Abstract
Aims
Despite the lower patency of venous compared with arterial coronary artery bypass grafts, ∼50% of grafts used are saphenous vein conduits because of their easier accessibility. In a search for ways to increase venous graft patency, we applied the results of a previous pharmacological study screening for non-toxic compounds that inhibit intimal hyperplasia of saphenous vein conduits in organ cultures. Here we analyse the effects and mechanism of action of leoligin [(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)tetrahydrofuran-3-yl]methyl (2Z)-2-methylbut-2-enoat, the major lignan from Edelweiss (Leontopodium alpinum Cass.).
Methods and results
We found that leoligin potently inhibits vascular smooth muscle cell (SMC) proliferation by inducing cell cycle arrest in the G1-phase. Leoligin induced cell death neither in SMCs nor, more importantly, in endothelial cells. In a human saphenous vein organ culture model for graft disease, leoligin potently inhibited intimal hyperplasia, and even reversed graft disease in pre-damaged vessels. Furthermore, in an in vivo mouse model for venous bypass graft disease, leoligin potently inhibited intimal hyperplasia.
Conclusion
Our data suggest that leoligin might represent a novel non-toxic, non-thrombogenic, endothelial integrity preserving candidate drug for the treatment of vein graft disease.
KEYWORDS: Lignan, Neointima, Therapy, Intimal hyperplasia
1. Introduction
Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) are the two invasive options to treat coronary artery disease (CAD), one of the leading causes of morbidity and mortality worldwide.1 The success of these approaches is, however, often limited by restenosis and graft failure. With respect to graft patency rates after CABG, the vessels of choice are clearly the internal mammary arteries.2 However, these arteries have limited availability; hence, saphenous vein grafts are more frequently used in CABG than arterial grafts (e.g. in 2004 at our institution, saphenous veins were used in 51% of bypass grafts3). Clinical optimization like graft handling (e.g. ‘no touch techniques’) and aggressive lipid-lowering therapy has impressively increased the patency rates of saphenous vein conduits; the current rates are ∼60% 10 years after CABG.3–5 The major reasons for a loss of patency at earlier time points are thromboses, neointima formation, and intimal hyperplasia, with 10–20% loss of patency occurring after the first year; loss at later periods are due to development of graft atherosclerosis.4,6,7
The causative factors and the pathophysiological processes that underlie vein graft disease are not well understood. It is thought that vein graft disease is the result of a variety of events initiated by vascular damage caused by surgical handling, ischaemia, or arterialization (blood pressure and blood flow) of the grafts. This initial damage is thought to provoke adaptive repair processes in the vessel wall, like tissue remodelling (positive and negative) and intimal hyperplasia.4,6–8 While this response is vital for the adaptation of the graft to the arterial environment, excessive response is believed to give rise to graft disease that ultimately results in graft failure.
Despite a complex array of intra- and inter-cellular signalling events in the development of graft disease after CABG and/or PCI, the core elements at the histological level are endothelial damage (denudation), and smooth muscle cell (SMC) proliferation and infiltration of the intima. Pro-inflammatory signalling, due to tissue damage and cellular necrosis and also as an element of adaptive tissue remodelling, is another highly relevant factor.9 Although the use of drug-eluting stents/matrices instead of purely mechanical devices will be the most likely approaches in PCI- and CABG-based prevention of restenosis and graft failure, there is a lack of drugs, screened or designed precisely for these applications. Currently used drugs are mainly agents developed for cancer or immunosuppressive therapy, which may be too aggressive or unspecific for the treatment of restenosis and graft disease; furthermore, endothelial healing, important for the prevention of thromboses, is also impaired by these drugs.
Keeping these issues in mind, we previously screened a set of compounds in an organ culture model for their ability to inhibit intimal hyperplasia in human saphenous veins in vitro. The concept underlying our screen was that the compounds of interest must inhibit intimal hyperplasia and neointima formation in saphenous vein organ cultures, without being toxic for endothelial cells (ECs).
2. Methods
2.1. General
All reagents used were of analytical grade quality and were purchased from Sigma Aldrich (Vienna, Austria) if not specified otherwise. Water was produced by reverse osmosis followed by distillation.
2.2. Study design
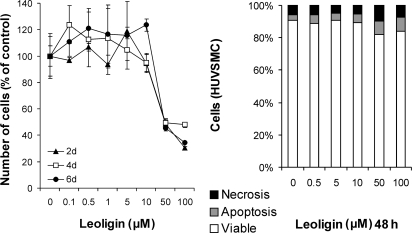
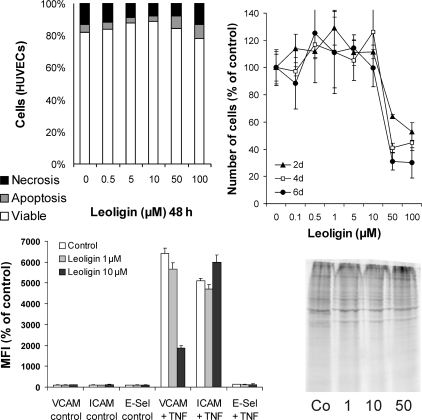
We had previously screened a set of plant extracts and compounds as candidate drugs for the treatment and prevention of cardiovascular diseases in a CABG setting. The initial screening was performed in organ cultures of human saphenous vein conduits (see below). The grafts were incubated with various plant extracts (for a list, see ref. 10), selected on the basis of their anti-inflammatory and anti-proliferative activity, as well as with six compounds and isolates with similar activities, such as histone deacetylase inhibitors (butyric acid and trichostatin A), curcumin, two bisabolane-type compounds, and leoligin. Of all extracts and compounds tested, leoligin proved to be the most efficient in inhibiting intimal hyperplasia of grafts without exerting any toxic effects and was therefore analysed further to elucidate its mechanisms of action as well as its in vivo applicability. A proliferation-inhibiting activity of leoligin in the absence of cell death induction was demonstrated both in SMCs and ECs (Figures 3 and 5). Furthermore, we examined the cell cycle-modulating activity of leoligin, and its effects on inhibitors of the cell cycle. Within the limits of the study, leoligin had no major side effects, since it exhibited no pro-thrombotic, cell death-inducing or protein synthesis-inhibiting activities.
Figure 3.
Leoligin inhibits the proliferation of smooth muscle cells (SMCs) without causing cell death. In order to investigate the effect of leoligin on the cellular level, isolated primary human vascular SMCs were incubated with the indicated concentrations of leoligin or solvent control (DMSO) for the indicated times. The right diagram shows a FACS analysis of cell viability determined by annexin V/propidium iodide staining. Values are means of a representative experiment performed in triplicate. The experiment was repeated three times, with similar results. The left diagram shows a cell proliferation analysis by the XTT assay. Values are means of three independent experiments ± SD.
Figure 5.
Leoligin is non-toxic for ECs and inhibits TNF-α-mediated VCAM-1 expression. Primary human vascular endothelial cells (ECs) were incubated with the indicated concentrations of leoligin for the indicated times. The upper left diagram shows a representative analysis of cell viability determined by the annexin V/propidium iodide staining and FACS analyses. Values are means of a representative experiment performed in triplicate. The upper right diagram shows EC proliferation as determined by the XTT assay. Values are means of three independent experiments ± SD. The impact of leoligin on TNF-α-induced surface expression of VCAM-1, ICAM-1, and E-selectin (E-Sel) is shown in the lower left diagram. Data shown are mean fluorescence intensities (MFI), % of control, of a representative experiment. The experiment was repeated three times. The lower right image shows a metabolic protein labelling of ECs in the presence of the indicated concentrations of leoligin. The experiment was repeated three times, giving similar results. A representative blot is shown.
2.3. Plant material, isolation, and purification of leoligin
Leoligin was re-isolated according to the procedure as described.11 The sub-aerial plant parts (5.30 kg) used for isolation were obtained from cultures. A voucher specimen (CH 5002) has been deposited at the herbarium of the Institute of Pharmacy/Pharmacognosy, University of Innsbruck. Purity of leoligin according to LC-DAD/MS- and NMR examination was >98%.
2.4. Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords (kindly donated by the Gynaecology and Obstetrics Department, Innsbruck Medical University) by enzymatic detachment using collagenase, as described previously.12 Human umbilical vein SMCs were isolated from the same umbilical cords.13 SMCs were routinely passaged in 0.2% gelatine-coated (Sigma, Steinheim, Germany) polysterene culture flasks (Becton–Dickinson, Meylan Cedex, France) in Medium 231 (Cascade Biologics, Paisley, UK). The isolation and analysis of human umbilical cord ECs and SMCs has been approved by the Ethics Committee of the Innsbruck Medical University.
2.5. Quantification of cell death and cellular DNA content
For detection and/or quantification of cell death, forward/sideward light scattering analysis, the AnnexinV/propidium iodide method, and staining of nuclear DNA content (cell cycle analyses) were used as described in.14
2.6. Analysis of cellular proliferation
Cell proliferation was measured by the XTT cell proliferation assay (Biomol, Hamburg, Germany) as described by the manufacturer.
2.7. Surface expression analyses of ICAM-1, VCAM-1 and E-selectin
FACS-based analyses of surface adhesion molecule expression were performed according to a protocol by Grabner et al.,15 using anti-VCAM-1 antibody (clone 1.4 C3; Neomarkers), anti-ICAM-1 antibody (clone 28; DAKO Cytomation), and anti-E-selectin antibody (clone 16G4; Novo castra).
2.8. Western blotting
Western blotting was performed as described previously,16 with the primary antibodies anti-p27/KIP-1 antibody (clone 57; BD Transduction Laboratories).
2.9. Metabolic labelling of proteins
Metabolic labelling with 35S-methionine/cysteine was performed as described previously.16 Briefly, cells were treated as indicated for 16 h. After washing the cells three times with labelling medium (nine parts of methionine-free RPMI 1640 + one part RPMI 1640 + 10% FCS), cells were incubated for 8 h with labelling medium containing 75 mCi/mL of 35S-methionine/cysteine mix (Hartmann Radiochemicals, Germany) in the presence or absence of leoligin. Thereafter, cells were washed twice with cold PBS and lysed (150 mM NaCl, 0.1% Triton X-100, 30 mM Tris, 1 mM PMSF, 10% glycerol, and peptide inhibitors). After protein preparation, total cellular proteins were separated on polyacrylamide gels, and the dried gels were analysed by exposure to X-ray films.
2.10. Human saphenous vein organ culture
For organ culture experiments, remnants (surgical waste) of saphenous veins from patients undergoing CABG were collected. The saphenous veins were opened longitudinally and attached to silicone patches (with the endothelium facing upwards). Tissue pieces were incubated with culture medium (RPMI 1640, 30% serum, heparin 8 mU/mL, antibiotics) for 2 weeks to induce intimal hyperplasia (for details, see ref. 17). To test for a potential intimal hyperplasia-inhibitory activity of leoligin, different concentrations of leoligin, or DMSO as a solvent control, were added to the cultures during the incubation period. The medium containing leoligin or DMSO was replaced by fresh medium every second day. Baseline samples were fixed directly after preparation of fresh tissue and represent the status of the vessel prior to organ culture. The use of human saphenous veins has been approved by the Ethics Committee of the Innsbruck Medical University and conforms to the principles of the Declaration of Helsinki.
2.11. Mouse model: vein graft disease
In the mouse model used, the vena cava of a donor mouse was interposed into the carotid artery of a recipient mouse. After transplantation and prior to wound closure, a 100 µL depot of 0.9% NaCl (control group) or 100 µM leoligin in 0.9% NaCl (leoligin group) was placed around the adventitia of the vein graft. Four weeks after the intervention, mice were sacrificed and the interposed venae cavae were harvested for analyses. Of the 14 animals in the control group and 12 in the leoligin group which survived the surgical procedure, four control and two leoligin-treated animals showed no blood flow due to thromboses and were excluded from further analyses. For details on the surgical procedure, see refs 17 and 18. In all experiments, male C57/BL6 mice were used. Animal experiments were approved by the Commission for Animal Testing of the Austrian Ministry for Science and Research.
2.12. Immunohistochemistry and morphometric analyses
Following fixation in 4% paraformaldehyde and dehydration of tissues from organ culture or in vivo experiments, tissues were embedded in paraffin (venae cavae from the in vivo experiments were embedded into mouse liver prior to fixation) and sections were prepared. Immunohistochemistry was performed with the Accustain Elastica Stain (HT25) kit (Sigma-Aldrich, USA) or the En Vision+ System-HRP (DAB) (DakoCytomation, Denmark) according to the manufacturers’ instructions. Primary antibodies used were anti-p27/KIP-1 antibody (clone 57; BD Transduction Laboratories) and anti-CD31/PECAM-1 antibody (clone JC70A; DakoCytomation). The images were analysed by blinded researchers using the Image J software of the National Institutes of Health (USA).
2.13. Statistics
Values for neointima thickness were statistically analysed using Microsoft Excel and SPSS 11.0 software. Values of individual groups were tested for a Gaussian distribution (Kolmogorov–Smirnov's test) and equality of variances (Levene's test). Further analyses were performed using ANOVA/Bonferroni adjustment (Figure 2) followed by individual t-test comparisons of the groups (Figures 2 and 6).
Figure 2.
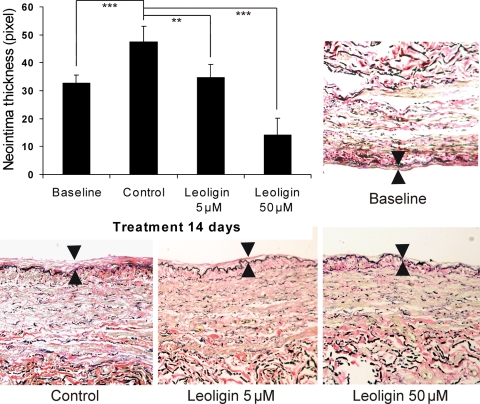
Leoligin inhibits intimal hyperplasia of human saphenous veins in vitro. Upper left diagram summarizes the data from experiments where human saphenous veins were induced to develop intimal hyperplasia in organ culture. Tissue samples were incubated with DMSO (solvent control) or various concentrations of leoligin for 2 weeks. Shown are mean values of intimal thickness (in pixel) ± SD. Over 25 measurements per sample were carried out, and a total of five samples (different donors) per concentration were analysed. All image analyses and quantification were performed by blinded observers. **P < 0.01; ***P < 0.001. The images (control, leoligin 5 µM, and leoligin 50 µM) show the effect of the presence of leoligin on organ culture-caused intimal thickening of a representative sample of a saphenous vein with mild pre-existing intimal hyperplasia (see image: baseline). The area between the arrows indicates the intimal thickness. Representative images are shown.
Figure 6.
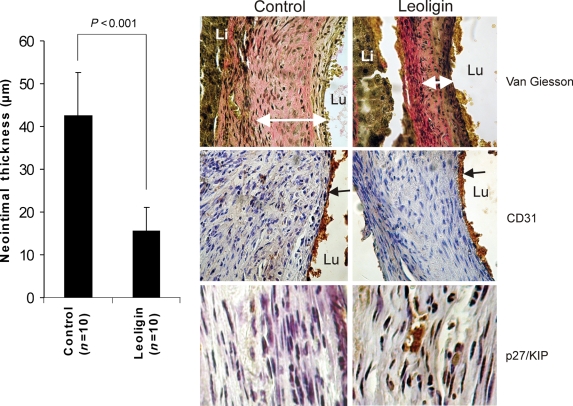
Leoligin inhibits neointima formation in vivo without causing endothelial damage. The left diagram displays a morphometric analysis of intimal thickness of vena cava interposed into the carotid artery of control-treated and leoligin-treated animals. Mean values of groups (µm) ± SD are shown. Group size was 10 animals in the control group and 10 animals in the leoligin group. Right side: upper two images show Elastica van Giesson staining of sections of the venous conduits. White arrows indicate neointimal thickness which was analysed by morphometry (left diagram). Central images display a staining with the endothelial cell CD31/PECAM-1 antigen (brown, black arrows) combined with a haematoxylin stain. Lower images display immunohistochemical staining of the sections with the cell cycle inhibitor p27/KIP-1 (brown) combined with a haematoxylin stain. Lu, lumen; Li, liver. All image analyses and quantification were performed by blinded observers. In all images shown, results of a representative experiment are depicted. Immunohistochemistry was performed on samples from all animals.
2.14. Platelet function test
Platelet function was assessed in vitro with the PFA-100® test system according to the manufacturer's instructions (Siemens healthcare Diagnostics, Eschborn, Germany).19,20 Five whole blood aliquots of two healthy volunteers with no reported medication were drawn into 3.8 mL buffered citrate (0.129 mol/L, pH 5.5) blood collection systems (S-Monovette for PFA 100, Sarstedt, Nürnberg, Germany). One sample was used as blank. One other sample was spiked with 3.8 µL DMSO prior to blood collection to serve as carrier control. The other three samples were spiked with 3.8 µL of one of three leoligin stock solutions (0.5, 5, and 50 mM, respectively). Both available PFA-100® sub-tests have been performed; i.e. platelet activators ADP (CADP) and epinephrine (CEPI).
3. Results
3.1. Leoligin, a compound from Edelweiss, potently inhibits intimal hyperplasia in a human saphenous vein in vitro model
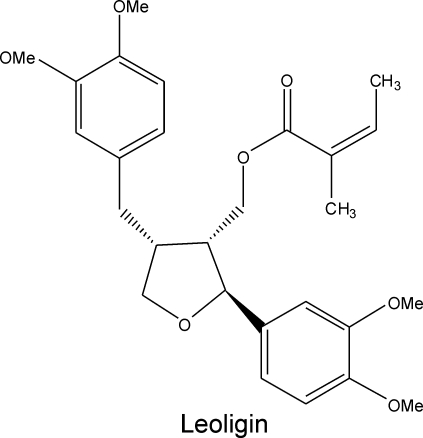
Leoligin [(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)tetrahydrofuran-3-yl]methyl (2Z)-2-methylbut-2-enoat (Figure 1), a compound that was previously isolated from the roots of Edelweiss (Leontopodium alpinum Cass.), is a lignan-type secondary plant metabolite. In an organ culture model for screening agents capable of inhibiting human saphenous vein intimal hyperplasia, leoligin showed a profound inhibitory activity (Figure 2). Leoligin potently inhibited intimal hyperplasia in a dose-dependent manner when added freshly every second day to organ cultures over a period of 2 weeks. Five micromolar leoligin completely inhibited intimal hyperplasia (P = 0.003), and 50 µM even reversed pre-existing intimal hyperplasia of saphenous veins (P < 0.001) (Figure 2). Intimal thickness was assessed by over 25 measurements per sample, and a total of five samples (different donors) per concentration were analysed. All image analyses and quantifications were performed by blinded observers.
Figure 1.
Leoligin is a constituent of Edelweiss (Leontopodium alpinum Cass.) roots. The popular alpine plant Edelweiss is used in folk medicine for the treatment of indigestion, fever, and ‘abdominal aches’. Leoligin (IUPAC name: [(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)tetrahydrofuran-3-yl]methyl (2Z)-2-methylbut-2-enoat) is a lignan, which we isolated from the roots of Edelweiss.
3.2. Leoligin inhibits smooth muscle cell proliferation by inducing cell cycle arrest in the G1-phase associated with accumulation and shift in molecular weight of p27/KIP protein
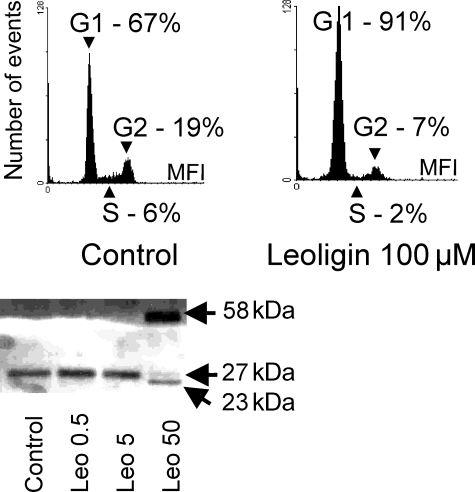
In order to understand the mechanism underlying leoligin-mediated inhibition and reversal of saphenous vein intimal hyperplasia, we analysed the effects of leoligin on isolated primary human vascular SMCs, which represent the central cell type in intimal thickening and intimal hyperplasia (SMC proliferation and migration). Our analyses clearly showed that leoligin causes only a marginal increase in cell death (apoptosis and necrosis) in SMCs after 48 h (Figure 3, right panel). In contrast, analyses of cell numbers by the XTT proliferation assay (Figure 3, left panel) as well as by Casy-based cell counting (data not shown) revealed a significant SMC proliferation inhibitory effect of leoligin. Subsequent measurements of nuclear DNA content (Figure 4, two upper panels) revealed that leoligin causes a massive accumulation of cells in the G1-phase of the cell cycle. Consequently, we performed western blot analysis of G1 arrest-relevant cell cycle regulators. The lower panel of Figure 4 shows a western blot demonstrating that leoligin induces a complete change in the appearance of p27/KIP protein, i.e. from one signal at 27 kDa to an intensive signal at 58 kDa, and three weak signals at 24, 27 (original signal) and 85 kDa (the 85 kDa signal was hardly visible and is not shown). All experiments were repeated at least three times. Cell cycle and the western blot analyses were repeated three times, and the results of representative experiments are shown.
Figure 4.
Leoligin induces a cell cycle arrest in the G1-phase and leads to accumulation of p27/KIP. The histograms show the results from a DNA content analysis of control-treated or leoligin-treated smooth muscle cells (SMCs) after 24 h of incubation (upper left histogram: solvent control; upper right histogram: leoligin 100 µM). The experiment was performed in duplicates and repeated three times. A representative experiment is shown. The lower panel shows a western blot analysis of p27/KIP of SMCs incubated for 24 h with the indicated concentrations of leoligin. The experiment was repeated three times, and a representative blot is shown.
3.3. Leoligin is not toxic for endothelial cells and inhibits TNF-α-mediated VCAM-1 expression
Since the integrity of the vascular endothelium plays a central role in vascular repair and healing, anti-thrombosis, and the graft atherosclerosis-relevant control of cell (macrophages) and compound (cholesterol) exchange between the blood and the vessel wall, we also analysed the effects of leoligin on the vascular endothelium. Leoligin inhibited the proliferation of ECs (Figure 5, upper right panel) without having any or only a low degree of toxic or cell death-inducing effects on them (Figure 5, upper left panel). Interestingly, leoligin inhibited TNF-α-mediated surface expression of VCAM-1 (Figure 5, lower left panel).21 To show that this is not due to a general phenomenon like inhibition of the endothelial translational machinery by leoligin, we performed metabolic protein labelling experiments (Figure 5, lower right panel) and analysed the effect of leoligin on other adhesion molecules (Figure 5, lower left panel). These data demonstrate that leoligin does not block protein synthesis and does not interfere with the translocation of proteins to the cell surface in general, but specifically inhibits stimulated VCAM-1 expression on the EC surface. All experiments were repeated at least three times, and the results of representative experiments are depicted.
3.4. Leoligin inhibits in vivo neointima formation without causing endothelial damage
To test the feasibility of applying leoligin in vivo, we analysed the effects of leoligin in a mouse model for vein graft disease (see Section 2). As in human saphenous vein bypass conduits, the transplant develops severe intimal hyperplasia within a couple of weeks.
Leoligin was applied after the transplantation directly as a periadventitial reservoir (100 µL of 100 µM leoligin in 0.9% NaCl) before surgical wound closure. Four weeks after transplantation, mice were sacrificed and the venous conduits were removed for further analyses. Thrombosed veins were excluded from further analyses and the remaining samples were subjected to immunohistochemical analysis. Figure 6 (left side) shows that leoligin treatment, in contrast to control treatment (0.9% NaCl), potently inhibited intimal hyperplasia in vivo. Vein grafts of mice treated with leoligin showed significantly reduced neointimal thickness (upper central and upper right image). A CD31 (endothelial marker) staining of the sections revealed that the endothelial layer of leoligin- and control-treated conduits was intact. Finally, a p27/KIP staining of the sections revealed that a large number of cells/nuclei in the leoligin-treated grafts stained positive for p27/KIP even 4 weeks after application of leoligin, indicating a long duration of drug effect. The treatment groups included 14 animals in the control group and 12 animals in the leoligin group. Four animals of the control group and two animals of the leoligin group were excluded from further analysis due to the presence of thrombosis in the grafts. Immunohistochemistry has been performed on samples from all animal; representative images are shown.
3.5. Leoligin is not thrombogenic and does not modulate normal platelet function
To examine potential pro-thrombotic activities of leoligin, we performed a standard platelet function test by the PFA-100® test system (see Section 2). No significant changes in the aperture closure time by leoligin (0.5, 5, and 50 µM) could be observed (data not shown). The test was performed in triplicates from blood samples of two volunteers with no reported medication.
4. Discussion
The popular alpine plant Edelweiss (L. alpinum Cass.) is used in folk medicine for the treatment of diarrhoea, fever, and ‘abdominal pain’. Edelweiss root extracts show a complex pattern of secondary plant metabolites of several compound classes like coumarins, lignans, sesquiterpenes, polyacetylens, diterpenes, and others.22 One of the major constituents of the roots is leoligin, a lariciresinol-type lignan. Lignans are polyphenolic plant metabolites derived from phenylalanine via dimerization of substituted cinnamic alcohols.
Until now, only a small number of studies have reported on the impact of lignans on the cardiovascular system, and only a few different lignans have been tested so far. The existing data suggest that lignans have a preventive effect on cardiovascular disease and are cell-protective agents with lipid-lowering, anti-oxidative, anti-hypertensive, anti-thrombotic, and anti-inflammatory activities; however, a large number of lignan-based cancer therapy studies (in vitro and in vivo) also have suggested a profound cytotoxic and cell death-inducing activity of this class of compounds.23,24 In line with this, also profound anti-angiogenic activity of certain lignans was demonstrated.25,26 Currently available data are not free of contradictions, which may be partially explained by the use of different lignans and cell types. Honokiol, for instance, a lignan constituent of the plant Magnolia officinalis, was shown to inhibit cell death in ECs,27 but also to potentiate cell death in vivo and in vitro in other systems.28 In SMCs, honokiol was demonstrated to induce an arrest in the G1-phase of the cell cycle, which is associated with up-regulation of p21/WAF1.29 Magnolol, another lignan isolated from M. officinalis, induces cell death in SMCs in a caspase-dependent manner;30 it inhibited both TNF-α-mediated VCAM-1 expression,31 and IL-6-induced STAT3 expression in ECs.32 An interesting study by Razuvaev et al.33 reported that the cyclolignan picropodophyllin inhibited intimal hyperplasia in vivo after balloon injury via interaction with the IGF-receptor and ERK signalling pathway. A few other lignan-type compounds, like flax seed lignans, have been tested in different model systems of inflammation, cancer, and cardiovascular diseases.
In general, however, due to a lack of knowledge concerning the specificities and characteristics of lignans, the mechanisms underlying the interaction of lignans with cells and organ systems, like the cardiovascular system, have not been well defined.
In contrast to previously analysed lignans, the present study demonstrated that leoligin is not toxic for SMCs and ECs. This may be due to the structural differences between individual lignans. Leoligin may represent a special type of lignan characterized by its cell cycle inhibitory activity and importantly also by its lack of toxicity and cell death-inducing activity. In contrast to honokiol, which causes a cell cycle arrest in the G1-phase by up-regulating p21/WAF1,29 our data suggest that leoligin causes a G1 arrest by increasing p27/KIP protein levels. Although the effect of both compounds is similar namely, a G1-phase arrest, the underlying signalling processes may differ. P27/KIP is well known to bind and thereby inactivate the cyclinE/cdk2 complex which phosphorylates pRB. Phosphorylated pRB loses its ability to inhibit transcription factors like E2F, which upon release, activates proliferation-promoting genes. The shift in the molecular weight of p27/KIP-1 at 50 µM leoligin from 27 to 58 kDa and 85 kDa may indicate the binding of p27/KIP-1 to the cyclinE/cdk2 complex or may also indicate an oligomerization of molecules (dimers/trimers). The above in vitro results were supported by our in vivo experiments, where leoligin was applied as a periadventitial reservoir to vein graft after the re-implantation. Immunohistochemistry of tissue samples taken 4 weeks after the treatment showed still a large number of SMCs in the leoligin group staining positive for p27/KIP. This may indicate that the mechanisms described above are active also in vivo and that a single dose of leoligin exerts a long-lasting effect in vivo. However, the precise signalling pathways activated by leoligin leading to the accumulation and change in the molecular weight of p27/KIP-1 protein remain to be elucidated.
To overcome or delay vein graft failure after CABG, the major therapeutic aims are the prevention of neointima formation and intimal hyperplasia as well as graft atherosclerosis. Although some progress has been made in past years, suitable drugs are still lacking. With improved understanding of the cellular and molecular mechanisms leading to vein graft failure after CABG, several treatment strategies emerged for inhibiting or blocking neointima formation and intimal hyperplasia as well as graft atherosclerosis. Numerous drugs like heparins, angiotensin-converting enzyme inhibitors, antagonists to growth factors such as terbinafine or trapidil, angiopeptin (a peptide analogue of somatostatin), and cytostatic agents such as etoposide or doxorubicin have been tested for their capability to prevent vascular proliferative diseases. However, the efficacy documented in animal studies could not be achieved in clinical trials.34 At present, anti-proliferative therapeutics targeting specific parts of the cell cycle are in the focus of interest. Promising agents include rapamycine, a macrocyclic triene antibiotic produced by Streptomyxes hygroscopius, paclitaxel, a cytotoxic bark constitutent of Taxus brevifolia, and flavopiridol, a semi-synthetic flavone derivative.
Leoligin is an interesting alternative. In contrast to most of these compounds, some of which are also clinically applied, leoligin is not toxic for ECs. Cytotoxic agents are too aggressive and often cause not only SMC apoptosis and inhibition of proliferation, but also significantly reduced endothelial viability. Since vascular endothelial wound closure after PCI or CABG is very important in vascular healing and anti-thrombosis, compounds that are less toxic for ECs are interesting agents for the prevention of graft failure and might play an important role in intra- and extravascular drug-eluting stents and matrices. Leoligin seems to possess precisely this characteristic. Although it inhibits ECs proliferation (Figure 5), which may reduce local endothelial healing, a wound repair via the circulation (EC precursors and circulating ECs) seems possible. Accordingly, leoligin seems to be a promising compound to treat and prevent intimal hyperplasia and thromboses after CABG.
Without doubt, additional studies are required to elucidate the applicability of leoligin in a clinical setting.
Funding
This project was supported by the Austrian National Bank (project 12697 to D.B.), and the Austrian Science Fund (FWF) (NFN project ‘DNTI’, S10703-B03). Funding to pay the Open Access charge was provided by the Austrian National Bank.
Acknowledgements
The authors would like to thank Pentapharm Ltd, Switzerland, for providing the plant material, T.M., Margit Lanthaler, and Sandra Frotschnig for excellent technical assistance, and Blair Henderson and Rajam Csordas-Iyer for linguistic corrections.
Conflict of interest: none declared.
References
- 1.WHO. Cardiovascular diseases. 2007 [Google Scholar]
- 2.Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93–101. doi: 10.1016/s0003-4975(03)01331-6. [DOI] [PubMed] [Google Scholar]
- 3.Schachner T, Laufer G, Bonatti H. The role of vein grafts in coronary surgery. European Surgery. 2007;39:72–75. [Google Scholar]
- 4.Lau GT, Lowe HC, Kritharides L. Cardiac saphenous vein bypass graft disease. Semin Vasc Med. 2004;4:153–159. doi: 10.1055/s-2004-835373. [DOI] [PubMed] [Google Scholar]
- 5.Tsui JC, Dashwood MR. Recent strategies to reduce vein graft occlusion: a need to limit the effect of vascular damage. Eur J Vasc Endovasc Surg. 2002;23:202–208. doi: 10.1053/ejvs.2002.1600. [DOI] [PubMed] [Google Scholar]
- 6.Hozumi T, Yoshikawa J, Yoshida K, Akasaka T, Takagi T, Honda Y, et al. Use of intravascular ultrasound for in vivo assessment of changes in intimal thickness of angiographically normal saphenous vein grafts one year after aortocoronary bypass surgery. Heart. 1996;76:317–320. doi: 10.1136/hrt.76.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin ML, Veith FJ, Panetta TF, Gordon RE, Wengerter KR, Suggs WD, et al. Saphenous vein biopsy: a predictor of vein graft failure. J Vasc Surg. 1993;18:407–414. [PubMed] [Google Scholar]
- 8.Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:I435–I440. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]
- 9.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmieder A, Schwaiger S, Csordas A, Backovic A, Messner B, Wick G, et al. Isogentisin—a novel compound for the prevention of smoking-caused endothelial injury. Atherosclerosis. 2007;194:317–325. doi: 10.1016/j.atherosclerosis.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Dobner MJ, Ellmerer EP, Schwaiger S, Narantuya S, Ododnchimeg B, Stütz M, et al. New lignan, benzofuran and sesquiterpen derivatives from the roots of Leontopodium alpinum and L. leontopodioides. Helvetica Chimica Acta. 2003;86:2284–2285. [Google Scholar]
- 12.Bernhard D, Pfister G, Huck CW, Kind M, Salvenmoser W, Bonn GK, et al. Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. FASEB J. 2003;17:2302–2304. doi: 10.1096/fj.03-0312fje. [DOI] [PubMed] [Google Scholar]
- 13.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Bernhard D, Ausserlechner MJ, Tonko M, Loffler M, Hartmann BL, Csordas A, et al. Apoptosis induced by the histone deacetylase inhibitor sodium butyrate in human leukemic lymphoblasts. FASEB J. 1999;13:1991–2001. doi: 10.1096/fasebj.13.14.1991. [DOI] [PubMed] [Google Scholar]
- 15.Grabner R, Till U, Heller R. Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion molecule-1 in formaldehyde-fixed endothelial cell monolayers. Cytometry. 2000;40:238–244. doi: 10.1002/1097-0320(20000701)40:3<238::aid-cyto9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Bernhard D, Skvortsov S, Tinhofer I, Hubl H, Greil R, Csordas A, et al. Inhibition of histone deacetylase activity enhances Fas receptor-mediated apoptosis in leukemic lymphoblasts. Cell Death Differ. 2001;8:1014–1021. doi: 10.1038/sj.cdd.4400914. [DOI] [PubMed] [Google Scholar]
- 17.Schachner T, Laufer G, Bonatti J. In vivo (animal) models of vein graft disease. Eur J Cardiothorac Surg. 2006;30:451–463. doi: 10.1016/j.ejcts.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Schachner T, Heiss S, Zipponi D, Tzankov A, Bernecker O, Kroell A, et al. Perivascular treatment with azathioprine reduces neointimal hyperplasia in experimental vein grafts. Heart Surg Forum. 2006;9:E515–E517. doi: 10.1532/HSF98.20051168. [DOI] [PubMed] [Google Scholar]
- 19.Favaloro EJ. Clinical application of the PFA-100. Curr Opin Hematol. 2002;9:407–415. doi: 10.1097/00062752-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Jilma B. Platelet function analyzer (PFA-100): a tool to quantify congenital or acquired platelet dysfunction. J Lab Clin Med. 2001;138:152–163. doi: 10.1067/mlc.2001.117406. [DOI] [PubMed] [Google Scholar]
- 21.Keiss HP, Schwaiger S, Dobner MJ, Stuppner H, Vollmar AM, Dirsch VM. A lignan from Leontopodium alpinum inhibits the TNF-a-induced expression of ICAM-1 and E-Selectin in a NF-kappaB-independent manner. Naunyn Schmiedebergs Arch Pharmacol. 2002;687(Suppl. 1) (Abstract) [Google Scholar]
- 22.Schwaiger S, Adams M, Seger C, Ellmerer EP, Bauer R, Stuppner H. New constituents of Leontopodium alpinum and their in vitro leukotriene biosynthesis inhibitory activity. Planta Med. 2004;70:978–985. doi: 10.1055/s-2004-832625. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Kim SB, You YJ, Ahn BZ. Deoxypodophyllotoxin; the cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 2002;68:271–274. doi: 10.1055/s-2002-23140. [DOI] [PubMed] [Google Scholar]
- 24.Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH, Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem. 2002;84:532–544. [PubMed] [Google Scholar]
- 25.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 26.Bergman JM, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Chen S, Wang Y. Honokiol up-regulates prostacyclin synthease protein expression and inhibits endothelial cell apoptosis. Eur J Pharmacol. 2007;554:1–7. doi: 10.1016/j.ejphar.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 28.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–633. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 29.Lee B, Kim CH, Moon SK. Honokiol causes the p21WAF1-mediated G(1)-phase arrest of the cell cycle through inducing p38 mitogen activated protein kinase in vascular smooth muscle cells. FEBS Lett. 2006;580:5177–5184. doi: 10.1016/j.febslet.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 30.Chen JH, Wu CC, Hsiao G, Yen MH. Magnolol induces apoptosis in vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:127–133. doi: 10.1007/s00210-003-0779-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen YH, Lin SJ, Chen JW, Ku HH, Chen YL. Magnolol attenuates VCAM-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br J Pharmacol. 2002;135:37–47. doi: 10.1038/sj.bjp.0704458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SC, Chang YL, Wang DL, Cheng JJ. Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and gene expression in endothelial cells. Br J Pharmacol. 2006;148:226–232. doi: 10.1038/sj.bjp.0706647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razuvaev A, Henderson B, Girnita L, Larsson O, Axelson M, Hedin U, et al. The cyclolignan picropodophyllin attenuates intimal hyperplasia after rat carotid balloon injury by blocking insulin-like growth factor-1 receptor signaling. J Vasc Surg. 2007;46:108–115. doi: 10.1016/j.jvs.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 34.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]