Abstract
Eph receptor tyrosine kinases, including EphA2, are expressed in the mammary gland. However, their role in mammary gland development remains poorly understood. Using EphA2-deficient animals, we demonstrate for the first time that EphA2 receptor function is required for mammary epithelial growth and branching morphogenesis. Loss of EphA2 decreased penetration of mammary epithelium into fat pad, reduced epithelial proliferation, and inhibited epithelial branching. These defects appear to be intrinsic to loss of EphA2 in epithelium, as transplantation of EphA2-deficient mammary tissue into wild-type recipient stroma recapitulated these defects. In addition, HGF-induced mammary epithelial branching morphogenesis was significantly reduced in EphA2-deficient cells relative to wild-type cells, which correlated with elevated basal RhoA activity. Moreover, inhibition of ROCK kinase activity in EphA2-deficient mammary epithelium rescued branching defects in primary three-dimensional cultures. These results suggest that EphA2 receptor acts as a positive regulator in mammary gland development, functioning downstream of HGF to regulate branching through inhibition of RhoA. Together, these data demonstrate a positive role for EphA2 during normal mammary epithelial proliferation and branching morphogenesis.
INTRODUCTION
Mammary epithelial morphogenesis is a complex developmental process during which an extensive network of branched ducts forms from a rudimentary epithelial bud (reviewed in Hennighausen and Robinson, 2005; Watson and Khaled, 2008). This process, termed branching morphogenesis, is most active during puberty. In response to hormonal stimuli, terminal end buds (TEBs) form at the tips of the ducts and invade into the surrounding stroma. New primary ducts then form by bifurcation of the TEBs and secondary side-branches sprout laterally from the trailing ducts. This process is reiterated through branching and tissue remodeling until the entire mammary fat pad is filled with a ductal tree in the virgin gland. During pregnancy, the mammary epithelium undergoes differentiation and expands drastically to meet the demand of milk production throughout lactation. After weaning, the mammary epithelium regresses through a process of programmed cell death.
Mammary gland branching morphogenesis is regulated by endocrine hormones and local paracrine interaction between the developing epithelial ducts and their adjacent mesenchymal stroma. Although the mediators of the complex interaction in mammary gland development are not fully characterized, receptor tyrosine kinases (RTKs) are among the critical regulators of branching morphogenesis (Sternlicht et al., 2006). Hepatocyte growth factor/scatter factor (HGF/SF), a mesenchymal-derived mitogen and morphogen, induces branching morphogenesis through its receptor c-Met, which is expressed on mammary epithelial cells (reviewed in Kamalati et al., 1999; Pollard, 2001). More recently, expression of multiple Eph family RTKs has been reported in the mammary gland (reviewed in Andres and Ziemiecki, 2003). However, their role in branching morphogenesis remains to be investigated.
The Eph RTK family is the largest family of RTKs identified in the genome, with at least 15 receptors and 9 ligands identified in vertebrates (reviewed in Murai and Pasquale, 2003; Brantley-Sieders and Chen, 2004). The family is subdivided into class A and class B based on homology and binding affinity for two distinct types of membrane-anchored ephrin ligands. Class B receptors generally bind to class B ephrins that are attached to the cell membrane by a transmembrane-spanning domain, while A class receptors normally interact with glycosyl-phosphatidylinositol (GPI)-linked class A ephrins, although interclass binding does occur among certain family members (reviewed in Murai and Pasquale, 2003; Brantley-Sieders and Chen, 2004). These molecules function in cell–cell communication during embryogenesis to regulate angiogenic remodeling processes, axon guidance, and tissue boundary formation (reviewed in Poliakov et al., 2004; Pasquale, 2005). In adult organisms, members of this RTK family have been linked to tumor progression and neovascularization (reviewed in Brantley-Sieders and Chen, 2004).
The first Eph receptors discovered in mammary gland are EphB4 (myk-1) and EphA2 (myk-2; Andres et al., 1994). The EphB4 receptor is expressed predominantly on myoepithelial cells surrounding the ducts and alveoli, whereas its cognate ligand, ephrin-B2, is expressed complementarily in luminal epithelial cells. Expression of both ligand and receptor is estrogen-dependent (Nikolova et al., 1998). More recently, a genome-wide transcript analysis identified EphA2 receptor and ephrin-B1 as the only two Eph molecules that are enriched in TEBs relative to ducts (Kouros-Mehr and Werb, 2006). Functionally, overexpression of EphB4 in mammary epithelial cells in MMTV-EphB4 transgenic mice disrupts the patterning of the normal mammary ductal tree, induces angiogenesis, accelerates tumor formation, and promotes metastasis when coexpressed with MMTV-Neu in bigenic mice (Munarini et al., 2002). EphA2 receptor overexpression has been associated with many types of cancer, including breast cancer (reviewed in Kinch and Carles-Kinch, 2003; Brantley-Sieders and Chen, 2004; Ireton and Chen, 2005). In addition, EphA2 has been shown to regulate HGF-induced Madin-Darby canine kidney (MDCK) cell branching morphogenesis in three-dimensional (3D) collagen gels (Miao et al., 2003). However, the role of EphA2 in mammary gland development remains unknown.
In this study we investigated the role of EphA2 in mammary gland branching morphogenesis in EphA2-deficient mice. We found that loss of EphA2 inhibits the proliferation of the mammary epithelium and delays ductal branching necessary for complete fat pad filling. At the cellular level, EphA2-deficiency resulted in marked reduction of branching in response to HGF stimulation. This defect, at least in part, is due to misregulation of Rho family GTPase function. These results suggest that EphA2 is required for mammary gland branching morphogenesis in vivo. Taken together, these data demonstrate a positive role for EphA2 in proliferation and branching morphogenesis of normal mammary epithelium.
MATERIALS AND METHODS
Animals
Animals were housed under pathogen-free conditions, and experiments were performed in accordance with AAALAC guidelines and with Vanderbilt University Institutional Animal Care and Use Committee approval. EphA2-deficient mice were backcrossed with FVB animals for 7–10 generations before analysis. Animals that were wild-type, heterozygous, or null for ephA2 (Brantley-Sieders et al., 2004, 2005) were identified by PCR analysis of genomic DNA from tail biopsy using the following primers: 5′-GGG TGC CAA AGT AGA ACT GCG-3′ (forward), 5′-GAC AGA ATA AAA CGC ACG GGT G-3′ (neo), and 5′-TTC AGC CAA GCC TAT GTA GAA AGC-3′ (reverse).
Reagents
Antibodies used include anti-EphA2 (SC-924 2 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA; 5 μg/ml, Zymed Laboratories, Burlingame, CA; D7 clone 2 μg/ml, Upstate Biotechnology, Lake Placid, NY), anti-Ephrin-A1 (clone P1 1:200, Immunex, Seattle, WA), normal rabbit IgG (Santa Cruz Biotechnology), anti-β-tubulin (1:500, Sigma-Aldrich, St. Louis, MO), biotinylated anti-proliferating cell nuclear antigen (PCNA; 1:500, BD Biosciences, San Jose, CA). Avidin peroxidase (ABC) reagents were purchased from Molecular Probes (Eugene, OR). 4′,6-Diamidino-2 phenylindole dihydrochloride (DAPI) was purchased from Sigma-Aldrich. Liquid 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate kit was purchased from Zymed Laboratories. Recombinant murine HGF was purchased from R&D Systems (Minneapolis, MN).
Whole-Mount Mammary Gland Analyses
Whole-mount hematoxylin staining of mammary glands was performed by taking no. 4 inguinal mammary glands and fixing in 10% buffered Formalin (Fisher Scientific, Pittsburgh, PA; SF93-4) overnight at 4°C. The glands were washed in acetone, equilibrated into 100% ethanol, and stained in Mayer's hematoxylin solution (VWR Scientific, West Chester, PA) for 1 h at room temperature, light protected. After staining the glands were destained in tap water and then further destained in 50% ethanol acidified with hydrochloric acid at a 0.05 M final concentration. The glands were then dehydrated in a graded ethanol series followed by xylenes and mounted on slides for photodocumentation.
Proliferation, Apoptosis, and Immunohistochemistry Assays
Proliferation and apoptosis in mammary gland in situ were assessed by PNCA immunohistochemistry or TUNEL analysis, as previously described (Brantley-Sieders et al., 2008). For immunohistochemistry, sections were de-waxed, rehydrated, and subjected to thermal antigen retrieval in citrate buffer (2 mM citric acid, 10 mM sodium citrate, pH 6.0) using a PickCell Laboratories 2100 Retriever (Leiden, The Netherlands) according to the manufacturer's instructions. Sections were incubated with primary anti-EphA2 antibody (5 μg/ml, Zymed Laboratories), anti-ephrin-A1 antibody (1:200, Immunex), or control rabbit IgG (5 μg/ml, Santa Cruz Biotechnology) overnight at 4°C, followed by 1-h room temperature incubation with biotinylated anti-rabbit antibodies (BD Biosciences). Sections were then treated incubated with avidin-peroxidase (Vector Laboratories, Burlingame, CA), followed by DAB substrate, counterstained with hematoxylin (Fisher Scientific) and mounted.
Mammary Fat Pad Clearing and Transplantation
Fat pad clearing and transplantation of EphA2-deficient mammary tissue into wild-type hosts, as well as wild-type tissue into EphA2-deficient hosts, was performed as described previously (Brantley et al., 2001). Briefly, the endogenous epithelium was surgically removed from the right no.4 inguinal mammary gland of 3-wk-old mice by excising the portion of the fat pad between the nipple and the lymph node, which contains the endogenous epithelium rudiment. A small portion (∼2 mm2) of donor tissue from 6-wk-old female mammary glands was engrafted into the remaining fat pad. Mammary glands harboring transplanted tissue were harvested 8 wk after transplantation and processed for whole-mount staining as described above. Engraftment of exogenous mammary epithelium was verified by radial outgrowth of epithelium, versus glands contaminated with endogenous epithelium that grows directionally from the nipple toward the body cavity.
Isolation and Culture of Primary Mammary Epithelial Cells
Primary mouse mammary epithelial cells (PMECs) were isolated from FVB wild-type and EphA2-deficient FVB female mice and cultured as follows: mammary glands were collected under sterile conditions and digested at 37°C for 4 h in 3 mg/ml collagenase A (Boehringer Mannheim, Indianapolis, IN; 103578) in PBS (pH 7.4), 100 U/ml hyaluronidase (Sigma; H-4272), and 1:1000 dilution of Fungizone (Invitrogen, Carlsbad, CA; 15290-018). The cell suspension was first plated on bacterial Petri dishes for 3–5 h to separate epithelial cells from fibroblasts, which adhere to Petri dishes. The epithelium-enriched cell suspension was then plated on dishes coated with collagen (Vitrogen, Cohesion Technologies, Palo Alto, CA) in 0.02 N acetic acid, washed with PBS before addition of cell culture media consisting of serum-free DMEM-F12 (50:50; Invitrogen), 5 ng/ml estrogen (Sigma; E-4389), 1 ng/ml progesterone (Sigma; P-7556), 5 ng/ml EGF (Sigma; E-4127), and 5 μg/ml insulin (Sigma; I-1882), and cultured at 37°C in 5% CO2.
In Vitro Branching Morphogenesis Assays
HGF-induced branching of PMECs isolated from wild-type or EphA2-deficient female mice was scored in 3D mammosphere culture using a modification of previously described methods (Debnath et al., 2003). Briefly, PMECs were trypsinized, and 100,000 cells were plated on a thin layer of growth factor–reduced Matrigel (BD Biosciences) in eight-well chamber slides (BD Falcon, Oxnard, CA). Cultures were maintained in normal PMEC medium supplemented with 2% growth factor-reduced Matrigel and 5% FCS in the presence or absence of 20 ng/ml recombinant murine HGF (R&D Systems) for 5 d, changing the media after 48 h. Cultures were photographed on day 5 using an Olympus CK40 inverted microscope with digital camera (Melville, NY), and branching was scored by counting branches in four independent 10× photographs per culture condition. For some experiments, PMECs were infected with 108 pfu/ml recombinant adenoviruses harboring constitutively active RhoA (Q63L, Cell Biolabs, San Diego, CA), EphA2, or control β-galactosidase (Brantley-Sieders et al., 2008) 24 h before mammosphere culture. For ROCK inhibition studies, PMECs were pre-treated with 0, 0.1 μM, 1 μM, or 10 μM Y27632 ROCK inhibitor (Calbiochem, San Diego, CA) or vehicle control for 1 h before mammosphere culture, and cultures were treated again after 48 h for 1 h. All experiments were performed three times with 4 independent cultures per condition in each experiment.
Rho Kinase Assay
Primary mammary epithelial cells were plated in 6 well plates and stimulated with HGF (25 ng/ml) for 15 min upon reaching ∼60% confluency. Cells were harvested in a 1.0% Triton X-100 lysis buffer consisting of 50 mM Tris-HCl, pH 8.0, 1.0% Triton X-100, 1 mM PMSF, 5 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 mM sodium orthovanadate. Harvested cells were sonicated and cleared lysates were assayed for ROCK activity using the CycLex Rho-Kinase Assay (ROCK Assay) Kit (MBL International, Woburn, MA) according to manufacturer's protocol. Briefly, 10 μL of each sample was incubated with a substrate corresponding to the C terminus of recombinant Myosin Binding Subunit of myosin phosphatase (MBS). The phosphorylated form of threonine 696 of the MBS was detected by an HRP conjugated detection antibody AF20, coupled with TMB color reaction. Absorbance was measured at 450 nm, which reflects relative amount of Rho-kinase activity. For some experiments, PMECs were infected with 108 pfu/ml recombinant adenoviruses harboring EphA2, or control β-galactosidase (Brantley-Sieders et al., 2008) 48 h prior the ROCK assay.
In Situ Rho/Rac Activity Detection Assays
Detection of GTP-bound Rho and Rac in mammary gland sections was performed as described previously (Muraoka et al., 2003). Briefly, paraffin-embedded tumor sections were rehydrated, treated with 0.01% trypsin (Cellgro; Mediatech, Herndon, VA) for 5 min, and blocked for 30 min in MOM (mouse-on-mouse) diluent (Molecular Probes). Sections were incubated with purified Pak-1–binding domain–glutathione S-transferase (PBD-GST), rhotekin-binding domain GST (RBD-GST) fusion protein, or GST control in MOM diluent containing 10 mM MgCl2 for 15 min at room temperature. Binding of GST fusion proteins was detected using an anti-GST antibody (Santa Cruz Biotechnology) followed by anti-rabbit Cy3. Sections were counterstained with DAPI and photographed, and binding of GST fusion proteins was quantified based on Cy3+ pixel area using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical Analyses
Statistical analysis of developmental and in vitro studies were performed using two-tailed, paired Student's t tests.
RESULTS
Loss of EphA2 Impairs Normal Development and Architecture of the Mammary Epithelial Tree
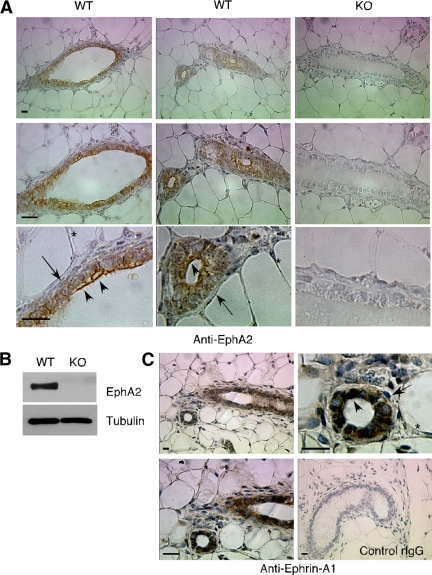
We first assessed expression of EphA2 and its preferred ligand, ephrin-A1, in normal mammary gland tissue isolated from 6-wk-old female mice. We observed EphA2 expression on the surface of luminal epithelial cells, whereas the surrounding myoepithelium and stroma did not express detectable protein under our staining conditions (Figure 1A). We confirmed specificity of staining, as well as loss of EphA2 expression in EphA2-deficient animals, by probing mammary gland tissue sections (Figure 1A) and mammary gland lysates (Figure 1B) with anti-EphA2 antibodies. Ephrin-A1 protein expression patterns were similar to those observed for EphA2, with protein detected on the surface of luminal epithelial cells (Figure 1C). These expression patterns are consistent with reported mRNA expression of EphA2 and ephrin-A1 in luminal epithelial cells (Kouros-Mehr and Werb, 2006), suggesting that this receptor–ligand pair may regulate epithelial morphogenesis.
Figure 1.
EphA2 receptor tyrosine kinase and ephrin-A1 ligand are expressed in luminal epithelial cells in virgin mammary gland tissue. (A) Immunohistochemical staining in virgin mammary tissue sections prepared from wild-type (WT) 6-wk-old virgin female mice revealed EphA2 protein expression on the surface of luminal epithelial cells (arrowheads), but no apparent expression in myoepithelial cells/fibroblasts surrounding ducts (arrows) or in fatty tissue (*). Staining specificity, as well as EphA2 deficiency, was confirmed by probing mammary tissue sections prepared from age-matched EphA2-deficient (KO) virgin female mice. (B) EphA2 protein deficiency in KO mice versus WT was also confirmed by immunoblot analysis of protein lysates prepared from whole mammary gland tissue. Uniform loading was confirmed by reprobing blots for expression of tubulin. (C) We also observed expression of ephrin-A1, the primary ligand for EphA2 receptor, in luminal epithelial cells (arrowheads), versus surrounding stromal cells (arrows) and fat (*) in tissue sections prepared from 6-wk-old wild-type virgin female mice. Staining specificity was validated by probing tissue with control rabbit IgG (rIgG). Scale bars, 10 μm.
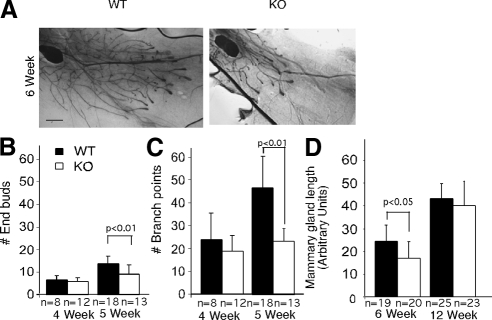
To determine the role of EphA2 in mammary gland morphogenesis, we performed whole-mount analysis of mammary glands from EphA2-deficient mice and wild-type littermate controls following a time course. In control animals, at the onset of puberty at ∼3.5 wk, the rudimentary mammary epithelium anlagen undergoes rapid proliferation and invades the stroma by directional growth and branching, giving rise to the characteristic mammary epithelial tree that populates the entire mammary fat pad at 10 wk of age. In contrast, the EphA2-deficient animals exhibit severe growth retardation (Figure 2). This defect is more prominent at 5 and 6 wk of age, with reduced branching activity and decreased numbers of TEBs (Figure 2, A–D). In addition, outgrowth of the mammary epithelial tree was also retarded in these animals (Figure 2, A and D). In mature animals at 12 wk, ∼50% of the EphA2-deficient animals exhibited a fully formed mammary ductal tree (Figure 2D). Failure of the mammary epithelium to fully penetrate the mammary fat pad persists in ∼30% of EphA2-deficient MMTV-Neu animals at 8 mo (Brantley-Sieders et al., 2008). We did not observe any defects in expansion of the mammary epithelium during pregnancy, in function during lactation, or in apoptosis during postlactational involution in EphA2-deficient animals relative to controls (data not shown).
Figure 2.
EphA2-deficiency impairs normal development and architecture of the mammary epithelial tree. (A) Whole-mount hematoxylin staining of number 4 inguinal mammary glands collected from WT and KO FVB female animals 5 and 6 wk after birth. Scale bar, 200 μm. Quantification of numbers of terminal end buds (B) and branching points (C) in mammary gland whole mounts from 4- and 5-wk-old mice. (D) Mammary gland whole mounts prepared from KO EphA2 and WT EphA2 animals at 6 wk of age and 12 wk of age were analyzed for epithelial penetration into the fat pad by measuring the distance between the lymph node to the tips of the epithelial tree in gland. Quantification of TEBs, branching points, and the degree of epithelial penetration through the mammary fat pad was performed by analyzing whole-mount preparations from >10 independent animals per developmental stage per genotype. Statistical analyses were performed using two-tailed, paired Student's t tests.
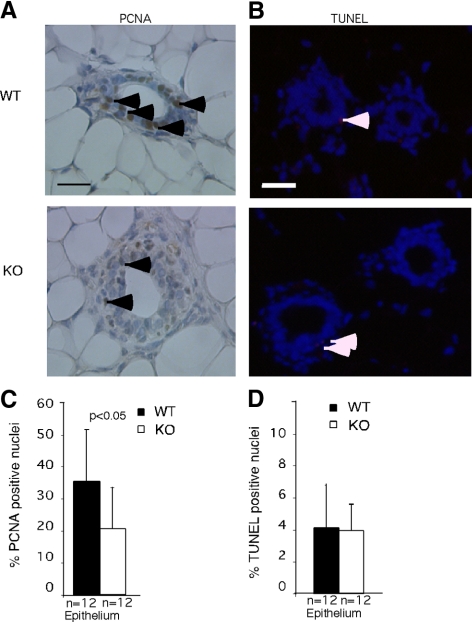
To dissect cellular mechanisms responsible for defects in outgrowth and branching in EphA2-deficient mice, we compared proliferation and apoptosis of mammary epithelial cells in these and control animals. Cell growth was assessed by quantifying expression of PCNA, a marker for actively dividing cells. As shown in Figure 3, high proliferative activity was observed in the mammary epithelium of 6-wk-old control wild-type mice. In contrast, cell proliferation was significantly reduced in EphA2-deficient littermates. We observed no significant difference in epithelial content and proliferation between control and EphA2-deficient mammary glands harvested from 12-wk-old mature female animals, indicating that mammary epithelial growth had recovered in EphA2-deficient animals (data not shown). Nor did we detect any difference in the levels of apoptosis in mammary gland between EphA2-null animals and wild-type control littermates, either at 6 or 12 wk of age (Figure 3C).
Figure 3.
EphA2 deficiency inhibits proliferation but has no effect on apoptosis. (A) PCNA immunohistochemistry in mammary gland tissue sections from wild-type and EphA2-deficient animals. Arrowheads indicate PCNA+ nuclei. Scale bars, 50 μm. (B) Proliferation was assessed by quantification of nuclear staining for PCNA in tissue sections. A significant reduction in the percentage of PCNA-positive nuclei relative to total nuclei was observed in KO EphA2 mammary glands, compared with WT controls (p < 0.05). (C) No significant change in the percentage of apoptotic nuclei, as assessed by TUNEL assay, was observed in KO mammary glands relative to WT controls. Quantification of the percentage of PCNA+ or TUNEL+ nuclei was performed by analyzing >10 independent animals per developmental stage per genotype. Five random 20× fields/tissue section were photographed for PCNA and TUNEL per each independent animal/genotype. Statistical analyses were performed using two-tailed, paired Student's t tests.
EphA2 Deficiency Inhibits HGF-induced Mammary Epithelial Cell Branching Morphogenesis
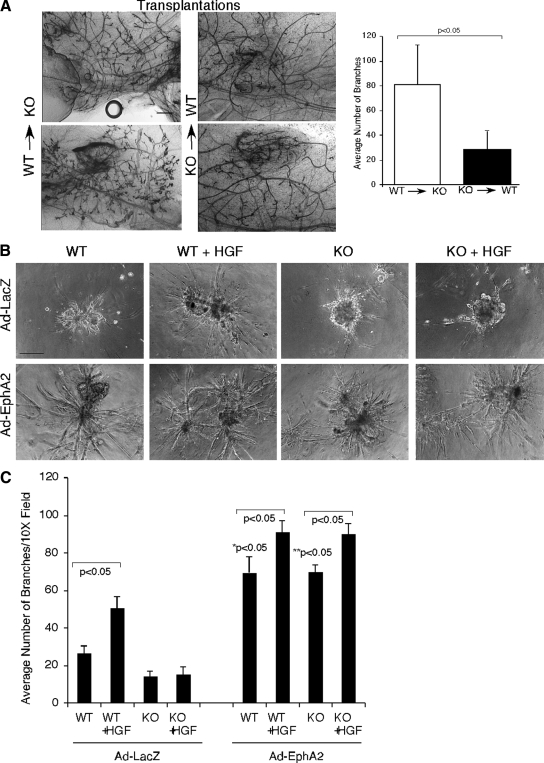
Although we did not detect expression of EphA2 in the mammary gland stroma in tissue sections (Figure 1A), lysates from primary mammary fibroblast cultures revealed low level EphA2 expression (data not shown). Therefore, reduced mammary gland branching morphogenesis observed in EphA2-null animals could be due to either defects in mammary epithelial cells or the surrounding mesenchymal stroma. To distinguish the role of EphA2 in epithelial cells versus stroma, we performed reciprocal transplantation experiments in which we grafted EphA2-deficient mammary gland tissue into the cleared fat pad of wild-type female mice, as well as wild-type mammary gland tissue into the cleared fat pad of EphA2-deficient female mice. Interestingly, we observed failed engraftment of EphA2-deficient mammary epithelium in four of 10 animals, versus one of 10 animals engrafted with wild-type epithelium. This observation is consistent with proliferation and outgrowth defects in endogenous EphA2-deficient epithelium (Figures 2 and 3). For animals in which the donor epithelium did engraft, we observed diminished branching in wild-type animals harboring EphA2-deficient epithelium, whereas wild-type epithelium grafted into EphA2-deficient animals displayed robust branching 8 wk after engraftment (Figure 4A). These data suggest that the branching defects observed in EphA2-deficient animals are due to loss of EphA2 function in mammary epithelium versus stroma.
Figure 4.
EphA2 activity is required in mammary epithelium for optimal branching in vivo, as well as for HGF-induced epithelial cell branching morphogenesis in Matrigel. (A) To determine if defective epithelial branching in EphA2-deficient (KO) mice was due to loss of EphA2 function in epithelium versus stroma, we transplanted KO mammary tissue into the cleared fat pads of WT female mice, as well as WT mammary tissue into the cleared fat pads of KO female mice. Photomicrographs display two independent whole-mount preparations of mammary fat pads harboring transplanted tissue. Although WT epithelium displayed robust branching in KO stroma 8-wk after transplantation, branching of KO epithelium was significantly diminished in WT hosts (p < 0.05; two-tailed, paired Student's t test). Scale bar, 200 μm. Quantification of branching was performed by analyzing whole-mount preparations from five independent transplants/condition. (B) Primary mammary epithelial cells isolated from WT or KO animals were transduced with adenoviruses expressing EphA2 (Ad-EphA2) or control LacZ (Ad-LacZ) and plated on growth-factor–reduced Matrigel with or without HGF for 5 d and photographed. HGF enhances branching morphogenesis in WT, but not KO, mammary epithelial cells. The defects in KO cells was rescued by reexpressing wild-type EphA2 receptor. Scale bar, 25 μm. (C) Branching morphogenesis was quantified by counting the number of branches per photograph in four independent samples per culture condition in three independent experiments. Statistical analyses were performed using two-tailed, paired Student's t tests.
To validate the data derived from reciprocal transplant experiments in an in vitro system where levels of specific branching morphogens may be manipulated, we isolated PMECs from wild-type and EphA2-deficient mice and tested their ability to branch and invade into matrix in a 3D basement membrane gel. We did not observe pronounced differences in low-level branching between wild-type and EphA2-deficient cells in untreated cultures. A number of factors acting in a paracrine manner are known to regulate mammary gland development via branching morphogenesis (Wiseman and Werb, 2002). In particular, HGF promotes ductal outgrowth and tubule formation in the mammary gland (Pollard, 2001). We thus analyzed the role of EphA2 in HGF-induced mammary epithelial cell branching morphogenesis. As shown in Figure 4, primary mammary epithelial cells form spheroid structures in growth factor reduced Matrigel, and EphA2-deficient cultures display diminished branching relative to wild-type control cells. In response to HGF stimulation, wild-type spheroids undergo extensive remodeling and branching. In contrast, EphA2-deficient spheroids fail to undergo branching morphogenesis, displaying significantly fewer branches relative to wild-type cells in response to HGF stimulation. Overexpression of wild-type EphA2 receptor in primary mammary epithelial cells via adenovirus transduction not only rescued phenotypes in EphA2-deficient cells, but also drastically enhanced branching morphogenesis in wild-type cells. Taken together, these data suggest that EphA2 receptor is required for HGF-induced branching morphogenesis.
Increased RhoA Activity in EphA2-deficient Cells Inhibits Mammary Epithelial Cell Branching
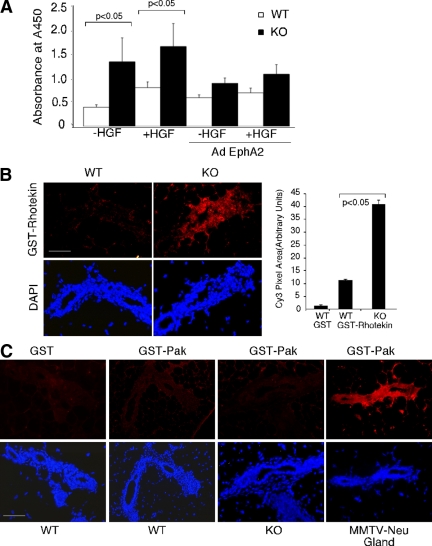
We next investigated the molecular mechanisms through which EphA2 regulates mammary epithelial cell branching morphogenesis. Dynamic regulation of the actin cytoskeleton is critical in a number of cellular processes including cell migration and branching morphogenesis. RhoA GTPases are key regulators of actin stress fiber formation and are necessary for cell migration (Ridley, 2001a,b; Connolly et al., 2002). Moreover, Ewald et al. (2008) recently reported that inhibition of ROCK kinase, a downstream effector of RhoA, results in hyperbranched mammary epithelium in organoid cultures, suggesting that RhoA is crucial for proper branching morphogenesis in mammary epithelial development. To investigate whether RhoA GTPase is involved in HGF/EphA2 induced branching of mammary epithelial cells, we measured ROCK kinase activity in wild-type and EphA2-deficient mammary epithelium. As shown in Figure 5A, HGF stimulation of primary mammary epithelial cells for 15 min induced ROCK kinase activity in wild-type cells. Interestingly, the basal level of ROCK activity is markedly increased in EphA2-deficient cells, and this level does not change in response to HGF stimulation. Experiments in which we restored expression of EphA2 with adenoviral infection EphA2-deficient cells rescued the phenotype by reducing ROCK activity to near wild-type levels, though levels of ROCK activity remained similar in wild-type cells upon adenoviral EphA2 overexpression (Figure 5A). To determine the level of active RhoA GTPase in vivo, we performed effector-binding assays on mammary gland tissue sections in situ (Muraoka et al., 2003). Consistent with results from ROCK assay in vitro, RhoA activity is markedly increased in EphA2-deficient mammary gland epithelium in 6-wk-old mice, compared with that in wild-type control littermates, as judged by GST-rhotekin binding detected by anti-GST antibodies (Figure 5B). As a balance of RhoA and other small Rho GTPases activities (such as Rac1 and Cdc42) often determines the biological outcome in branching morphogenesis, we assayed Rac1 and Cdc42 activity in situ using GST-Pak proteins. We did not detect any significant levels of Rac1 and Cdc42 activities in the 6-wk-old mammary gland sections (Figure 5C).
Figure 5.
Increased RhoA activity in EphA2-deficient mammary epithelial cells. (A) Rho kinase activity was measured by an immune-based kinase assay, as described in Materials and Methods. Rho kinase activity was significantly increased in EphA2-deficient mammary epithelial cells (p < 0.05; two-tailed, paired student's t test). Data are a representation of three independent experiments. (B) Rho GTPase activities were analyzed in 6-wk-old mammary glands in situ by incubating tissue sections with GST-rhotekin or GST-Pak for the detection of RhoA and Rac1/Cdc42 activities, respectively; followed by detection using a Cy3-conjugated anti-GST antibody. Scale bars, 50 μm. Rho activity was quantified based on Cy3-positive pixel area using NIH ImageJ software. Quantification of Rho activity was performed in four 40× fields/section in tissue sections from three independent wild-type or EphA2-deficient mammary animals. Statistical analyses were performed using two-tailed, paired Student's t tests.
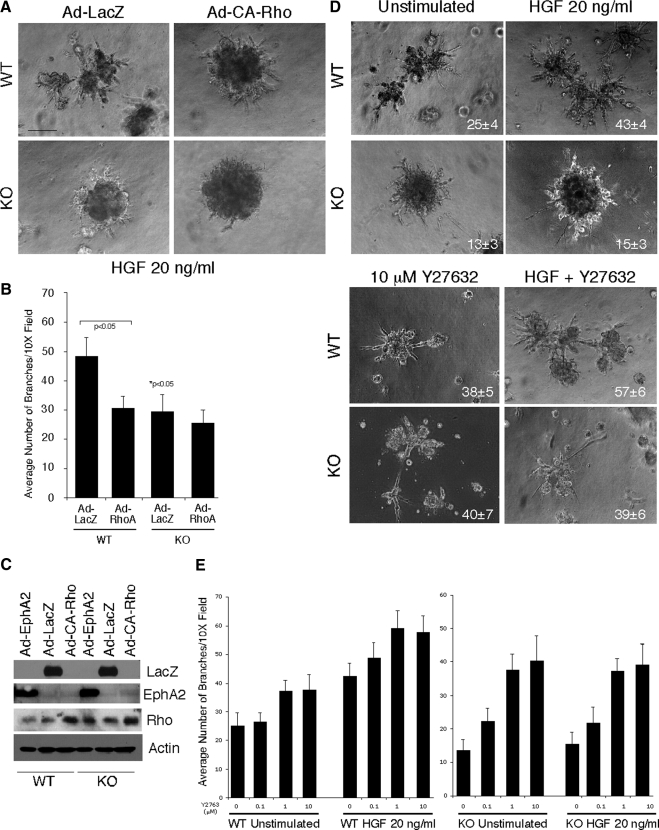
To determine the functional relevance of RhoA activity in EphA2-mediated branching morphogenesis, we first examined HGF-induced branching of primary mammary epithelial cells in cells expressing control adenovirus LacZ versus adenovirus harboring constitutively active RhoA mutant (CA-Rho). CA-RhoA significantly inhibited branching in wild-type cultures and slightly reduced branching in EphA2-deficient cultures (Figure 6, A and B). We also assessed branching in the presence or absence of Y27632, an inhibitor of ROCK kinase. Treatment with Y27632 rescued branching defects in EphA2-deficient cells in both the presence and absence of HGF (Figure 6D). HGF stimulation enhanced Y27632-mediated branching in wild-type cells in a dose-dependent manner (Figure 6E). Interestingly, HGF did not affect Y27632-mediated branching in EphA2-deficient cells. As HGF-mediated branching is impaired in EphA2-deficient mammary epithelium (Figure 4) and as the ROCK kinase inhibitor rescues the branching defects in EphA2-deficienct cells in either the presence or absence of HGF (Figure 6E), these data support a model (Figure 7) in which EphA2 functions downstream of HGF to regulate mammary epithelial branching through inhibition of RhoA.
Figure 6.
EphA2-dependent branching morphogenesis is dependent on RhoA activity. (A) Primary mammary epithelial cells isolated from WT or KO animals were transduced with adenoviruses expressing a constitutively activated RhoA (CA-Rho) or control LacZ. Cells were plated on growth-factor reduced Matrigel with 20 ng/ml HGF for 5 d and photographed. Branching in KO LacZ control cells was significantly diminished relative to WT LacZ control cells in response to HGF (* p < 0.05; two-tailed, paired Student's t test). Expression of the Q63L RhoA inhibited HGF-induced branching morphogenesis in WT cells. Scale bar, 25 μm. (B) Branching morphogenesis was quantified by counting the number of branches per photograph in four independent samples per culture condition in three independent experiments. Statistical analyses were performed using two-tailed, paired Student's t tests. (C) Expression of wild-type EphA2, CA-Rho, and β-galactosidase via adenovirus transduction was confirmed by immunoblot analysis. (D) Branching was also assessed in WT and KO primary mammary epithelial cells in the presence or absence of 20 ng/ml HGF and upon treatment with the Rho kinase inhibitor 10 mM Y27632. As observed previously, WT, but not KO, cells displayed elevated branching in response to HGF (p < 0.05 WT untreated vs. WT + HGF). Branching was elevated in both WT and KO cells in the presence of Y27632. Although the addition of HGF enhanced Y27632-mediated branching in WT cells, adding HGF had no effect on Y27632-mediated branching in KO cells (p < 0.05 WT vs. WT Y27632 and WT Y27632 vs. Y27632 + HGF; p < 0.05 KO vs. KO Y27632; two-tailed, paired Student's t tests). (E) The differential effects of HGF Rho-dependent branching for WT versus KO cells was confirmed by a dose response assay in which cultures were treated with 0, 0.1, 1, or 10 μM Y27632 in the presence or absence of HGF.
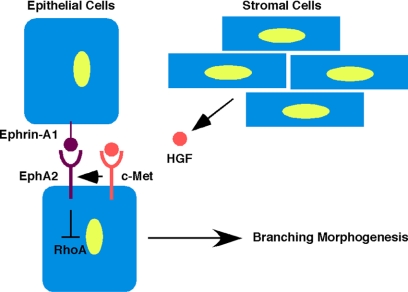
Figure 7.
Model for HGF-mediated regulation of EphA2 in mammary epithelial branching morphogenesis. HGF produced by mesenchymal cells within mammary stroma binds to c-Met receptor, expressed on mammary epithelium. Through activation of EphA2 receptor function by an as yet unidentified mechanism, Rho activity is then down-regulated to promote branching morphogenesis of the developing mammary epithelium during puberty.
DISCUSSION
Expression of several Eph receptor tyrosine kinases in mammary gland has been reported (Andres and Ziemiecki, 2003). However, their role in mammary gland development remains poorly understood. Using EphA2-null animals, we demonstrated for the first time that EphA2 receptor function is required for mammary gland branching morphogenesis. Loss of EphA2 resulted in decreased penetration of mammary epithelium into the fat pad, reduced proliferation of epithelial cells, and inhibition of mammary epithelial branching. In addition, HGF-induced mammary epithelial cell migration and branching morphogenesis was significantly reduced in EphA-deficient cells, compared with that in wild-type cells. These results suggest that EphA2 receptor acts as a positive regulator in mammary gland development.
Other studies, however, suggest that Eph receptors may inhibit mammary gland morphogenesis. Overexpression of EphB4 in mammary epithelium under the control of MMTV promoter/enhancer led to less branching activity, reduced alveolar buds, and a decrease in proliferation of mammary epithelial cells (Munarini et al., 2002). Although not observed in mammary epithelium, this phenotype is reminiscent of the effect of EphA receptor activation seen in MDCK cells in response to HGF-induced branching morphogenesis in collagen gels (Miao et al., 2003). In this model, costimulation of MDCK cells with ephrin-A1 and HGF inhibited sprouting and induced the collapse of preexisting branches. These results suggest that high levels of Eph receptor signaling above the endogenous level in epithelial cells, either by overexpression of receptor or exogenous stimulation with a large dose of ephrin ligand, may inhibit branching morphogenesis. However, low levels of endogenous EphA2 receptor are required for proper mammary gland development in vivo based on our data.
Epithelial branching morphogenesis is a fundamental biological process underlying the development of many organs. In breast tissue, epithelial branching morphogenesis is driven by endocrine hormonal stimuli that elicit local paracrine interactions between the developing epithelial ducts and their adjacent mesenchymal stroma. Cytokines and growth factors, such as HGF, FGF, TGF-β, and amphiregulin are among molecules that are critical in local regulation of branching morphogenesis (Pollard, 2001; Sternlicht et al., 2006). A common pathway activated downstream of these signaling molecules is the Rho family of small GTPases. These proteins cycle between an inactive, GDP-bound state and an active, GTP-bound state, regulated by guanine nucleotide exchange factors (GEFs) that exchange GDP for GTP and GTPase activating proteins (GAPs) that promote hydrolysis of GTP to GDP (Ridley, 2001a,b). Recently, RhoA, Rac-1, and Cdc42 GTPases have emerged as critical mediators of Eph signal transduction, as association of several Eph RTK family members with GEFs concomitant with activation of Rho family GTPases has been observed in a variety of cell types. Interactions between Eph RTKs and the GEFs ephexin, intersectin, and kalarin link Eph-mediated activation of Rho family GTPases to growth-cone collapse (ephexin) or dendritic spine morphogenesis (intersectin and kalarin) in neuronal patterning during embryogenesis (reviewed in Murai and Pasquale, 2003; Brantley-Sieders and Chen, 2004; Pasquale, 2005). More recently, Eph signaling through Rho family GTPases has been linked to vascular remodeling and angiogenesis (Brantley-Sieders et al., 2004; Hunter et al., 2006). In MDCK epithelial cells, Miao et al. (2003) reported that ephrin-A1 has no effect on RhoA but inhibits Rac1 activation induced by HGF. Here we show that EphA2-dependent RhoA activity modulates HGF-induced branching morphogenesis in mammary epithelial cells. First, loss of EphA2 led to a constitutive higher level of RhoA GTPase activity, which is insensitive to HGF stimulation (Figure 5, A and B). Interestingly, Rac1 activity did not appear to be significantly altered in EphA2-deficient mice, indicating that RhoA activity is a primary target regulated by the EphA2 receptor (Figure 5C). Furthermore, a ROCK kinase inhibitor, Y27632, rescued branching defects in EphA2-deficient cells in 3D culture (Figure 6), functionally linking EphA2-dependent RhoA activity and branching morphogenesis.
Regulation of Rho GTPase in mammary epithelium is complex. For example, targeted disruption of p190-B RhoGAP inhibits mammary epithelial ductal outgrowth, and heterozygous female mice display reduced proliferation within TEBs and delayed outgrowth of mammary ducts (Chakravarty et al., 2000). Because deletion of this GAP, which negatively regulates Rho, results in elevated Rho activity, these data are consistent with our findings that elevated RhoA function in virgin mammary epithelium is associated with decreased proliferation and ductal outgrowth in EphA2-deficient mammary epithelium. Interestingly, overexpression of the same RhoGAP in mammary epithelium elevates branching in transgenic animals, though ductal elongation is still delayed (Vargo-Gogola et al., 2006). These data suggest that disruption of Rho activity in mammary epithelium, either positive or negative, has a profound impact on epithelial morphogenesis in vivo. This hypothesis is consistent with the apparent differential regulation of Rho by EphA2 signaling in normal versus transformed mammary epithelium, which is influenced by cooperative signaling pathways. In the present study, loss of EphA2 results in elevated RhoA activity in mammary epithelium that is accompanied by suppression of ductal outgrowth in vivo. Moreover, impaired HGF-mediated branching in primary mammary epithelial cells derived from EphA2-deficient mice is rescued by inhibition of the downstream effector ROCK in vitro. These data are consistent with reported hyperbranching of mammary epithelial organoid cultures upon treatment with the same ROCK inhibitor (Ewald et al., 2008). In the normal mammary development, EphA2 may serve to restrict levels of active RhoA, enabling HGF to maintain levels of activity that promote the proper balance between branching and ductal outgrowth necessary for normal epithelial morphogenesis. By contrast, elevated EphA2 expression in normal mammary epithelial cells via adenoviral transduction enhances branching. These data are consistent with reports that EphA2 overexpression in nontransformed MCF10A cells confers malignant transformation and tumor forming potential in vivo (Zelinski et al., 2001; Carles-Kinch et al., 2002), and overexpression in 4T1 mouse mammary adenocarcinoma cells enhances tumor progression and metastasis in vivo (Fang et al., 2005). In MMTV-Neu mice, loss of EphA2 diminishes tumorigenesis and metastasis, as well as reducing levels of active RhoA (Brantley-Sieders et al., 2008). Thus, overexpression of EphA2, as well as cooperation with other oncogenic pathways in the context of mammary epithelial neoplasia, appears to enhances RhoA.
Interestingly, we did observe diminished RhoA activity in EphA2-deficient mammary epithelium relative to wild-type controls in 5-wk-old mice (data not shown), during which time the epithelium is still actively growing and branching. This observation suggests that EphA2 might also regulate Rho activity differentially at different stages of mammary epithelial development and/or malignant progression. For example, in normal mammary epithelial cells, ephrin-A ligands, such as ephrin-A1, may engage EphA2 receptors on adjacent cells and disrupt activation of Rho GTPase, thus alleviating inhibition of epithelial branching morphogenesis. We previously reported that EphA2 physically and functionally interacts with ErbB2 and that coexpression of ErbB2 was sufficient to induce phosphorylation of EphA2 in the absence of exogenous ephrin-A1 ligand. Moreover, EphA2 deficiency results in impaired Rho activation in MMTV-Neu tumors and diminished motility of primary tumor cells (Brantley-Sieders et al., 2008). Cell motility in EphA2-deficient MMTV-Neu tumor cells was rescued by overexpression of activated RhoA. Thus, EphA2 receptor activation by ErbB2 and/or other receptor tyrosine kinases might enhance Rho activity, cell motility, and malignancy in the context of cancer cells in which weakened cell–cell contacts could impair interaction between EphA2 and endogenous ligands. This might also be true in 5-wk-old mammary glands, in which active growth might also diminish cell–cell contact and interaction between EphA2 and ephrin-A1 on adjacent luminal epithelial cells. It will be of great interest to investigate differential regulation of Rho family GTPases by EphA2 in normal mammary epithelium versus mammary adenocarcinoma.
In conclusion, we provide the first evidence for EphA2 receptor regulation of normal growth and branching morphogenesis in normal mammary epithelial development. Based on reciprocal transplantation and primary epithelial culture studies, EphA2 function specifically in mammary epithelium, rather than mesenchymal stroma, appears to mediate these processes. Our data suggest that EphA2 receptor functions downstream of HGF to regulate mammary epithelial branching through inhibition of RhoA GTPase.
ACKNOWLEDGMENTS
We thank Yoonha Hwang for expert technical assistance. This work was supported by National Institutes of Health (NIH) Grants CA95004 and CA114301 (J.C.), NIH Grant CA1179151 (D.B.-S.), and Department of Defense predoctoral fellowship W81XWH-08-1-0249 (D.V.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0378) on March 25, 2009.
REFERENCES
- Andres A. C., Reid H. H., Zurcher G., Blaschke R. J., Albrecht D., Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–1467. [PubMed] [Google Scholar]
- Andres A. C., Ziemiecki A. Eph and ephrin signaling in mammary gland morphogenesis and cancer. J. Mammary Gland Biol. Neoplasia. 2003;8:475–485. doi: 10.1023/B:JOMG.0000017433.83226.22. [DOI] [PubMed] [Google Scholar]
- Brantley D. M., Chen C. L., Muraoka R. S., Bushdid P. B., Bradberry J. L., Kittrell F., Medina D., Matrisian L. M., Kerr L. D., Yull F. E. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol. Biol. Cell. 2001;12:1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., Caughron J., Hicks D., Pozzi A., Ruiz J. C., Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J. Cell Sci. 2004;117:2037–2049. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., Fang W. B., Hicks D. J., Zhuang G., Shyr Y., Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–1886. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles-Kinch K., Kilpatrick K. E., Stewart J. C., Kinch M. S. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- Chakravarty G., Roy D., Gonzales M., Gay J., Contreras A., Rosen J. M. P190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ. 2000;11:343–354. [PubMed] [Google Scholar]
- Connolly J. O., Simpson N., Hewlett L., Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol. Biol. Cell. 2002;13:2474–2485. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S. K., Brugge J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W. B., Brantley-Sieders D. M., Parker M. A., Reith A. D., Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Robinson G. W. Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hunter S. G., Zhuang G., Brantley-Sieders D., Swat W., Cowan C. W., Chen J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol. Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton R. C., Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr. Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- Kamalati T., Niranjan B., Yant J., Buluwela L. HGF/SF in mammary epithelial growth and morphogenesis: in vitro and in vivo models. J. Mammary Gland Biol. Neoplasia. 1999;4:69–77. doi: 10.1023/a:1018756620265. [DOI] [PubMed] [Google Scholar]
- Kinch M. S., Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin. Exp. Metastasis. 2003;20:59–68. doi: 10.1023/a:1022546620495. [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H., Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Nickel C. H., Cantley L. G., Bruggeman L. A., Bennardo L. N., Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J. Cell Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munarini N., Jager R., Abderhalden S., Zuercher G., Rohrbach V., Loercher S., Pfanner-Meyer B., Andres A. C., Ziemiecki A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J. Cell Sci. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- Murai K. K., Pasquale E. B. ‘Eph’ective signaling: forward, reverse and crosstalk. J. Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Muraoka R. S., Koh Y., Roebuck L. R., Sanders M. E., Brantley-Sieders D., Gorska A. E., Moses H. L., Arteaga C. L. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol. Cell Biol. 2003;23:8691–8703. doi: 10.1128/MCB.23.23.8691-8703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova Z., Djonov V., Zuercher G., Andres A. C., Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J. Cell Sci. 1998;111(Pt 18):2741–2751. doi: 10.1242/jcs.111.18.2741. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Poliakov A., Cotrina M., Wilkinson D. G. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pollard J. W. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3:230–237. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001a;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho GTPases and cell migration. J. Cell Sci. 2001b;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Kouros-Mehr H., Lu P., Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T., Heckman B. M., Gunther E. J., Chodosh L. A., Rosen J. M. P190-B Rho GTPase-activating protein overexpression disrupts ductal morphogenesis and induces hyperplastic lesions in the developing mammary gland. Mol. Endocrinol. 2006;20:1391–1405. doi: 10.1210/me.2005-0426. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Khaled W. T. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- Wiseman B. S., Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski D. P., Zantek N. D., Stewart J. C., Irizarry A. R., Kinch M. S. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]