Abstract
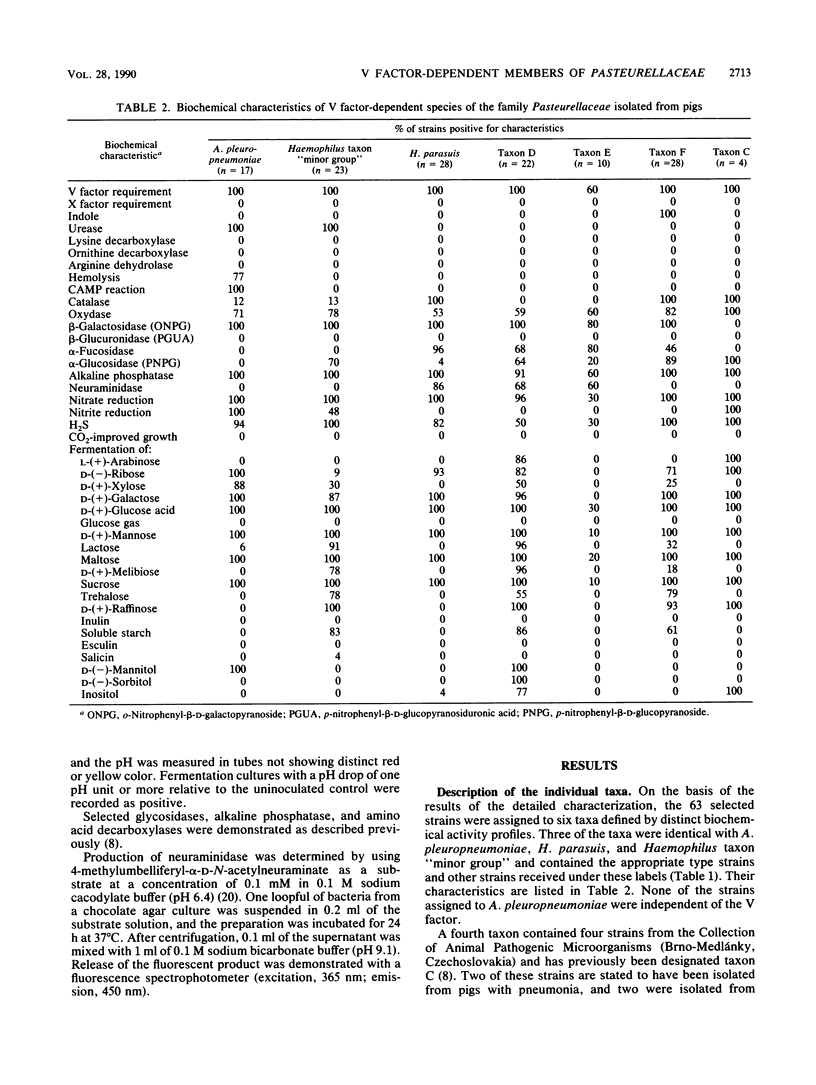
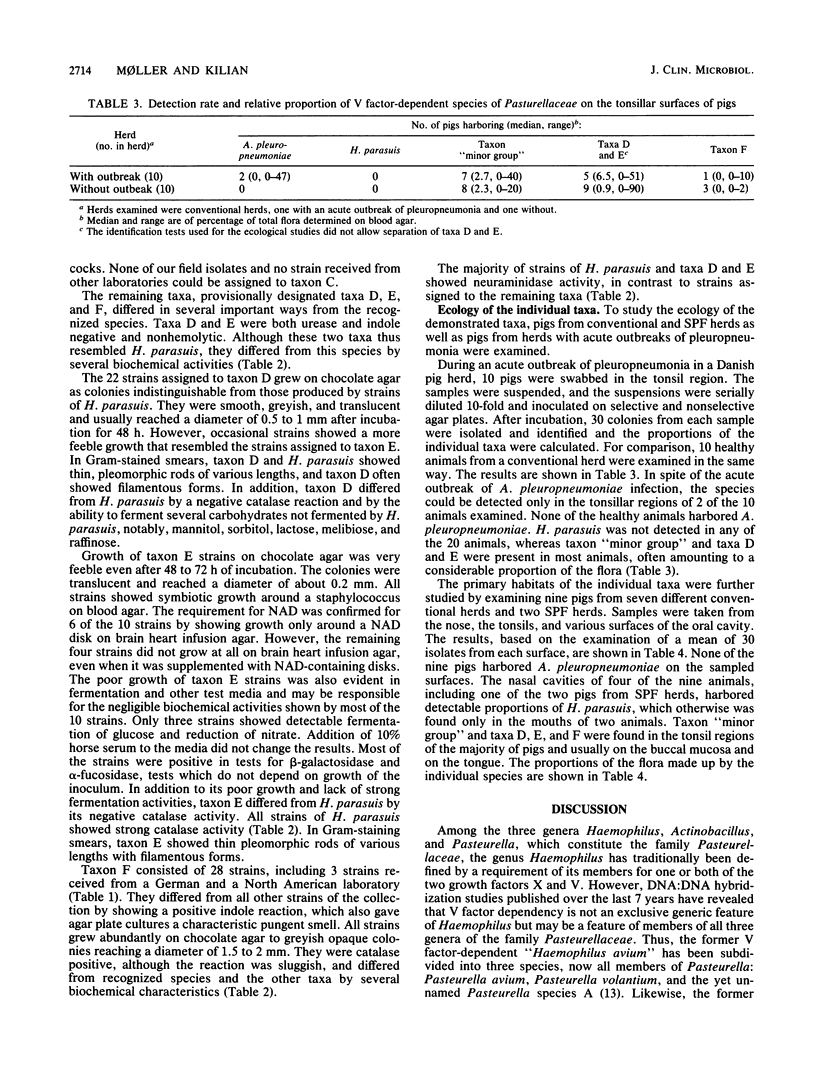
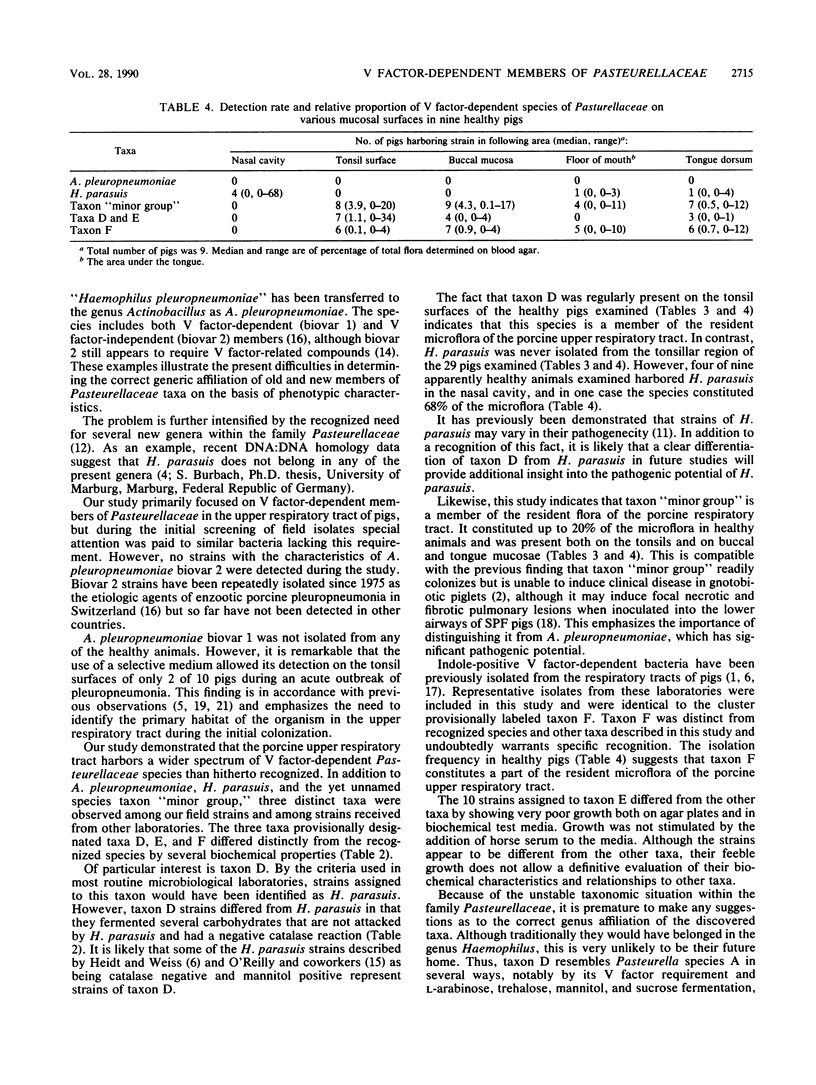
A study was performed to obtain a better understanding of the diversity and ecology of members of the family Pasteurellaceae in the porcine respiratory tract. A collection of 132 V factor-dependent strains of Pasteurellaceae selected from porcine field isolates mainly from the respiratory tract were subjected to detailed characterization. In addition to the three hitherto recognized species Actinobacillus pleuropneumoniae, Haemophilus parasuis, and Haemophilus taxon "minor group," three distinct taxa were observed. Some of these taxa, which are provisionally designated taxa D, E, and F, would by traditional criteria be mistaken for H. parasuis but differed by several biochemical characteristics. To study the ecology of the V factor-dependent species, swabs from the nasal and oral cavities of 29 pigs were cultivated on selective and nonselective media. By studying approximately 30 isolates from each sample, the distribution and relative proportion of the individual taxa were determined. A. pleuropneumoniae was detected in samples from the tonsil areas of only two acutely ill animals. H. parasuis was isolated from the nasal cavities of four out of nine healthy pigs but from the oral cavities of only two animals. In contrast, taxon "minor group" and taxa D, E, and F were present in the oral cavities of the majority of pigs but were not detected in samples from their nasal cavities. The results indicate that all the observed V factor-dependent species of Pasteurellaceae except A. pleuropneumoniae, are members of the resident microflora of various mucosal surfaces of the porcine upper respiratory tract.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke E., Weiss R., Seipp A. Untersuchungen des Aminosäure- und Kohlenhydrat-Stoffwechsels porciner Haemophilus-Stämme mittels Dünnschichtchromatographie. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):495–505. [PubMed] [Google Scholar]
- Brandreth S. R., Smith I. M. Lack of pathogenicity of haemophili of the 'minor group' taxon for the gnotobiotic piglet. Res Vet Sci. 1986 Mar;40(2):273–275. [PubMed] [Google Scholar]
- De Ley J., Mannheim W., Mutters R., Piechulla K., Tytgat R., Segers P., Bisgaard M., Frederiksen W., Hinz K. H., Vanhoucke M. Inter- and intrafamilial similarities of rRNA cistrons of the Pasteurellaceae. Int J Syst Bacteriol. 1990 Apr;40(2):126–137. doi: 10.1099/00207713-40-2-126. [DOI] [PubMed] [Google Scholar]
- Gilbride K. A., Rosendal S. Evaluation of a selective medium for isolation of Haemophilus pleuropneumoniae. Can J Comp Med. 1983 Oct;47(4):445–450. [PMC free article] [PubMed] [Google Scholar]
- Heidt M., Weiss R. Zum Vorkommen bakterieller Infektionserreger in pathologisch-anatomisch veränderten Lungen von Schweinen unter besonderer Berücksichtigung der Gattung Haemophilus. 2. Biochemische Untersuchungen an Haemophilus-Stämmen. Berl Munch Tierarztl Wochenschr. 1982 Dec 1;95(23):453–458. [PubMed] [Google Scholar]
- Kilian M. A rapid method for the differentiation of Haemophilus strains. The porphyrin test;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):835–842. doi: 10.1111/j.1699-0463.1974.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Morozumi T., Nicolet J. Morphological variations of Haemophilus parasuis strains. J Clin Microbiol. 1986 Jan;23(1):138–142. doi: 10.1128/jcm.23.1.138-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly T., Rosendal S., Niven D. F. Porcine haemophili and actinobacilli: characterization by means of API test strips and possible taxonomic implications. Can J Microbiol. 1984 Oct;30(10):1229–1238. doi: 10.1139/m84-195. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Young T. F. Characterization of Haemophilus spp. isolated from healthy swine and evaluation of cross-reactivity of complement-fixing antibodies to Haemophilus pleuropneumoniae and Haemophilus taxon "minor group". J Clin Microbiol. 1985 Dec;22(6):945–950. doi: 10.1128/jcm.22.6.945-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Varki A., Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983 Oct 25;258(20):12465–12471. [PubMed] [Google Scholar]
- Willson P. J., Falk G., Klashinsky S. Detection of Actinobacillus pleuropneumoniae Infection in Pigs. Can Vet J. 1987 Mar;28(3):111–116. [PMC free article] [PubMed] [Google Scholar]


