bud neck might interact with actin filaments and migrate down them to the plus end. Filaments with the correct polarity would be fed into the mother cell and support the transport of secretory vesicles into the bud. Filaments with the wrong polarity would be pulled into the bud and removed from the pool of filaments supporting secretory vesicle transport. In this way, Myo1p localized at the neck can enhance the frequency of correctly oriented filaments which can then support polarized growth.
Finally, because cells expressing only Bnr1p FH1-FH2-COOH and headless Myo1p are still viable, there must be additional mechanisms that also contribute to the polarity of actin cables polarity. Considering the importance of polarity, it is not surprising that multiple mechanisms exist to ensure the establishment and maintenance of actin cable orientation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Dave Pruyne for abundant helpful suggestions throughout this study, Dr. Matthew Lord (University of Vermont) for the MYO1 plasmids, and Dr. Erfei Bi (University of Pennsylvania) for the myo1Δ strain. This work was supported by PHS grant GM39066 to AB.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0194) on March 18, 2009.
REFERENCES
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. W., Vaughan A. N., Cramer L. P. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol. Biol. Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Maddox P., Lew D. J., Salmon E. D., McMillan J. N., Yeh E., Pringle J. R. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery S. M., Yoshida S., Pellman D. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol. Biol. Cell. 2007;18:1826–1838. doi: 10.1091/mbc.E06-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Mino A., Kikyo M., Takahashi K., Shimizu K., Takai Y. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell. 1998;9:1221–1233. doi: 10.1091/mbc.9.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D., Welch M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Goode B. L., Eck M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Henson J. H., Svitkina T. M., Burns A. R., Hughes H. E., MacPartland K. J., Nazarian R., Borisy G. G. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol. Biol. Cell. 1999;10:4075–4090. doi: 10.1091/mbc.10.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckaba T. M., Gay A. C., Pantalena L. F., Yang H. C., Pon L. A. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckaba T. M., Lipkin T., Pon L. A. Roles of type II myosin and a tropomyosin isoform in retrograde actin flow in budding yeast. J. Cell Biol. 2006;175:957–969. doi: 10.1083/jcb.200609155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kamei T., Tanaka K., Hihara T., Umikawa M., Imamura H., Kikyo M., Ozaki K., Takai Y. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:28341–28345. doi: 10.1074/jbc.273.43.28341. [DOI] [PubMed] [Google Scholar]
- Karpova T. S., McNally J. G., Moltz S. L., Cooper J. A. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J. Cell Biol. 1998;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo M., Tanaka K., Kamei T., Ozaki K., Fujiwara T., Inoue E., Takita Y., Ohya Y., Takai Y. An FH domain-containing Bnr1p is a multifunctional protein interacting with a variety of cytoskeletal proteins in Saccharomyces cerevisiae. Oncogene. 1999;18:7046–7054. doi: 10.1038/sj.onc.1203184. [DOI] [PubMed] [Google Scholar]
- Lippincott J., Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Laves E., Pollard T. D. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol. Biol. Cell. 2005;16:5346–5355. doi: 10.1091/mbc.E05-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos D., Metodiev M., Muller E., Stone D., Rose M. D. Pheromone-induced polarization is dependent on the Fus3p MAPK acting through the formin Bni1p. J Cell Biol. 2004;165:99–109. doi: 10.1083/jcb.200309089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L. Differential activities and regulation of Saccharomyces cerevisiae formin proteins Bni1 and Bnr1 by Bud6. J. Biol. Chem. 2005;280:28023–28033. doi: 10.1074/jbc.M503094200. [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 2006;70:605–645. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsa V., Klebl F., Tanner W. Purification and characterization of the Saccharomyces cerevisiae BGL2 gene product, a cell wall endo-beta-1,3-glucanase. J. Bacteriol. 1993;175:2102–2106. doi: 10.1128/jb.175.7.2102-2106.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R. D., Heuser J. A., Pollard T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K., Yamamoto Y., Nohara H., Kinoshita M., Fujiwara T., Irie K., Takai Y. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:827–839. doi: 10.1128/MCB.21.3.827-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 2000;113:571–585. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. Role of formins in actin assembly: nucleation and barbed end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Gao L., Bi E., Bretscher A. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell. 2004a;15:4971–4989. doi: 10.1091/mbc.E04-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004b;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Pruyne D. W., Schott D. H., Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. The J. Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 2002a;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 2002b;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D. H., Collins R. N., Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., Li R. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 2002;12:1864–1870. doi: 10.1016/s0960-9822(02)01238-1. [DOI] [PubMed] [Google Scholar]
- Vallen E. A., Caviston J., Bi E. Roles of Hof1p, Bni1p, Bnr1p, and Myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:593–611. doi: 10.1091/mbc.11.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. C., Pon L. A. Actin cable dynamics in budding yeast. Proc. Natl. Acad. Sci. USA. 2002;99:751–756. doi: 10.1073/pnas.022462899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.