Abstract
Aims
Angiotensin II (ANG II)-induced inflammatory and oxidative stress responses contribute to the pathogenesis of hypertension. In this study, we determined whether nuclear factor-kappa B (NF-κB) activation in the hypothalamic paraventricular nucleus (PVN) increases oxidative stress and contributes to the ANG II-induced hypertensive response.
Methods and results
Rats were infused intravenously with ANG II (10 ng/kg per min) or saline for 4 weeks. These rats received either vehicle or losartan (LOS, 20 µg/h), an angiotensin II type 1 receptor (AT1-R) antagonist; pyrrolidine dithiocarbamate (PDTC, 5 µg/h), a NF-κB inhibitor; tempol (TEMP, 80 µg/h), a superoxide scavenger; LOS (20 µg/h), and PDTC (5 µg/h); or TEMP (80 µg/h) and PDTC (5 µg/h), given intracerebroventricularly (ICV) via osmotic minipump. ANG II infusion resulted in increased mean arterial pressure, renal sympathetic nerve activity, plasma proinflammatory cytokines (PIC), norepinephrine, and aldosterone. These rats also had higher levels of Fra-LI (an indicator of chronic neuronal activation), PIC, phosphorylated IKKβ, NF-κB subunits, AT1-R, superoxide, and gp91phox (a subunit of NADP(H) oxidase) and lower levels of IκBα in the PVN than control animals. ICV treatment with LOS, PDTC, or TEMP attenuated these changes, and combined treatment with ICV LOS and PDTC, or ICV TEMP and PDTC prevented these ANG II-induced hypertensive responses.
Conclusion
These findings suggest that an ANG II-induced increase in the brain renin–angiotensin system activates NF-κB in the PVN and contributes to sympathoexcitation in hypertension. The increased superoxide in the PVN contributes to NF-κB activation and neurohumoral excitation in hypertension.
Keywords: Hypertension, Nuclear factor-kappa B, Brain, Angiotensin II, Oxidative stress
1. Introduction
Angiotensin II (ANG II), the major biologically active component of the renin–angiotensin system (RAS) and a potent vasoconstrictor, plays important roles in the maintenance of cardiovascular function and in the development of hypertension.1 The angiotensin II type 1 receptor (AT1-R) plays a predominant role in the central regulation of arterial blood pressure (BP).2 ANG II is a large peptide and does not readily cross the blood–brain barrier; it exerts its actions by binding to neuronal AT1-R in the circumventricular organs, including the subfornical organ and organum vasculosum lamina terminalis, where the blood–brain barrier is weak or absent, and subsequently activating hypothalamic and brain stem sites such as the paraventricular nucleus (PVN) and ventrolateral medulla, contributing to sympathoexcitation and hypertensive response.3
Considerable evidence suggests that the PVN is an important centre for integrating neural signals of the pressor response to ANG II.4 The binding of ANG II to AT1-R in the PVN modulates sympathetic outflow, which could be a trigger of the hypertensive response.2 Although the actions of ANG II in the PVN have been associated with sympathoexcitation and hypertension, the mechanism by which peripheral ANG II modulates central nervous system cytokines, particularly in the PVN, and contributes to hypertension is not clear.
A growing body of evidence indicates that hypertension is an inflammatory state wherein proinflammatory cytokines (PIC), such as tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), contribute to the hypertensive effect.5 In vitro and in vivo evidence suggests that ANG II infusion increases PIC and that RAS blockade attenuates circulating and tissue levels of cytokines in cardiovascular disease.6 These findings suggest a cross-talk between the RAS and PIC. Recent findings from our laboratory and others suggest that PIC are produced in the PVN of heart failure rats and contribute to sympathoexcitation.7 However, it is not known whether ANG II induces the production of PIC in the PVN to contribute to pressor response.
It is well known that ANG II8 and PIC9 induce oxidative stress in vascular tissue and contribute to hypertensive response. Inhibition of the nicotinamide-adenine dinucleotide phosphate [NADP(H)] oxidase complex attenuates ANG II-induced increases in superoxide production,10 thus implicating the involvement of reactive oxygen species (ROS) in the pathogenesis of ANG II-induced hypertension. Recent studies have shown that ROS are increased in the hypothalami of hypertensive rats and that ROS blockade decreases sympathetic activity;11 this may be one potential mechanism by which brain ROS contribute to hypertensive response. However, it is not known whether ANG II, PIC, and ROS interact within the PVN and contribute to hypertensive response.
Despite the abundant evidence indicating that TNF-α contributes significantly to cardiac dysfunction in animal models, the results of two large clinical trials using etanercept, a truncated, soluble TNF receptor antagonist (RENAISSANCE), and infliximab (ATTACH), a TNF-α blocking antibody, were largely negative.12 The failure of these trials could be due to the targeting of one cytokine, when it is known that several PIC are activated in heart failure. One of the most important downstream molecules involved in the activation of PIC is the transcriptional factor nuclear factor-kappa B (NF-κB). NF-κB is involved in both the production of PIC and the induction of oxidative stress.13 However, it is not known whether peripheral infusion of ANG II upregulates brain NF-κB and contributes to hypertensive response. Therefore, we hypothesized that ANG II infusion upregulates oxidative stress to induce NF-κB activation in the PVN and contributes to sympathoexcitation and hypertension. Our result suggests that central blockade of NF-κB using pyrrolidine dithiocarbamate (PDTC) decreases PIC, RAS, and oxidative stress in the PVN, and attenuates sympathoexcitation, whereas a combined treatment of an AT1-R blocker and PDTC or tempol (TEMP) and PDTC normalizes oxidative stress in the PVN in ANG II-induced hypertension.
2. Methods
2.1. Animals
Experiments were performed on adult male Sprague–Dawley rats (275–300 g). Rats were housed in a climate-controlled room with a 12 h light–dark cycle and allowed access to standard rat chow and tap water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committees of both Louisiana State University and Shanxi Medical University. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. General experimental protocol
Baseline BP and heart rate (HR) were recorded for 3 days, then ANG II (dissolved in saline) was continuously infused intravenously at a rate of 10 ng/kg per min for 4 weeks by an Alzet osmotic minipump.14,15 In control rats, normal saline (NS) alone was infused. All rats were implanted with intracerebroventricular (ICV) cannulae for continuous infusion (0.25 µL/h) of the NF-κB inhibitor PDTC (5 µg/h), the AT1-R antagonist losartan (LOS, 20 µg/h), the superoxide dismutase mimetic TEMP (80 µg/h), or LOS (20 µg/h) and PDTC (5 µg/h), or TEMP (80 µg/h) and PDTC (5 µg/h), or artificial cerebrospinal fluid (aCSF, vehicle) by Alzet osmotic minipump for 4 weeks. ANG II, PDTC, LOS, and TEMP were from Sigma-Aldrich. The doses used in this study are based on our previous studies13,16 and other studies demonstrating the effectiveness of these drugs in blocking neurohumoral excitation.17–19 At the end of 4 weeks, rats were sacrificed to collect blood and brain tissue for molecular and immunohistochemical studies. Some rats were anaesthetized with urethane (1.5 g/kg, i.p.) for terminal electrophysiological studies.
2.3. Implantation of arterial and venous catheters and intracerebroventricular cannulae
Surgical procedures were performed after administration of pentobarbital sodium (50 mg/kg, i.p.). Catheters were placed into the femoral artery and advanced into the abdominal aorta for the measurement of arterial pressure, and into the femoral vein and advanced into the vena cava for minipump infusion of ANG II as previously described.14,15 All rats had cannulae implanted in the lateral cerebral ventricle as previously described.13 For combined treatments, bilateral cannulae were implanted into the lateral ventricle and two osmotic minipumps used for drug infusions.
2.4. Mean arterial pressure measurement
The femoral artery cannula was flushed with 0.1 mL heparinized saline (50 U/mL) and connected to a pressure transducer attached to a digital BP monitor and a polygraph. Mean arterial pressure (MAP) and HR data were collected for 30 min between 8 and 11 AM and averaged.
2.5. Sympathetic neural recordings
The general methods have been described previously.7 Under urethane anaesthesia (1.5 g/kg, i.p.), the left renal nerves were isolated via retroperitoneal laparotomy. The recordings of rectified and integrated renal sympathetic nerve activity (RSNA), MAP, and HR were analysed using methods described previously.7 For each animal, the net RSNA was normalized using methods recorded by Xu et al.20
2.6. Collection of blood and tissue samples
Rats were decapitated while still under anaesthesia to collect trunk blood and tissue samples. Trunk blood was collected in chilled ethylenediaminetetraacetic acid tubes. Plasma samples were separated and stored at −80°C until assayed for PIC, norepinephrine (NE), and aldosterone (ALDO) levels.
2.7. Biochemical assays
Plasma and tissue TNF-α and interleukin-1β (IL-1β) levels were measured as previously described,21 and plasma and tissue IL-6 levels were measured using a BioSource (Invitrogen) rat IL-6 ELISA kit according to the manufacturer's specifications. The minimum detectable concentration of IL-6 was <7 pg/mL. Inter-assay coefficients of variation were: TNF-α, ≤4.3%; IL-1β, ≤9.7%; and IL-6, ≤10%. Intra-assay coefficients of variation were: TNF-α, ≤2.7%; IL-1β, ≤8.2%; and IL-6, ≤5%.
Plasma ALDO was measured using ELISA techniques as previously described.21 Plasma NE was measured using HPLC as described previously.22
2.8. Immunohistochemistry and immunofluorescence
Immunohistochemical studies were performed as described previously.13,23 A double-staining protocol was used for Fra-like (Fra-LI) activity (Santa Cruz Biotechnology) plus PIC (Santa Cruz Biotechnology) staining in the PVN. For each animal, labelled neurons within the bilateral borders of the PVN were counted manually in two representative 40 µm transverse sections at about −1.80 mm from bregma, and an average value was reported. Superoxide generation was determined by fluorescent-labelled dihydroethidium (DHE; Molecular Probes) staining as previously described.22 Protein immunofluorescence staining was performed as previously described.21 The primary NF-κB p50 and gp91phox antibodies were from Santa Cruz Biotechnology, and the phosphorylated IKKβ (p-IKKβ) antibody was from Cell Signalling Technology. Positive immunofluorescent-staining cells were counted under confocal microscopy in four view fields (equal area) randomly selected from bilateral PVN transverse sections at about −1.80 mm from bregma. One sample consisted of the average of four view fields from a section.
2.9. Western blot
Protein extracted from the PVN was used for the measurement of p-IKKβ, gp91phox, and AT1-R expression by western blot.24 The gp91phox and p-IKKβ antibodies used for western blot were the same antibodies used for immunofluorescence; the AT1-R antibody was from Abcam. Protein loading was controlled by probing all blots with β-actin antibody (Santa Cruz Biotechnology) and normalizing p-IKKβ, gp91phox, and AT1-R protein intensities to that of β-actin. The bands were analysed using NIH Image J software.
2.10. Analysis of mRNA expression by real-time reverse transcriptase–polymerase chain reaction
NF-κB p65, IκBα, and AT1-R mRNA expression were determined by real-time RT–PCR as previously described.25 The primer sequences used were as follows: NF-κB p65 subunit forward 5′-CATCAAGATCAATGGCTACA-3′, NF-κB p65 subunit reverse 5′-CACAAGTTCATGTGGATGAG-3′; IκBα forward 5′-CCCTGGAAAATCTTCAGACG-3′, IκBα reverse 5′-ACAAGTCCACGTTCCTTTGG-3′; AT1-R forward 5′-CAACCTCCAGCAATCCTTTC-3′, AT1-R reverse 5′-CCCAAATCCATACAGCCACT-3′. Gene expression levels of NF-κB p65, IκBα, and AT1-R mRNA were normalized to GAPDH levels.
2.11. Statistical analysis
All data are expressed as mean ± SEM. The significance of differences between mean values was analysed by ANOVA followed by a post hoc Tukey test. BP data were analysed by repeated measures ANOVA. A probability value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Mean arterial pressure, renal sympathetic nerve activity, and angiotensin II type 1 receptor in the paraventricular nucleus
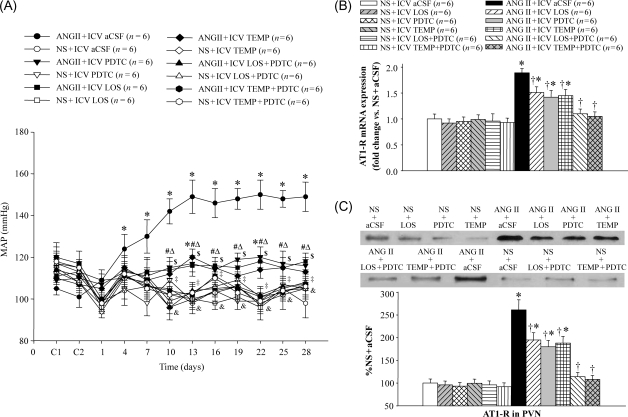
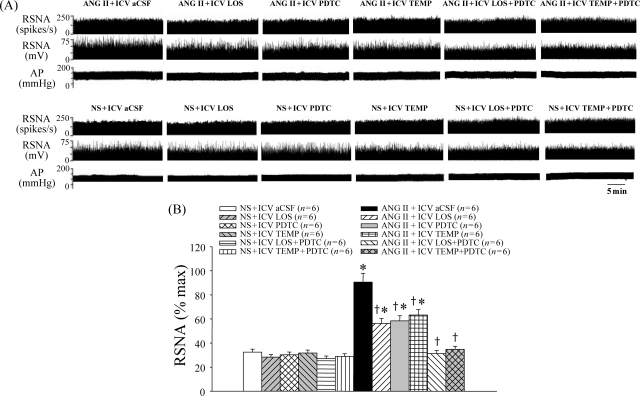
ANG II infusion induced a significant increase in MAP compared with controls from Day 4; MAP remained elevated throughout Day 28 of the study (Figure 1A). ICV treatment with LOS, PDTC, or TEMP attenuated ANG II-induced pressor response, RSNA (% max), and AT1-R in the PVN (Figures 1 and 2). In contrast, ICV treatment with LOS + PDTC or TEMP + PDTC prevented the increases in MAP, RSNA, and AT1-R in the PVN in ANG II-infused rats (Figures 1 and 2).
Figure 1.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on mean arterial pressure (MAP) and angiotensin II type 1 receptor (AT1-R) in the paraventricular nucleus (PVN) of angiotensin II (ANG II)-infused rats and control rats. (A) Mean arterial pressure in different groups. (B) Angiotensin II type 1 receptor mRNA expression in different groups. (C) Western blot of angiotensin II type 1 receptor in the paraventricular nucleus in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF); †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF; #P < 0.05 ANG II + ICV PDTC vs. ANG II + ICV aCSF; ΔP < 0.05 ANG II + ICV LOS vs. ANG II + ICV aCSF; $P < 0.05 ANG II + ICV TEMP vs. ANG II + ICV aCSF; ‡P < 0.05 ANG II + ICV LOS + PDTC vs. ANG II + ICV aCSF; &P < 0.05 ANG II + ICV TEMP + PDTC vs. ANG II + ICV aCSF.
Figure 2.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on renal sympathetic nerve activity (RSNA: % max) in angiotensin II (ANG II)-infused rats and control rats. (A) Renal sympathetic nerve activity in different groups. (B) Bar graph comparing renal sympathetic nerve activity in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF). †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
3.2. Proinflammatory cytokines in the paraventricular nucleus
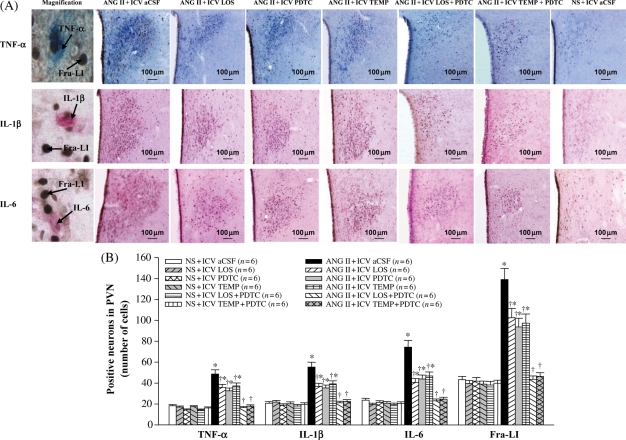
Immunohistochemistry studies revealed that ANG II-infused rats had significantly more Fra-LI-, TNF-α-, IL-1β-, and IL-6-positive neurons in the PVN than saline-infused rats (Figure 3). TNF-α-, IL-1β-, and IL-6-positive neurons in ANG II-infused rats were distributed among Fra-LI positive neurons, with 32.4% of Fra-LI positive neurons also positive for TNF-α, 39.3% of Fra-LI positive neurons also positive for IL-1β, and 36.5% of Fra-LI positive neurons also positive for IL-6. There were fewer positive neurons of Fra-LI, TNF-α, IL-1β, and IL-6 in the PVN of ANG II + ICV LOS, ANG II + ICV PDTC, or ANG II + ICV TEMP rats than in ANG II + ICV aCSF rats, and numbers were still higher than those of saline-infused rats (Figure 3). ANG II + ICV LOS, ANG II + ICV PDTC, and ANG II + ICV TEMP-treated rats had fewer Fra-LI positive PVN neurons also positive for TNF-α (ANG II + ICV LOS 16.2%, ANG II + ICV PDTC 14.6%, and ANG II + ICV TEMP 14.9%), IL-1β (ANG II + ICV LOS 18.7%, ANG II + ICV PDTC 20.1%, and ANG II + ICV TEMP 17.9%), and IL-6 (ANG II + ICV LOS 15.6%, ANG II + ICV PDTC 12.8%, and ANG II + ICV TEMP 14.1%) in the PVN. ICV treatment with LOS + PDTC or TEMP + PDTC prevented the ANG II-induced increases in Fra-LI, TNF-α, IL-1β, and IL-6 in the PVN. ELISA studies showed that the levels of TNF-α, IL-1β, and IL-6 in the PVN of ANG II-infused rats were higher than in saline-infused rats (Table 1), that ICV treatment with LOS, PDTC, or TEMP reduced the levels of TNF-α, IL-1β, and IL-6 in the PVN, and that ICV treatment with LOS + PDTC or TEMP + PDTC normalized the levels of TNF-α, IL-1β, and IL-6 in the PVN of ANG II-infused rats (Table 1).
Figure 3.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on Fra-like (Fra-LI), tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) expression in the paraventricular nucleus (PVN) of angiotensin II (ANG II)-infused rats and control rats. (A) Immunohistochemistry for Fra-like (black dots), tumour necrosis factor-alpha (blue), interleukin-1β (pink), and interleukin-6 (pink) positive neurons in different groups. (B) Bar graph comparing Fra-like, tumour necrosis factor-alpha, interleukin-1β, and interleukin-6 positive neurons in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF). †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
Table 1.
Paraventricular nucleus levels of proinflammatory cytokines and plasma humoral indicators
| Group | PVN (pg/mg protein, n = 6) |
plasma (pg/mL, n = 6) |
|||||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-6 | IL-1β | IL-6 | NE | ALDO | |
| NS + ICV aCSF | 3.0 ± 0.2 | 17.3 ± 1.5 | 18.9 ± 1.6 | 20.7 ± 1.9 | 40.3 ± 3.8 | 201.4 ± 14.6 | 219.3 ± 16.9 |
| NS + ICV LOS | 2.8 ± 0.2 | 18.1 ± 1.6 | 18.2 ± 1.5 | 18.5 ± 1.5 | 38.5 ± 3.4 | 195.2 ± 13.4 | 215.8 ± 16.2 |
| NS + ICV PDTC | 2.7 ± 0.1 | 15.3 ± 1.2 | 15.2 ± 1.2 | 16.8 ± 1.2 | 36.6 ± 3.1 | 190.1 ± 12.9 | 213.2 ± 15.6 |
| NS + ICV TEMP | 3.1 ± 0.2 | 16.8 ± 1.4 | 17.4 ± 1.5 | 21.3 ± 1.8 | 37.9 ± 3.2 | 187.2 ± 12.4 | 210.7 ± 15.3 |
| NS + ICV LOS + PDTC | 2.6 ± 0.2 | 14.2 ± 1.2 | 14.7 ± 1.2 | 15.9 ± 1.3 | 34.8 ± 3.0 | 181.5 ± 11.6 | 202.5 ± 14.6 |
| NS + ICV TEMP + PDTC | 2.7 ± 0.2 | 15.1 ± 1.3 | 15.0 ± 1.2 | 16.7 ± 1.3 | 35.1 ± 3.2 | 185.8 ± 11.9 | 207.8 ± 14.8 |
| ANG II + ICV aCSF | 6.8 ± 0.6* | 45.6 ± 4.2* | 54.3 ± 4.5* | 53.8 ± 4.7* | 125.5 ± 11.8* | 356.1 ± 28.2* | 398.7 ± 30.2* |
| ANG II + ICV LOS | 4.9 ± 0.4*† | 32.7 ± 2.8*,† | 37.6 ± 3.0*,† | 40.4 ± 3.5*,† | 83.7 ± 7.2*,† | 265.7 ± 19.3*,† | 284.1 ± 23.0*,† |
| ANG II + ICV PDTC | 4.7 ± 0.4*† | 30.4 ± 2.6*,† | 35.2 ± 2.7*,† | 37.3 ± 2.8*,† | 75.5 ± 6.4*,† | 274.8 ± 22.6*,† | 288.5 ± 23.8*,† |
| ANG II + ICV TEMP | 5.0 ± 0.4*† | 34.4 ± 3.0*,† | 39.1 ± 3.2*,† | 39.6 ± 3.1*,† | 78.3 ± 6.7*,† | 270.3 ± 21.3*,† | 291.4 ± 24.5*,† |
| ANG II + ICV LOS + PDTC | 3.2 ± 0.2† | 18.3 ± 1.7† | 19.0 ± 1.7† | 21.9 ± 1.9† | 40.5 ± 3.9† | 206.5 ± 14.9† | 221.4 ± 17.1† |
| ANG II + ICV TEMP + PDTC | 3.3 ± 0.3† | 19.1 ± 1.8† | 19.5 ± 1.8† | 22.4 ± 2.0† | 41.7 ± 4.2† | 217.4 ± 16.7† | 235.7 ± 18.6† |
*P < 0.05 vs. control (NS + Treated or NS + ICV aCSF).
†P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
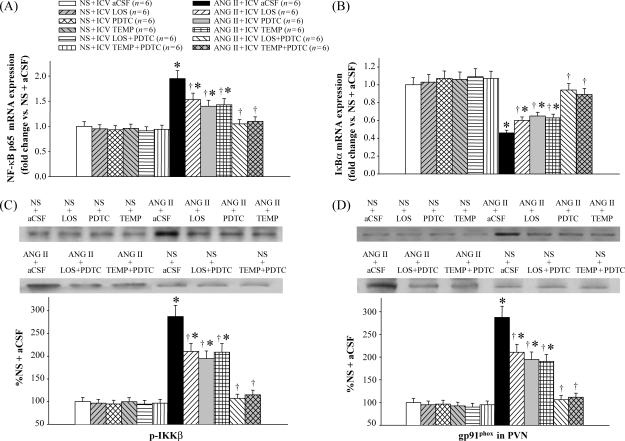
3.3. Nuclear factor-kappa B in the paraventricular nucleus
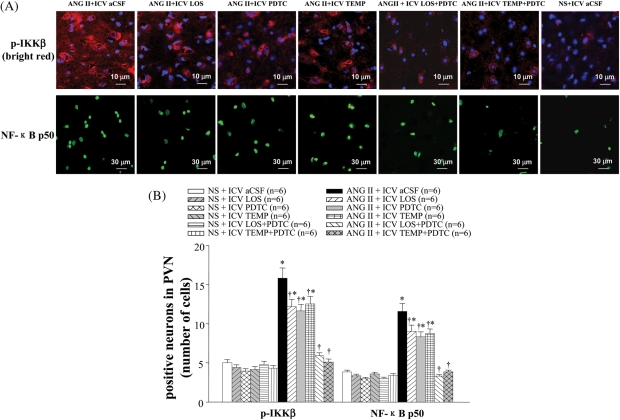
Immunofluorescence studies showed that the levels of p-IKKβ and NF-κB p50 in the PVN of ANG II-infused rats were higher than saline-infused rats (Figure 4). ANG II-infused rats treated with LOS, PDTC, or TEMP had fewer p-IKKβ- or NF-κB p50-positive neurons in the PVN than ANG II + ICV aCSF rats, but had more than saline-infused rats (Figure 4). Western blot also showed lower expression of p-IKKβ in the PVN of LOS-, PDTC-, or TEMP-treated hypertensive rats than in saline-treated hypertensive rats (Figure 5C). ICV treatment with LOS + PDTC or TEMP + PDTC normalized the levels of p-IKKβ and NF-κB p50 in the PVN in ANG II-infused rats.
Figure 4.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on phosphorylated IKKβ (p-IKKβ) and NF-κB p50 in the paraventricular nucleus (PVN) of angiotensin II (ANG II)-infused rats and control rats. (A) Immunofluorescence for phosphorylated IKKβ (bright red) and NF-κB p50 (bright green) positive neurons in different groups. Neuronal nuclei are shown in blue. (B) Bar graph comparing paraventricular nucleus phosphorylated IKKβ and NF-κB p50 positive neurons in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF). †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
Figure 5.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on mRNA expression for NF-κB p65 and IκBα, and protein expression for phosphorylated IKKβ (p-IKKβ) and gp91phox in the paraventricular nucleus (PVN) of angiotensin II (ANG II)-infused rats and control rats. (A) NF-κB p65 mRNA expression in the paraventricular nucleus in different groups. (B) IκBα mRNA expression in the paraventricular nucleus in different groups. (C) Western blot of phosphorylated IKKβ in the paraventricular nucleus in different groups. (D) Western blot of the NADP(H) oxidase subunit gp91phox in the paraventricular nucleus in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF). †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
ANG II-infused rats had more NF-κB p65 and less IκBα mRNA expression in the PVN as determined by real-time RT–PCR (Figure 5A and B). ICV treatment with LOS, PDTC, or TEMP attenuated these changes, and ICV treatment with LOS + PDTC or TEMP + PDTC normalized NF-κB p65 and IκBα mRNA expression in the PVN of ANG II-infused rats (Figure 5A and B).
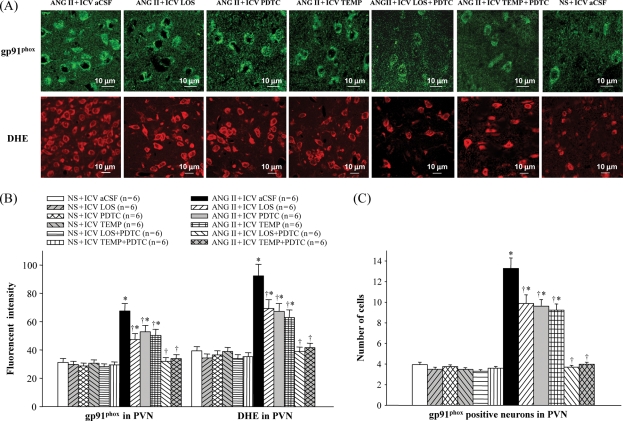
3.4. Superoxide and NADP(H) oxidase in the paraventricular nucleus
Immunofluorescence revealed that ANG II-infused rats had more superoxide in the PVN, as determined by fluorescent-labelled DHE and the NAD(P)H oxidase subunit gp91phox, when compared with saline-infused rats (Figure 6). ICV treatment with LOS, PDTC, or TEMP decreased gp91phox and DHE in the PVN of ANG II-infused rats (Figure 6). Western blot further demonstrated that ANG II-infused rats had higher expression of gp91phox in the PVN than saline-infused rats, and that ICV treatment with LOS, PDTC, or TEMP reduced gp91phox levels in the PVN of ANG II-infused rats (Figure 5D). ICV treatment with LOS + PDTC or TEMP + PDTC normalized the levels of superoxide and gp91phox in the PVN of ANG II-infused rats (Figures 5D and 6).
Figure 6.
Effect of intracerebroventricular (ICV) treatment with artificial cerebrospinal fluid (aCSF), losartan (LOS), pyrrolidine dithiocarbamate (PDTC), tempol (TEMP), LOS + PDTC, or TEMP + PDTC on superoxide and NADP(H) oxidase in the paraventricular nucleus (PVN) of angiotensin II (ANG II)-infused rats and control rats. (A) Immunofluorescence for the NADP(H) oxidase subunit gp91phox (bright green) and superoxide as determined by fluorescent-labelled dihydroethidium (DHE, bright red) in the paraventricular nucleus in different groups. (B) Immunofluorescent intensity of gp91phox and dihydroethidium in the paraventricular nucleus of different groups of rats. (C) Comparison of gp91phox positive neurons in the paraventricular nucleus in different groups. *P < 0.05 vs. control (NS + Treated or NS + ICV aCSF). †P < 0.05 ANG II + Treated vs. ANG II + ICV aCSF.
3.5. Plasma humoral factors
Plasma levels of IL-1β, IL-6, NE, and ALDO in ANG II-infused rats were higher than in saline-infused rats. ANG II-infused rats treated with LOS, PDTC, or TEMP had lower levels of plasma IL-1β, IL-6, NE, and ALDO than ANG II-infused rats treated with aCSF, but these values remained higher than those in saline-infused rats (Table 1). ICV treatment with LOS + PDTC or TEMP + PDTC normalized the levels of plasma IL-1β, IL-6, NE, and ALDO in ANG II-infused rats (Table 1).
4. Discussion
The novel findings of this study are: (i) ANG II infusion activates NF-κB in the PVN, and increases sympathoexcitation and hypertensive response, which are associated with the increases of PIC, AT1-R, and oxidative stress in the PVN; (ii) central blockade of AT1-R, NF-κB, or superoxide attenuates sympathoexcitation and BP, and decreases PIC, markers of NF-κB activation, AT1-R, and oxidative stress in the PVN of ANG II-infused rats; and (iii) combined treatment with central blockade of AT1-R and NF-κB, or central blockade of superoxide and NF-κB, normalizes BP, PIC, markers of NF-κB activation, AT1-R, and oxidative stress in the PVN and sympathetic activity of ANG II-infused rats.
RAS activation, and the subsequent increase in local ANG II production, is one major mechanism by which chronic ANG II infusion induces hypertension.26 A growing body of evidence indicates that ANG II upregulates pressor response by acting on the cardiovascular centres of the central nervous system and increasing sympathetic activity.26 ANG II also induces production of PIC, such as TNF-α, in the periphery by its direct effects on immune cell activation.27 The PVN is an important centre regulating sympathetic drive and fluid homeostasis.28 AT1-R is the primary receptor inducing the action of ANG II in the PVN; AT1-R blockade inhibits the effects of ANG II at this site.26 Recently, we demonstrated that increased PIC in the PVN contribute to sympathetic activation in heart failure rats.13 In addition, both in vitro and in vivo studies have demonstrated the existence of cross-talk between the RAS and TNF-α.24,29 Chronic AT1-R blockade significantly reduces circulating TNF-α levels in hypertensive patients.30 Further, studies from our laboratory using TNF-α knockout mice suggest that some effects of ANG II are, at least in part, mediated by TNF-α.25 Felder's laboratory recently showed that myocardial infarction increases PVN cytokines, modulates COX-2, and contributes to neurohumoral excitation.21 In heart failure, multiple neurohormones and modulators are activated; hence a clear role played by ANG II in the PVN cannot be determined in those studies. Thus, in this study, we examined the interplay between RAS, NF-κB, and oxidative stress in the PVN and its contribution to the ANG II-induced pressor response and sympathetic activity. ANG II infusion significantly increased sympathoexcitation, and elevated MAP, PIC, NF-κB, and oxidative stress in the PVN, whereas ICV treatment with LOS attenuated MAP, RSNA and PIC, NF-κB, and oxidative stress in the PVN of ANG II-infused rats. These results demonstrate that the brain RAS contributes to hypertension by modulating NF-κB and oxidative stress in the PVN.
NF-κB plays an important role in the pathogenesis of cardiovascular diseases, including hypertension. However, the mechanism by which NF-κB in the PVN contributes to the progression of hypertension is not known. Recent studies suggest that RAS components (angiotensinogen and the AT1-R) are the important products mediated by cytoplasmic NF-κB.31 Functional NF-κB p50/p65 complexes are present in essentially all cell types in the nervous system. ANG II, PIC, and ROS can effectively activate NF-κB in various types of cells. After phosphorylation of IκB by an IκB kinase (IKK)-containing signalsome, translocation of activated NF-κB to nuclei is the major regulator facilitating the synthesis of several different PIC, including TNF-α, IL-6, and AT1-R, in neurons.32 Biochemical and genetic ablation studies indicate that p-IKKβ is an important regulator of NF-κB activation.33,34 We reported that activated NF-κB in the PVN contributes to exaggerated sympathetic activity in heart failure rats.13 Furthermore, recent unpublished findings from our laboratory, using NF-κB knockout mice, demonstrated that these heart failure animals had attenuated oxidative stress in the PVN and brain stem, which was accompanied by decreased plasma NE. Interestingly, these animals also had attenuated expression of AT1-R protein and mRNA, suggesting an interaction between RAS, NF-κB, and oxidative stress in the cardiovascular regulatory centres in the brain. Since ANG II and PIC have been shown to upregulate NF-κB, we wanted to determine whether blocking brain NF-κB attenuated ANG II-induced responses. Treatment of ANG II-infused rats with PDTC decreased MAP and RSNA, and reduced expression of PIC and markers of NF-κB activation in the PVN of these rats, suggesting that NF-κB activation in the PVN contributes to hypertension. ICV treatment with LOS also decreased NF-κB activation in the PVN of hypertensive rats, indicating that, in the PVN, NF-κB plays an important role in modulating oxidative stress and pressor response to ANG II.
In addition to regulating PIC synthesis, NF-κB also contributes to NAD(P)H oxidase-dependent oxidative stress.34 Zimmerman et al.35 recently found that NAD(P)H-dependent superoxide in the central nervous system contributed to the mechanism of hypertension by promoting sympathoexcitation. Other recent studies indicate that ROS production is increased in humans with hypertension and also in several hypertensive animal models, and that ANG II activates NAD(P)H oxidase to induce the production of O2·−, which acts directly as a vasoconstrictor.10 In this study, we found that combined treatment with central blockade of AT1-R and NF-κB with ICV LOS + PDTC, or central blockade of superoxide and NF-κB with ICV TEMP + PDTC, normalized BP, PIC, NF-κB activation, and oxidative stress in the PVN of ANG II-infused rats. This suggests that the interaction between brain RAS, superoxide, and NF-κB plays a critical role in ANG II-induced sympathoexcitation and pressor response by modulating PIC synthesis in the PVN.
Since ANG II7 and PIC13,22 are known to induce oxidative stress, we examined whether increased superoxide in the PVN contributed to NF-κB activation by administering TEMP ICV to scavenge superoxide. TEMP treatment attenuated NF-κB activation in the PVN, suggesting that increased superoxide in the PVN after ANG II infusion contributes to the activation of NF-κB and PIC in the PVN and that this is accompanied by increased sympathetic activity in hypertensive rats. However, TEMP alone did not normalize these changes in the PVN, but when combined with PDTC, there was complete normalization of hypertension and sympathoexcitation.
From the above discussion, it is evident that multiple mechanisms contribute to ANG II-induced sympathoexcitation and BP response. In addition, there is considerable redundancy built into this proposed system, as both NF-κB and AT1-R can be upregulated by superoxide, so blockade of NF-κB alone may not be sufficient to bring about a complete inhibition of AT1-R protein synthesis in the PVN. In addition, it has been shown, at least in the periphery, that other transcription factors might contribute to the upregulation of AT1-R, e.g. activator protein-1, CREB, or ERK-1/2,36,37 which were not explored in this study. Thus, it is possible that mechanisms that have not yet been explored in the central nervous system could also contribute to sympathoexcitation and BP response.
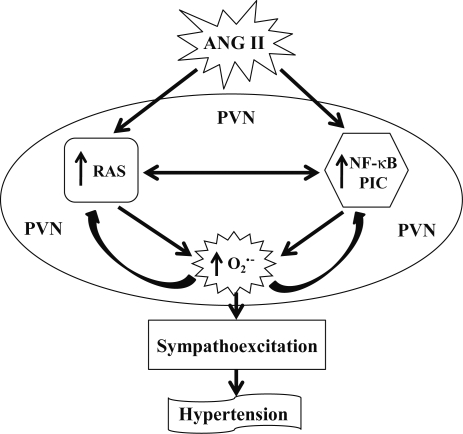
In summary, ANG II infusion resulted in a significant upregulation of oxidative stress, RAS, PIC, and NF-κB in the PVN. The interplay between RAS, PIC, and NF-κB resulted in a sustained increase in oxidative stress in the PVN, thereby contributing to the pathogenesis of hypertension. Individual blockade of these components attenuated, but did not normalize, oxidative stress and pressor response. Combination treatment with an AT1-R blocker and PDTC or PDTC and TEMP resulted in complete normalization of NF-κB activation and oxidative stress in the PVN. These findings suggest that an interaction between the RAS, NF-κB, and PIC induces superoxide in the PVN, contributing to sympathoexcitation and the pressor response in hypertension (Figure 7). Manipulations designed to inhibit superoxide and NF-κB activation may be effective adjuncts to the current treatment of hypertension.
Figure 7.
The schematic of the hypothesis showing the mechanism by which angiotensin II modulates nuclear factor-kappa B activation in the paraventricular nucleus and contributes to neurohumoral excitation and pressor response in hypertension.
Conflict of interest: none declared.
Funding
Supported by US National Institutes of Health (NIH) Grant RO1-HL-080544-01 (PI: J.F.), National Natural Science Foundation of China (No. 30770867), and Shantou University Medical College Grant.
References
- 1.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veerasingham SJ, Raizada MK. Brain renin–angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32:248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- 4.Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol. 2004;97:1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]
- 5.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 6.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- 7.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–R927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 8.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathway. J Hypertens. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megumi F, Katsuyuki A, Ai N, Toshiro F. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–367. doi: 10.1161/HYPERTENSIONAHA.107.091009. [DOI] [PubMed] [Google Scholar]
- 12.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 13.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin–angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgley AJ, Kett MM, Anderson WP. Evidence for renal vascular remodeling in angiotensin II-induced hypertension. J Hypertens. 2003;21:1401–1406. doi: 10.1097/00004872-200307000-00031. [DOI] [PubMed] [Google Scholar]
- 15.Reckelhoff JF, Zhang H, Srivastava K, Roberts LJ, 2nd, Morrow JD, Romero JC. Subpressor doses of angiotensin II increase plasma F(2)-isoprostanes in rats. Hypertension. 2000;35:476–479. doi: 10.1161/01.hyp.35.1.476. [DOI] [PubMed] [Google Scholar]
- 16.Kang YM, Ma Y, Guo YL, He RL, Guo ZW, Elks C, et al. Activation of microglia in paraventricular nucleus of hypothalamus: a source of central cytokines induced by nuclear factor-kappaB or oxidative stress in hypertension (Abstract) Circulation. 2008;118:S295–S296. [Google Scholar]
- 17.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension. 2008;52:679–686. doi: 10.1161/HYPERTENSIONAHA.108.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin–angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–H1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 19.Okada S, Yamaguchi-Shima N, Shimizu T, Arai J, Yorimitsu M, Yokotani K. Brain nuclear factor kappa B is involved in the corticotropin-releasing factor-induced central activation of sympatho-adrenomedullary outflow in rats. Eur J Pharmacol. 2008;584:207–212. doi: 10.1016/j.ejphar.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Collister JP, Osborn JW, Brooks VL. Endogenous ANG II supports lumbar sympathetic activity in conscious sodium-deprived rats: role of area postrema. Am J Physiol. 1998;275:R46–R55. doi: 10.1152/ajpregu.1998.275.1.R46. [DOI] [PubMed] [Google Scholar]
- 21.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 22.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, et al. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–H609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNFalpha. Am J Physiol Cell Physiol. 2004;286:C779–C784. doi: 10.1152/ajpcell.00398.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorbea-Oppliger VJ, Fink GD. Cerebroventricular injection of angiotensin II antagonist: effects on blood pressure responses to central and systemic angiotensin II. J Pharmacol Exp Ther. 1995;273:611–616. [PubMed] [Google Scholar]
- 27.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 28.de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- 29.Sasamura H, Nakazato Y, Hayashida T, Kitamura Y, Hayashi M, Saruta T. Regulation of vascular type 1 angiotensin receptors by cytokines. Hypertension. 1997;30:35–41. doi: 10.1161/01.hyp.30.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Cottone S, Vadalà A, Vella MC, Nardi E, Mulé G, Contorno A, et al. Changes of plasma endothelin and growth factor levels, and of left ventricular mass, after chronic AT1-receptor blockade in human hypertension. Am J Hypertens. 1998;11:548–553. doi: 10.1016/s0895-7061(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 31.Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem. 2002;277:5719–5724. doi: 10.1074/jbc.M107515200. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 33.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-κB as a potential biomarker for oxidative stress. Br J Nutr. 2001;86:S121–S127. doi: 10.1079/bjn2001340. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 36.Holownia A, Braszko J. The effect of angiotensin II and IV on ERK1/2 and CREB signalling in cultured rat astroglial cells. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:157–163. doi: 10.1007/s00210-007-0192-4. [DOI] [PubMed] [Google Scholar]
- 37.Lu D, Yang H, Raizada MK. AT1 receptor-mediated nuclear translocation of Raf-1 in brain neurons. J Neurochem. 1998;70:424–427. doi: 10.1046/j.1471-4159.1998.70010424.x. [DOI] [PubMed] [Google Scholar]