Abstract
Aims
Neutrophils/platelet interactions are involved in abdominal aortic aneurysm (AAA). The intraluminal thrombus (ILT) is a human model of platelet/neutrophil interactions. The present study focused on mediators involved in neutrophil recruitment in AAA.
Methods and results
Conditioned media from luminal, intermediate, and abluminal layers of 29 human ILTs were analysed for neutrophil markers [elastase/α1-antitrypsin and MMP9/NGAL complexes, myeloperoxidase (MPO), and α-defensin peptides], RANTES, platelet factor 4 (PF4), and interleukin-8 (IL-8). Their time-dependent release into serum from clots generated in vitro and their plasma concentrations in AAA patients and controls were determined. Immunohistochemistry for neutrophils, platelets, IL-8, PF4, and RANTES on AAA sections was performed; and molecules involved in ILT neutrophil chemotactic function were analysed in vitro. Neutrophils and platelets colocalized in the luminal layer of the thrombus. Consistently, neutrophil markers and platelet-derived RANTES and PF4 were released predominantly by the luminal thrombus pole, where their concentrations were significantly correlated. The luminal ILT layer was also the main source of IL-8, whose immunostaining colocalized with neutrophils. All were also released time dependently from clots and were increased in plasma of AAA patients. Luminal ILT layers displayed potent neutrophil chemotactic activity in vitro, which was inhibited by RANTES- and IL-8-blocking antibodies as well as by reparixin, an antagonist of the IL-8 receptors CXCR1 and CXCR2.
Conclusion
Taken together, these results suggest that platelet-derived RANTES and neutrophil-derived IL-8 are involved in attracting neutrophils to the luminal layer of AAA ILT.
KEYWORDS: Intraluminal thrombus, Interleukin-8, RANTES, PF4, MMP9/NGAL, MPO, Elastase/α1-antitrypsin, α-Defensins
1. Introduction
Progressive dilatation of the aortic wall in abdominal aortic aneurysm (AAA) is associated with extracellular matrix breakdown.1 Such tissue-degrading remodelling involves proteolytic enzymes whose levels are increased in AAA.2,3 Neutrophils produce large amounts of proteases, and recent experimental studies in mice have pointed out the determinant role of neutrophils in AAA development.4–6 In AAA, neutrophils are mainly found within the luminal layer of the non-occlusive intraluminal thrombus (ILT) that develops at the interface with the circulating blood,7,8 and leucocyte elastase prevents ILT recolonization by mesenchymal cells.9
Morphological characteristics of the thrombus10,11 are linked to the risk of rupture;12,13 and more degradation is observed in the part of the aneurysmal wall lined by a thrombus than in the adjacent part in contact with the flowing blood,14 indirectly suggesting a role of ILT in AAA pathology. ILT stores large amounts of proteases,8,15–17 including neutrophil-derived proteases.7–9 Interestingly, inhibition of platelet aggregation or activation limited aneurysmal enlargement18–20 and was associated with a reduced accumulation of neutrophils within the thrombus, suggesting links between platelet activation and neutrophil ILT recruitment. In parallel, neutrophils which themselves produce neutrophil chemotactic factors, including interleukin-8 (IL-8)21 may amplify their own recruitment.22
The present study aims at investigating molecular mediators involved in ILT neutrophil recruitment. For this purpose, we explored the topological distribution of neutrophil- and platelet-derived chemokines IL-8, RANTES, and platelet factor 4 (PF4) in the ILT and wall of human AAAs by immunohistochemical and biochemical approaches. This was completed by the measurement of the release of neutrophil markers, IL-8, RANTES, and PF4 into serum during blood clot retraction and lysis in vitro. Related-plasma levels in AAA patients and healthy controls were determined. Finally, the study assessed the neutrophil chemotactic activity of ILT tissue-culture media ex vivo and the involvement of IL-8 and RANTES in this phenomenon.
2. Methods
2.1. Plasma and tissue samples
Blood was collected 24 h before surgery on sodium citrate from 24 patients (male, aged 69.6 ± 8.7 years, range 60–82) with degenerative AAA,20 and 24 healthy male controls (aged 68.7 ± 8.5 years, range 58–78) examined at ‘Centre d’Investigation Preventative et Clinique’ (IPC, Paris).23 A significant ILT was present in all the AAA studied. Circulating aortic channels were measured at the level of maximal dilatation on CT scan. ILT proportion was calculated as followed: (maximal aortic diameter−circulating channel)/maximal aortic diameter and was expressed as percentage. The mean maximal AAA diameter was 54.0 ± 8.55 mm. The mean ILT thickness was 25.5 ± 8.4 mm, representing 46.8 ± 11.8% of the AAA dilatation.
Samples of AAA thrombus and residual wall were obtained during AAA surgical repair from these 24 and five additional patients. ILT (n = 29) were dissected into luminal, intermediate, and abluminal layers9,20 and the wall was separated into media and adventitia (n = 10). The thrombus layers, media, and adventitia were cut into small pieces (1 mm3) and separately incubated in RPMI-1640 medium (Gibco) for 24 h at 37°C (2 ml/g wet tissue). The tissue-culture media containing released material were then collected and stored at −80°C after determining the protein concentration by the Bradford assay (Bio-Rad). Ethics Committee advice and patient and healthy volunteer informed consent were obtained (RESAA and AMETHYST studies, CPP Paris-Cochin no 2095, 1930, and 1931). The investigation conforms with the principles outlined in the Declaration of Helsinki.
In vitro serum from three healthy volunteers was obtained 2 h and 1, 2, 3, 4, and 7 days after clotting of blood at 37°C. Paired citrated plasma samples were used directly or recalcified (3×10−2 M CaCl2) to induce fibrin formation in the absence of blood cells. Sera, plasma, and recalcified plasma were then kept at −80°C until ELISAs were performed.
2.2. Imunohistochemistry
AAA samples (n = 8) were fixed in 3.7% paraformaldehyde, embedded in paraffin, and sectioned at 5 µm. Immunohistochemistry was performed using anti-IL-8 (dilution 1:50, Santa Cruz Biotechnologies), anti-RANTES (dilution 1:5, R&D Systems), anti-PF4 (dilution 1:100, Abcam), anti-NGAL (dilution 1:100, Hycult), anti-proteinase-3 (dilution 1:50, Hycult), anti-CD66b (neutrophils, dilution 1:25, Immunotech), anti-CD68 (macrophages, dilution 1:50, Dako), anti-CD41 (integrin αIIb, platelets, dilution 1:100, Santa Cruz Biotechnologies), anti-CD3 (T-lymphocytes, dilution 1:10, Dako), and anti-CD20 (B-lymphocytes, dilution 1:100, Dako) as primary antibodies and a peroxidase Dako LSAB kit for detection. Control irrelevant antibodies (Dako) were applied at the same concentration in order to assess non-specific staining.
2.3. ELISA tests
Concentrations of MPO (Hycult), α-defensins (Hycult), elastase/α1-antitrypsin complexes (Calbiochem), IL-8 (R&D Systems and Sanquin), RANTES (R&D Systems), MMP-9/NGAL complexes (R&D Systems), and PF4 (American Diagnostica) were determined by ELISA kits, according to the manufacturers’ instructions. For intra- and inter-assay coefficients of variation, see Supplementary material online, TableS1.
2.4. Neutrophil chemotaxis assay
Neutrophils were obtained from heparinized venous blood of volunteers by sedimentation on a separating medium containing 9% Dextran T500 (Pharmacia LKB, Uppsala, Sweden) and 38% Radioselectan (Schering, Lannoy, France). After two washes in phosphate buffered saline (PBS), neutrophils were counted on a Hemalog H1 device (Technicon Instruments Corporation, Tarrytown, NY, USA) and adjusted to 105 neutrophils/µL in PBS.
Neutrophil chemotaxis was determined according to Vicioso et al.24 Eight series of three, star-shaped wells, 24 mm in diameter and 24 mm apart were cut in agarose plates [0.7% agarose (Indubiose A37, BioSepra, Villeneuve-La-Garenne, France) in Kreb’s buffer pH 6.8 containing 10% foetal calf serum (Bio Whittacker, Lagny, France)]. The centre well of each three-well series received 5 µL of neutrophil suspension. The outer well received 5 µL of chemotactic factor, i.e. formyl-methionyl-leucyl-phenylalanine (10−7 M, fMLP, Sigma), recombinant human IL-8 (20 ng/mL, R&D Systems), recombinant human RANTES (0.1 µg/mL, R&D Systems), or thrombus luminal and abluminal conditioned media. In some experiments, conditioned media of luminal thrombus, IL-8, and RANTES were pretreated with 0.1 µg/mL human IL-8 (R&D Systems), RANTES (R&D Systems) blocking antibodies (n = 6), or neutrophils were incubated with reparixin (10−6 M, generously provided by Dompé pharma, Italy) (n = 7), a specific non-competitive allosteric inhibitor of CXCR1 and CXCR2 receptors,25 for 20 min at room temperature. The inner well received 5 µL of RPMI-1640 as a non-chemotactic control of spontaneous migration. The dishes were incubated at 37°C in a humidified 5% CO2 incubator for 4 h, and migration was quantified using an inverted microscope with an ocular micrometer, by measuring (in millimetres) the linear distances the cells had moved from the margin of the wells towards the chemotactic factor (oriented migration) and the control medium (spontaneous migration). Results were expressed as a ‘chemotactic differential’ (oriented migration−spontaneous migration).
2.5. Statistical analysis
Results are expressed as mean ± SD. The paired Wilcoxon and Mann–Whitney non-parametric tests (Statview software, version 4.57) were used to compare the results between each AAA compartment of the same sample and between AAA and control plasma, respectively. Results are presented as box plots, in which the median is shown. Upper and lower limits of boxes represent inter-quartiles (25th and 75th), whereas upper and lower bars show percentiles (10th and 90th). Correlations were established by Spearman’s rank test. Statistical significance was accepted for P < 0.05.
3. Results
3.1. Neutrophil distribution in abdominal aortic aneurysm
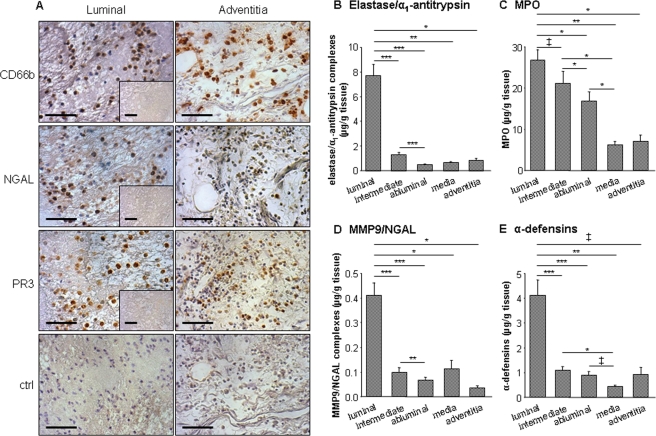
Neutrophils, revealed by positive immunostaining of CD66b, NGAL, and proteinase-3, were detected within the most luminal part (blood interface layer) of the ILT and in the adventitia (Figure 1A). No neutrophils were detected within the deeper thrombus layers (Figure 1A, insets). Consistently, soluble neutrophil markers measured by ELISA, i.e. elastase/α1-antitrypsin, MMP9/NGAL, MPO, and α-defensins, were all essentially found in supernatants of the luminal ILT layer with a significant negative gradient from the luminal to the abluminal poles (Figure 1B–E). Significant positive correlations were found between all these neutrophil markers released by the ILT luminal pole, whereas only α-defensins significantly correlated with maximal ILT thickness (Table 1).
Figure 1.
Release of neutrophil markers by abdominal aortic aneurysm compartments and immunolocalization of neutrophils in abdominal aortic aneurysm. (A) Within the thrombus (n = 8), neutrophil CD66b and neutrophil-derived NGAL and proteinase-3 staining were detected at the luminal pole, whereas no staining was observed in deeper layers, devoid of cells (insets). Within the wall (n = 8), CD66b-positive staining was observed in the adventitia. NGAL and proteinase-3 staining were associated with CD66b within the adventitia. Negative controls for luminal thrombus layer and adventitia. Bar = 100 µm. Photomicrographs presented are representative of all tissues analysed. (B–E) Conditioned media from luminal, intermediate, and abluminal layers of abdominal aortic aneurysm thrombi (n = 29) and from the medial layer and adventitia of the remaining wall (n = 10) were analysed by ELISA for elastase/α1-antitrypsin complexes (B), myeloperoxidase (C), MMP9/NGAL complexes (D), and α-defensins (E). All these markers were predominantly released by the thrombus where a negative gradient from the luminal to the abluminal pole was found. ‡P < 0.05, *P < 0.01, **P < 0.001, and ***P < 0.0001.
Table 1.
Correlations between neutrophil and platelet markers and interleukin-8 released by the thrombus, maximal aortic diameter, and intraluminal thrombus thickness
| Elastase/α1-antitrypsin complexes | MPO | MMP9/NGAL complexes | α-Defensins | IL-8 | RANTES | PF4 | |
|---|---|---|---|---|---|---|---|
| MPO | r = 0.670 | ||||||
| P < 0.001 | |||||||
| MMP9/NGAL complexes | r = 0.838 | r = 0.587 | |||||
| P < 0.0001 | P < 0.001 | ||||||
| α-Defensins | r = 0.462 | r = 0.347 | r = 0.609 | ||||
| P < 0.05 | P < 0.01 | P < 0.05 | |||||
| IL-8 | r = 0.892 | r = 0.834 | r = 0.896 | r = 0.765 | |||
| P < 0.003 | P < 0.02 | P < 0.006 | P < 0.03 | ||||
| RANTES | r = 0.784 | r = 0.577 | r = 0.690 | r = 0.170 | r = 0.783 | ||
| P < 0.001 | P < 0.003 | P < 0.0001 | P = 0.8 | P < 0.001 | |||
| PF4 | r = 0.641 | r = 0.425 | r = 0.599 | r = 0.190 | r = 0.838 | r = 0.811 | |
| P < 0.0001 | P < 0.01 | P < 0.001 | P = 0.6 | P < 0.003 | P < 0.0001 | ||
| Maximal aortic diameter | r = 0.228 | r = 0.3636 | r = 0.089 | r = 0.114 | r = 0.167 | r = 0.408 | r = 0.230 |
| P = 0.8 | P = 0.4 | P = 0.7 | P = 0.06 | P = 0.5 | P = 0.8 | P = 0.6 | |
| Maximal ILT thickness | r = 0.486 | r = 0.459 | r = 0.252 | r = 0.413 | r = 0.371 | r = 0.262 | r = 0.370 |
| P = 0.2 | P = 0.2 | P = 0.7 | P < 0.01 | P = 0.5 | P = 0.8 | P = 0.7 |
3.2. Interleukin-8 distribution in abdominal aortic aneurysm
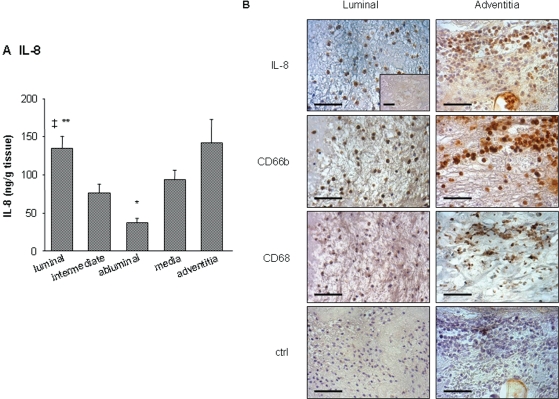
A negative gradient from the luminal to the abluminal ILT layer was observed, with significantly more IL-8 released by the luminal layer (Figure 2A). IL-8-positive staining was associated with neutrophils present at the luminal pole, whereas no staining was detected in deeper thrombus layers (Figure 2B). Moreover, IL-8 released by ILT strongly correlated with elastase/α1-antitrypsin, MMP9/NGAL, MPO, and α-defensins, but it did not with maximal aortic diameter and ILT thickness (Table 1). Aneurysmal media and adventitia also released IL-8, and concentrations in the adventitia were similar to those in the luminal ILT layer (Figure 2A). However, the total amount of IL-8 released by ILT was four-fold higher when compared with the wall (1830 ± 325 and 459 ± 84 ng for ILT and the wall, respectively, P < 0.01), taking into account the weight difference between them. Within the wall, IL-8 staining was found in macrophage- and neutrophil-positive areas of the adventitia (Figure 2B).
Figure 2.
Interleukin-8 (IL-8) release by abdominal aortic aneurysm compartments and immunolocalization. (A) Conditioned media from luminal, intermediate, and abluminal layers of abdominal aortic aneurysm thrombi (n = 29) and from media and adventitia of the wall (n = 10) were analysed for interleukin-8 antigen by ELISA. Interleukin-8 antigen was found in supernatants of all compartments. ‡P < 0.05 vs. intermediate, *P < 0.01 vs. media and adventitia and **P < 0.001 vs. abluminal. (B) Immunohistochemical staining of interleukin-8. Within the thrombus (n = 8), interleukin-8 accumulated at the luminal pole, whereas no staining was observed in deeper layers (inset). At the luminal pole, interleukin-8 staining colocalized with CD66b-positive neutrophils. Note the absence of macrophages in the luminal part of the thrombus. In adventitia (n = 8), interleukin-8 staining was observed in neutrophil- and macrophage-rich areas. Bar = 100 µm. Photomicrographs presented are representative of all tissues analysed.
3.3. RANTES and platelet factor 4 distribution in abdominal aortic aneurysm
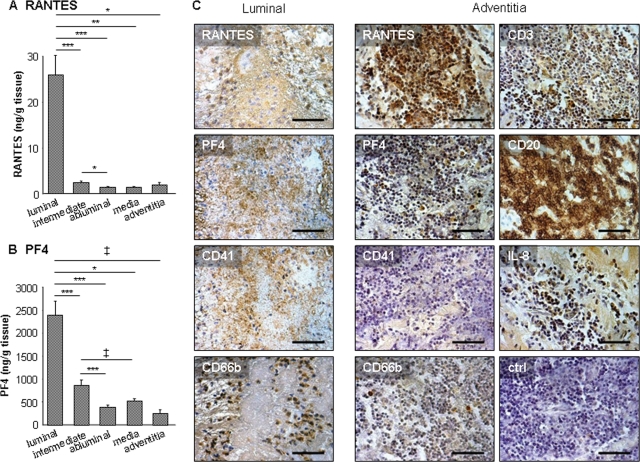
A similar distribution pattern was observed for RANTES and PF4 (Figure 3A and B). The luminal layer released significantly more RANTES and PF4 than the other ILT layers or the media and adventitia. In addition, a strong positive correlation was found between the two chemokines in the tissue-culture media from the luminal ILT pole (Table 1). Moreover, RANTES and PF4 immunostaining colocalized within the ILT, where they were associated, at the luminal pole, with platelet immunoreactivity revealed by CD41-positive staining, consistent with their platelet origin (Figure 3C). Interestingly, platelets and neutrophils colocalized in the luminal layer of the thrombus (Figure 3C), and strong positive correlations were found for both RANTES and PF4 and with elastase/α1-antitrypsin, MMP9/NGAL, and MPO or IL-8 (Table 1). In contrast, RANTES and PF4 did not correlate with α-defensin levels or maximal aortic diameter and ILT thickness. Within the wall, RANTES and PF4 staining was localized in lymphocyte-rich areas of the adventitia, whereas platelets were not observed (Figure 3C). In such areas, IL-8-positive staining was present, as well as some neutrophils (Figure 3C).
Figure 3.
Release of RANTES and platelet factor 4 by abdominal aortic aneurysm compartments and immunolocalization. (A and B) Conditioned media from luminal, intermediate, and abluminal layers of abdominal aortic aneurysm thrombi (n = 29) and from media and adventitia of the remaining wall (n = 10) were analysed by ELISA for RANTES (A) and platelet factor 4 (B). Both RANTES and platelet factor 4 were mainly released by the luminal thrombus layer when compared with other abdominal aortic aneurysm compartments. ‡P < 0.05, *P < 0.01, **P < 0.001, and ***P < 0.0001. (C) Immunolocalization of RANTES, platelet factor 4, and CD41 within the luminal pole of abdominal aortic aneurysm mural thrombus and adventitia (n = 8). Within intraluminal thrombus, RANTES, and platelet factor 4 were observed at the luminal pole in fibrin rich-areas and colocalized with platelet CD41 staining. Note the colocalization of platelet CD41- and neutrophil CD66b-positive staining at the luminal pole of intraluminal thrombus. Within the diseased wall, no staining was detected for CD41. RANTES and platelet factor 4 were localized in CD3- and CD20-positive areas within the adventitia, where interleukin-8-positive staining was also observed. Some neutrophils can be found in such areas. Bar = 100 µm. Photomicrographs presented are representative of all tissues analysed.
3.4. Release of neutrophil markers, interleukin-8, RANTES, and platelet factor 4 in sera during clot lysis
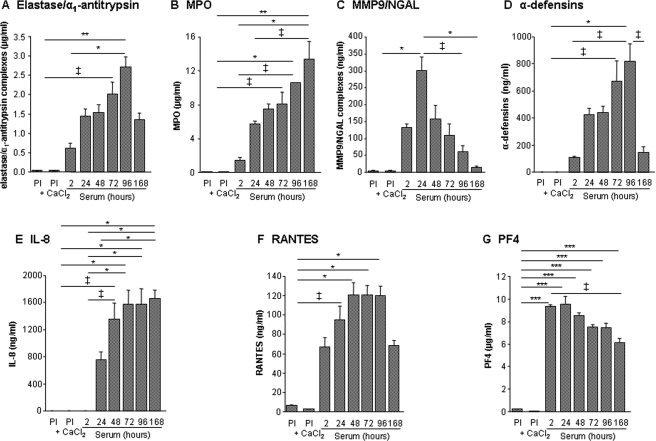
Neutrophil markers, IL-8, RANTES, and PF4 were found in higher concentrations in serum from three healthy volunteers when compared with paired plasma or plasma-derived serum (Figure 4). Serum concentrations of elastase/α1-antitrypsin, MMP9/NGAL, α-defensins, and RANTES increased during clot retraction and lysis, reaching their maximal levels between 24 and 96 h, and then decreased. In contrast, no decrease in MPO and IL-8 concentration was observed, whereas maximal serum concentrations of PF4 were reached as early as 2 h and decreased after 24 h (Figure 4).
Figure 4.
Time-dependent release of neutrophil markers, interleukin-8, RANTES, and platelet factor 4 from blood clots. Levels of elastase/α1-antitrypsin complexes (A), myeloperoxidase (B), MMP9/NGAL complexes (C), α-defensins (D), interleukin-8 (E), RANTES (F), and platelet factor 4 (G) were measured in serum after incubation at 37°C for 2, 24, 48, 72, 96, and 168 h in plasma (Pl) and recalcified plasma (Pl+CaCl2) from three healthy volunteers. ‡P < 0.05, *P < 0.01, **P < 0.001, and ***P < 0.0001.
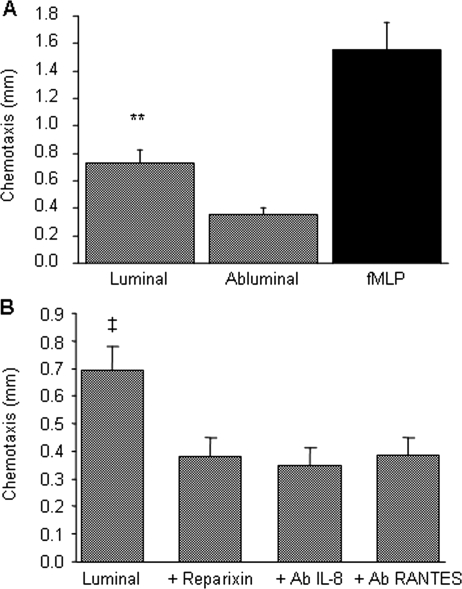
3.5. Neutrophil chemotaxis
Tissue-culture media from both luminal and abluminal thrombus layers induced neutrophil chemotaxis, with a significantly stronger activity displayed by the luminal part (Figure 5A). A 50% inhibition of the neutrophil chemotaxis induced by the ILT luminal pole was obtained with IL-8-blocking antibody and reparixin, an inhibitor of IL-8 receptors,25 as well as with RANTES-blocking antibody (Figure 5B).
Figure 5.
Interleukin-8 and RANTES-dependent neutrophil chemotactic activity of the luminal thrombus layer. (A) Conditioned media from luminal and abluminal thrombus layers (n = 29) were tested for their neutrophil chemotactic ability, as described in Methods section. fMLP (10−7 M) was used as a positive control for neutrophil chemotaxis. (B) Reparixin (10−6 M) added to neutrophils (n = 7) or interleukin-8- and RANTES-blocking antibodies added to conditioned media (n = 6) inhibited neutrophil chemotaxis induced by conditioned media from the luminal layer of the thrombus. ‡P < 0.05 vs. reparixin, interleukin-8, and RANTES-blocking antibodies, **P < 0.001 vs. abluminal.
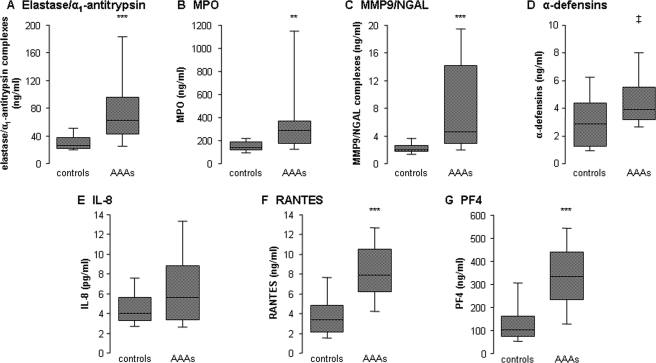
3.6. Plasma concentrations of neutrophil markers in abdominal aortic aneurysm patients
Elevated-plasma concentrations of the four neutrophil markers (elastase/α1-antitrypsin, MPO, MMP9/NGAL, and α-defensins) were found in AAA patients against healthy controls (Figure 6). In AAA plasma, strong association was found between levels of each neutrophil markers (Table 2). IL-8, RANTES, and PF4 plasma levels were also increased in AAA patients (Figure 6), but did not reach statistical significance for IL-8. Interestingly, plasma concentrations of platelet PF4 and RANTES were correlated, suggesting the platelet origin of RANTES in plasma (Table 2). Plasma levels of neutrophil markers in AAA patients were strongly correlated with platelet PF4 and RANTES, whereas IL-8 levels were correlated with RANTES, PF4, and the neutrophil markers, elastase/α1-antitrypsin and α-defensins (Table 2). In contrast, plasma levels of neutrophil and platelet markers did not correlate with either maximal aortic diameter or maximal ILT thickness, whereas IL-8 plasma levels correlated with maximal aortic diameter (Table 2). Similarly, no correlation was found between plasma levels of these markers and their concentrations in tissue conditioned media.
Figure 6.
Plasma concentrations of neutrophil markers, interleukin-8, RANTES, and platelet factor 4. Levels of elastase/α1-antitrypsin complexes (A), myeloperoxidase (B), MMP9/NGAL complexes (C), α-defensins (D), interleukin-8 (E), RANTES (F), and platelet factor 4 (G) were measured in plasma from 24 abdominal aortic aneurysm patients and 24 healthy controls. ‡P < 0.05, **P < 0.001, and ***P < 0.0001 vs. healthy controls.
Table 2.
Correlations between plasma concentrations of neutrophil markers, interleukin-8, RANTES, platelet factor 4, and maximal aortic diameter and intraluminal thrombus thickness in abdominal aortic aneurysm patients
| Elastase/α1-antitrypsin complexes | MPO | MMP9/NGAL complexes | α-Defensins | IL-8 | RANTES | PF4 | |
|---|---|---|---|---|---|---|---|
| MPO | r = 0.856 | ||||||
| P < 0.001 | |||||||
| MMP9/NGAL complexes | r = 0.883 | r = 0.824 | |||||
| P < 0.0001 | P < 0.0001 | ||||||
| α-Defensins | r = 0.597 | r = 0.763 | r = 0.490 | ||||
| P < 0.01 | P < 0.05 | P < 0.01 | |||||
| IL-8 | r = 0.249 | r = 0.200 | r = 0.195 | r = 0.339 | |||
| P < 0.02 | P = 0.2 | P = 0.3 | P < 0.02 | ||||
| RANTES | r = 0.358 | r = 0.456 | r = 0.293 | r = 0.646 | r = 0.298 | ||
| P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.02 | |||
| PF4 | r = 0.549 | r = 0.623 | r = 0.601 | r = 0.566 | r = 0.316 | r = 0.742 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.01 | P < 0.02 | P < 0.0001 | ||
| Maximal aortic diameter | r = 0.118 | r = 0.104 | r = 0.138 | r = 0 | r = 0.588 | r = 0.164 | r = 0.242 |
| P = 0.7 | P = 0.1 | P = 0.44 | P = 0.5 | P < 0.05 | P = 0.4 | P = 0.3 | |
| Maximal ILT thickness | r = 0.063 | r = 0.054 | r = 0.063 | r = 0.202 | r = 0.670 | r = 0.031 | r = 0.273 |
| P = 0.9 | P = 0.9 | P = 0.5 | P = 0.1 | P = 0.055 | P = 0.8 | P = 0.9 |
4. Discussion
The contribution of neutrophils to AAA evolution has been recently highlighted by studies showing that neutropenia limited AAA development after elastase perfusion in mice,4 whereas L-selectin5 or dipeptidyl peptidase I deficiencies6 prevented both neutrophil recruitment and murine AAA development. Although neutrophils are present in aneurysmal adventitia (Cohen et al.26 and the present study), neutrophils predominantly accumulate within the luminal pole of the ILT9,20,27 at the interface with circulating blood. Elastase/α1-antitrypsin, MMP9/NGAL, MPO, and α-defensins, used as neutrophil markers, were all essentially released by the luminal ILT layer, as reported for neutrophil-derived elastase, MMP-8, uPA, and MMP9/NGAL,7–9 and strong positive correlations were found between them.
The interface of ILT with the flowing blood is also characterized by the presence of activated platelets,20 involved in fibrin formation28 and ILT renewal. The link between ILT platelet deposition and neutrophil accumulation was further supported by the presence of P-selectin,20 the recently reported correlation between neutrophil-derived uPA and platelet-derived PAI-1,8 and by the present data showing the immunocolocalization of neutrophils and platelets within the ILT and the strong positive correlations between neutrophil markers and RANTES and PF4. RANTES and PF4 are produced by platelets29 and were mainly released by the luminal ILT layer where they colocalized with CD41 staining. In addition to promoting leucocyte adhesion via P-selectin expression,30,31 activated platelets also release chemokines involved in leucocyte recruitment to the site of injury, including RANTES and PF4. Tissue-culture media from the luminal pole of ILT induced neutrophil chemotaxis and RANTES-blocking antibody inhibited 50% of neutrophil chemotaxis. Neither purified PF4 nor PF4-blocking antibody influenced neutrophil chemotaxis in our experiments (data not shown). Conflicting results have been reported concerning the neutrophil chemotactic activity of PF4.32,33 Interestingly, within the wall, where no CD41-positive staining was observed, RANTES and PF4 staining were localized in lymphocyte-rich areas of the adventitia, consistent with their expression by T-lymphocytes34,35 and the reported overexpression of RANTES in AAA wall.36 In such areas, some neutrophils can be found, supporting a role of RANTES in neutrophil attraction.
IL-8 is a potent neutrophil chemotactic factor whose overexpression in human AAA36–38 has been reported. Our data confirm these previous results showing IL-8-positive staining in inflammatory areas of the adventitia, rich in neutrophils and macrophages, and the release of IL-8 into tissue-culture media from wall compartments. We mainly describe, for the first time, the presence of IL-8 within the ILT, where positive staining colocalized with neutrophils. Moreover, significant positive correlations were found between IL-8 and neutrophil markers released by ILT. Interestingly, IL-8 distribution in AAA reflected the localization of inflammation, which corresponded to neutrophils at the thrombus luminal pole and predominantly to macrophages within the adventitia. Although similar concentrations of IL-8 were released in tissue-culture media from the luminal ILT layer and the adventitia, four-fold higher amounts of IL-8 were released by ILT than by the wall, taking into account the weight difference between them. Thus, ILT is the main source of IL-8 in the AAA lesion. Consistent with the presence of IL-8 within the ILT, neutrophil chemotactic activity of the luminal thrombus layer was efficiently inhibited by IL-8 blocking antibody and by reparixin, a synthetic antagonist of IL-8 receptors. Similar to the ILT, blood clots generated in vitro released, in a time-dependent manner, platelet-derived RANTES and PF4, as well as the neutrophil markers and IL-8. This highlights the ability of the thrombus to trap and activate neutrophils, thus inducing the release of IL-8, which can amplify neutrophil ILT recruitment.
In spite of the preferential accumulation of platelets and neutrophils within the thrombus, no correlation between levels of platelet markers released by the luminal pole and either maximal aortic diameter or maximal thrombus thickness was observed. This absence of correlation was also observed for neutrophil markers, with the exception of α-defensins, whose levels correlated with maximal ILT thickness. This may indicate that cellular and biochemical markers of the thrombus activities do not parallel its morphological parameters.
Elevated-plasma concentrations of neutrophil and platelet markers were observed in AAA patients, as already described in AAA for markers of platelet activation20 and RANTES39 and in atherosclerosis for neutrophil elastase40 and PF4.41 However, plasma concentrations of platelet and neutrophil markers were not associated with AAA diameter or ILT thickness, suggesting that plasma levels of these markers are not directly linked to AAA morphology and could not be uniquely linked to ILT and AAA wall biological activities. A link could also exist with peripheral circulating platelet and neutrophil activation, as recently demonstrated in aneurysms of the ascending aorta, which are devoid of any ILT.42 As reported in coronary artery disease,43 increased IL-8 plasma levels were found in AAA patients compared with controls, but this increase did not reach statistical significance, although IL-8 plasma levels significantly correlated with maximal aortic diameter, as also previously reported.44 As observed in tissue-culture media from the thrombus luminal layer, strong positive correlations were found between plasma concentrations of neutrophil markers and platelet-derived RANTES and PF4, highlighting the relationship between platelet and neutrophil activation, whatever tissue or peripheral activation. Nevertheless, a possible link between plasma and ILT enrichment in neutrophil and platelet markers was supported, at least in part, by findings from clots generated in vitro, showing that serum contains higher amounts of these markers than plasma, thus demonstrating that clot formation followed by retraction and lysis induces the release of neutrophil and platelet markers. Therefore, since elevated-plasma levels of neutrophil and platelet markers could be related to the accumulation of neutrophil and platelets within ILT, a contribution of systemic activation of platelets and leucocytes to these high plasma levels in AAA patients cannot be excluded.45,46
In conclusion, the present study shows a preferential accumulation of neutrophils at the luminal pole of the mural thrombus and suggests the involvement of platelet-derived RANTES and neutrophil-derived IL-8 in ILT neutrophil recruitment. Initiation of neutrophil recruitment may depend on platelet factors, expressed and released during ILT formation and renewal, and may be amplified by IL-8 released from neutrophils. Elevated-plasma concentrations of platelet and neutrophil markers in AAA patients may also reflect platelet and neutrophil interactions. In view of this suspected role of platelet/neutrophil interactions, antagonism of IL-8 and RANTES receptors may represent an attractive novel strategy to control AAA pathology in human.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
X.H. was supported by Fondation Lefoulon-Delalande and P.R. by grants from the Fondation pour la Recherche Médicale and the Fondation de France. This study was supported by grants from Leducq Foundation, Fondation pour la Recherche Médicale, and FP-7 European Union integrated project ‘Fighting Aneurysmal Disease’ (FAD). Funding to pay the Open Access charge was provided by INSERM.
Supplementary Material
Acknowledgements
We thank the vascular medico/surgical staff from the Centre Cardiologique du Nord and Hôpital Européen Georges Pompidou for providing us with plasma and aneurysmal samples, the medical staff of the IPC for plasma of healthy controls, and Mary Osborne-Pellegrin for editing this manuscript.
Conflict of interest: none declared.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JJ. The pathobiology of aortic aneurysms. J Surg Res. 2004;117:163–175. doi: 10.1016/j.jss.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Hobeika MJ, Thompson RW, Muhs BE, Brooks PC, Gagne PJ. Matrix metalloproteinases in peripheral vascular disease. J Vasc Surg. 2007;45:849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 4.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 5.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, et al. L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112:241–247. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]
- 6.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkesson M, Kazi M, Zhu C, Silveira A, Hemdahl AL, Hamsten A, et al. Presence of NGAL/MMP-9 complexes in human abdominal aortic aneurysms. Thromb Haemost. 2007;98:427–433. [PubMed] [Google Scholar]
- 8.Houard X, Rouzet F, Touat Z, Philippe M, Dominguez M, Fontaine V, et al. Topology of the fibrinolytic system within the mural thrombus of human abdominal aortic aneurysms. J Pathol. 2007;212:20–28. doi: 10.1002/path.2148. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine V, Touat Z, Mtairag el M, Vranckx R, Louedec L, Houard X, et al. Role of leukocyte elastase in preventing cellular re-colonization of the mural thrombus. Am J Pathol. 2004;164:2077–2087. doi: 10.1016/s0002-9440(10)63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satta J, Laara E, Juvonen T. Intraluminal thrombus predicts rupture of an abdominal aortic aneurysm. J Vasc Surg. 1996;23:737–739. doi: 10.1016/s0741-5214(96)80062-0. [DOI] [PubMed] [Google Scholar]
- 11.Wolf YG, Thomas WS, Brennan FJ, Goff WG, Sise MJ, Bernstein EF. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg. 1994;20:529–535. doi: 10.1016/0741-5214(94)90277-1. discussion 535–528. [DOI] [PubMed] [Google Scholar]
- 12.Roy J, Labruto F, Beckman MO, Danielson J, Johansson G, Swedenborg J. Bleeding into the intraluminal thrombus in abdominal aortic aneurysms is associated with rupture. J Vasc Surg. 2008;48:1108–1113. doi: 10.1016/j.jvs.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Siegel CL, Cohan RH, Korobkin M, Alpern MB, Courneya DL, Leder RA. Abdominal aortic aneurysm morphology: CT features in patients with ruptured and nonruptured aneurysms. AJR Am J Roentgenol. 1994;163:1123–1129. doi: 10.2214/ajr.163.5.7976888. [DOI] [PubMed] [Google Scholar]
- 14.Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 15.Carrell TW, Burnand KG, Booth NA, Humphries J, Smith A. Intraluminal thrombus enhances proteolysis in abdominal aortic aneurysms. Vascular. 2006;14:9–16. doi: 10.2310/6670.2006.00008. [DOI] [PubMed] [Google Scholar]
- 16.Fontaine V, Jacob MP, Houard X, Rossignol P, Plissonnier D, Angles-Cano E, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2002;161:1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gacko M, Glowinski S. Activities of proteases in parietal thrombus of aortic aneurysm. Clin Chim Acta. 1998;271:171–177. doi: 10.1016/s0009-8981(97)00246-5. [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Louedec L, Philippe M, Michel JB, Houard X. Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. J Vasc Surg. 2008 doi: 10.1016/j.jvs.2008.09.057. in press. [DOI] [PubMed] [Google Scholar]
- 19.Lindholt JS, Sorensen HT, Michel JB, Thmsen HF, Henneberg EW. Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc Endovasc Surg. 2008;42:329–334. doi: 10.1177/1538574408315205. [DOI] [PubMed] [Google Scholar]
- 20.Touat Z, Ollivier V, Dai J, Huisse MG, Bezeaud A, Sebbag U, et al. Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution. Am J Pathol. 2006;168:1022–1030. doi: 10.2353/ajpath.2006.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strieter RM, Kasahara K, Allen R, Showell HJ, Standiford TJ, Kunkel SL. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem Biophys Res Commun. 1990;173:725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- 22.McCain RW, Dessypris EN, Christman JW. Granulocyte/macrophage colony-stimulating factor stimulates human polymorphonuclear leukocytes to produce interleukin-8 in vitro. Am J Respir Cell Mol Biol. 1993;8:28–34. doi: 10.1165/ajrcmb/8.1.28. [DOI] [PubMed] [Google Scholar]
- 23.Rossignol P, Cambillau M, Bissery A, Mouradian D, Benetos A, Michel JB, et al. Influence of blood sampling procedure on plasma concentrations of matrix metalloproteinases and their tissue inhibitors. Clin Exp Pharmacol Physiol. 2008;35:464–469. doi: 10.1111/j.1440-1681.2008.04897.x. [DOI] [PubMed] [Google Scholar]
- 24.Vicioso MA, Garaud JJ, Reglier-Poupet H, Lebeaut A, Gougerot-Pocidalo MA, Chollet-Martin S. Moderate inhibitory effect of interleukin-10 on human neutrophil and monocyte chemotaxis in vitro. Eur Cytokine Netw. 1998;9:247–253. [PubMed] [Google Scholar]
- 25.Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JR, Keegan L, Sarfati I, Danna D, Ilardi C, Wise L. Neutrophil chemotaxis and neutrophil elastase in the aortic wall in patients with abdominal aortic aneurysms. J Invest Surg. 1991;4:423–430. doi: 10.3109/08941939109141172. [DOI] [PubMed] [Google Scholar]
- 27.Houard X, Ollivier V, Louedec L, Michel JB, Back M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009 doi: 10.1096/fj.08-116202. Epub ahead of print 9 January 2009, doi:fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 28.Sarda-Mantel L, Coutard M, Rouzet F, Raguin O, Vrigneaud JM, Hervatin F, et al. 99mTc-annexin-V functional imaging of luminal thrombus activity in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:2153–2159. doi: 10.1161/01.ATV.0000237605.25666.13. [DOI] [PubMed] [Google Scholar]
- 29.von Hundelshausen P, Petersen F, Brandt E. Platelet-derived chemokines in vascular biology. Thromb Haemost. 2007;97:704–713. doi: 10.1160/th07-01-0066. [DOI] [PubMed] [Google Scholar]
- 30.de Gaetano G, Cerletti C, Evangelista V. Recent advances in platelet-polymorphonuclear leukocyte interaction. Haemostasis. 1999;29:41–49. doi: 10.1159/000022459. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi S, Watanabe N, Nakazawa K, Suzuki J, Tsushima K, Tamatani T, et al. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circulation. 2000;102:1710–1717. doi: 10.1161/01.cir.102.14.1710. [DOI] [PubMed] [Google Scholar]
- 32.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA. 1993;90:3574–3577. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deuel TF, Senior RM, Chang D, Griffin GL, Heinrikson RL, Kaiser ET. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci USA. 1981;78:4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasagni L, Grepin R, Mazzinghi B, Lazzeri E, Meini C, Sagrinati C, et al. PF-4/CXCL4 and CXCL4L1 exhibit distinct subcellular localization and a differentially regulated mechanism of secretion. Blood. 2007;109:4127–4134. doi: 10.1182/blood-2006-10-052035. [DOI] [PubMed] [Google Scholar]
- 35.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 36.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Koch AE, Kunkel SL, Pearce WH, Shah MR, Parikh D, Evanoff HL, et al. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993;142:1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi M, Higashikata T, Ishibashi-Ueda H, Sasaki H, Ogino H, Iihara K, et al. Sustained upregulation of inflammatory chemokine and its receptor in aneurysmal and occlusive atherosclerotic disease: results form tissue analysis with cDNA macroarray and real-time reverse transcriptional polymerase chain reaction methods. Circ J. 2005;69:1490–1495. doi: 10.1253/circj.69.1490. [DOI] [PubMed] [Google Scholar]
- 39.Jedynak M, Siemiatkowski A, Gacko M, Mroczko B, Borkowski J. Serum concentrations of MCP-1 and RANTES in patients during aortic surgery: the relationship with ischemia-reperfusion. Arch Immunol Ther Exp (Warsz) 2004;52:201–207. [PubMed] [Google Scholar]
- 40.Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, et al. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Caimi G, Hoffmann E, Montana M, Incalcaterra E, Dispensa F, D’Amico T, et al. Plasma markers of platelet and polymorphonuclear leukocyte activation in young adults with acute myocardial infarction. Clin Hemorheol Microcirc. 2005;32:67–74. [PubMed] [Google Scholar]
- 42.Touat Z, Lepage L, Ollivier V, Nataf P, Hvass U, Labreuche J, et al. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol. 2008;28:940–946. doi: 10.1161/ATVBAHA.107.158576. [DOI] [PubMed] [Google Scholar]
- 43.Romuk E, Skrzep-Poloczek B, Wojciechowska C, Tomasik A, Birkner E, Wodniecki J, et al. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur J Clin Invest. 2002;32:657–661. doi: 10.1046/j.1365-2362.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 44.Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–1164. doi: 10.1515/CCLM.2000.178. [DOI] [PubMed] [Google Scholar]
- 45.Balduini CL, Salvini M, Montani N, Noris P, Spedini P, Belletti S, et al. Activation of the hemostatic process in patients with unruptured aortic aneurysm before and in the first week after surgical repair. Haematologica. 1997;82:581–583. [PubMed] [Google Scholar]
- 46.Tanaseanu C, Neagu M, Popescu M, Manda G, Cojocaru M. Peripheral leukocytes activation in aortic aneurysmal disease. Rom J Intern Med. 1998;36:167–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.