Abstract
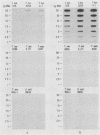
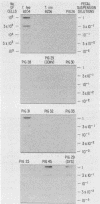
Oligodeoxynucleotide probes (17 and 28 bases long) complementary to a unique region of Treponema hyodysenteriae 16S rRNA were developed. These probes bound specifically to partially purified rRNA and whole-cell rRNA of T. hyodysenteriae. No binding to partially purified rRNA or whole-cell rRNA of Treponema innocens, Treponema succinifaciens, Treponema bryantii, or Escherichia coli occurred under stringent conditions. The 28-base probe was 5 to 10 times more sensitive than the 17-base probe when hybridized with T. hyodysenteriae rRNA. The 28-base probe detected T. hyodysenteriae in the feces of experimentally inoculated pigs exhibiting clinical signs of swine dysentery.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwyk W. M., Canale-Parola E. Treponema succinifaciens sp. nov., an anaerobic spirochete from the swine intestine. Arch Microbiol. 1979 Sep;122(3):231–239. doi: 10.1007/BF00411285. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., DeLong E. F., Olsen G. J., Pace N. R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Geiser A., Stanbridge E. J. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J Gen Microbiol. 1987 Jul;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Hunter D., Saunders C. N. Diagnosis of swine dysentery using an absorbed fluorescent antiserum. Vet Rec. 1977 Oct 8;101(15):303–304. doi: 10.1136/vr.101.15.303. [DOI] [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Kinyon J. M., Kaeberle M. L. Microtitration agglutination for detection of Treponema hyodysenteriae antibody. J Clin Microbiol. 1978 Sep;8(3):293–298. doi: 10.1128/jcm.8.3.293-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Nord N. A., Kinyon J. M., Egan I. T. Enzyme-linked immunosorbent assay for detection of antibody to Treponema hyodysenteriae antigens. J Clin Microbiol. 1982 Feb;15(2):249–252. doi: 10.1128/jcm.15.2.249-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle R. A., Kinyon J. M. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol. 1988 Nov;26(11):2357–2360. doi: 10.1128/jcm.26.11.2357-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin T. E. Use and applications of nucleic acid probes in the clinical laboratory. Clin Chem. 1989 Sep;35(9):1819–1825. [PubMed] [Google Scholar]
- Pace B., Matthews E. A., Johnson K. D., Cantor C. R., Pace N. R. Conserved 5S rRNA complement to tRNA is not required for protein synthesis. Proc Natl Acad Sci U S A. 1982 Jan;79(1):36–40. doi: 10.1073/pnas.79.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Trust T. J. Rapid identification of Campylobacter species using oligonucleotide probes to 16S ribosomal RNA. Mol Cell Probes. 1989 Jun;3(2):133–142. doi: 10.1016/0890-8508(89)90024-8. [DOI] [PubMed] [Google Scholar]
- Saunders C. N., Hunter D. A fluorescent antibody staining technique for the diagnosis of swine dysentery. Vet Rec. 1974 May 25;94(21):491–492. doi: 10.1136/vr.94.21.491. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Kinyon J. M., Harris D. L. Selective medium for isolation of Treponema hyodysenteriae. J Clin Microbiol. 1976 Jul;4(1):57–60. doi: 10.1128/jcm.4.1.57-60.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch Microbiol. 1980 Sep;127(2):145–156. doi: 10.1007/BF00428018. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Cornell C. P. Erythrocytes as a source of essential lipids for Treponema hyodysenteriae. Infect Immun. 1987 Feb;55(2):304–308. doi: 10.1128/iai.55.2.304-308.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Blitchington R., Hindenach B., Greene R. C. Species-specific oligonucleotide probes for rRNA of Clostridium difficile and related species. J Clin Microbiol. 1988 Dec;26(12):2484–2488. doi: 10.1128/jcm.26.12.2484-2488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. C., Wilt G. R., Reed R. B., Powe T. A. Use of an enzyme-linked immunosorbent assay for detection of Treponema hyodysenteriae infection in swine. J Clin Microbiol. 1989 Mar;27(3):411–416. doi: 10.1128/jcm.27.3.411-416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]