Abstract
Fibroblast growth factors (FGFs) and their receptors (FGFRs) initiate diverse cellular responses that contribute to the regulation of oligodendrocyte (OL) function. In order to understand the mechanisms by which FGFRs elicit these cellular responses, we investigated the phosphorylation of signal transduction proteins and the role of cholesterol-glycosphingolipid-enriched “lipid raft” microdomains in differentiated OLs. Surprisingly, we found that the most abundant tyrosine-phosphorylated protein in OLs was the 120-kd isoform of FGFR2 and that it was phosphorylated even in the absence of FGF2, suggesting a potential ligand-independent function for this receptor. Furthermore, FGFR2, but not FGFR1, was associated with lipid raft microdomains in OLs and myelin (but not in astrocytes). This provides the first evidence for the association of FGFR with TX-100-insoluble lipid raft fractions. FGFR2 phosphorylated the key downstream target, FRS2 in OLs. Raft disruption resulted in loss of phosphorylated FRS2 from lipid rafts, coupled with the loss of Akt but not of Mek or Erk phosphorylation. This suggests that FGFR2-FRS2 signaling in lipid rafts operates via the PI3-Kinase/Akt pathway rather than the Ras/Mek/Erk pathway, emphasizing the importance of microenvironments within the cell membrane. Also present in lipid rafts in OLs and myelin, but not in astrocytes, was a novel 52-kd isoform of FGFR2 that lacked the extracellular ligand-binding region. These results demonstrate that FGFR2 in OLs and myelin possess unique characteristics that are specific both to receptor type and to OLs and provide a novel mechanism to elicit distinct cellular responses that mediate both FGF-dependent and -independent functions.
Keywords: oligodendrocyte, myelin, lipid rafts, FGF
INTRODUCTION
Fibroblast growth factors (FGFs) are important regulators of nervous system development and physiology. FGF signaling is mediated by four receptors (FGFR1-FGFR4) and a subset of ligand FGFs that are expressed in the brain from a family of 22 members (Miyake et al., 1996; Ford-Peressis et al., 2001; Bansal et al., 2003; Itoh and Ornitz, 2004). These FGF receptors and ligands play diverse roles crucial in the regulation of normal physiological and pathological processes (reviewed in Unsicker, 1996; Bansal, 2002; Eswarakumar et al., 2005).
The basic structure of the FGF receptors includes three extracellular immunoglobulin-like domains, a hydrophobic transmembrane domain, an intracellular tyrosine kinase domain, and a C-terminal tail (Johnson et al., 1990). FGF receptor genes can be alternatively spliced in different cell types, resulting in multiple isoforms of the receptors, including those that contain all three Ig-like domains (three-Ig-like) and those that lack the first Ig loop (two-Ig-like) (Johnson et al., 1990; Mohammadi et al., 2005; Zhang et al., 2006). In addition to the conventional high molecular weight isoforms, several low molecular weight isoforms of FGFRs that are soluble and extracellular have been reported (Hanneken et al., 2001). Activation of FGF receptors requires heparan sulfate proteoglycan co-receptors (HSPGs) to regulate signaling potency (Ibrahimi et al., 2004). Further, the FGF receptors themselves can be differentially N-glycosylated, which provides another level of regulation of receptor activity (Duchesne et al., 2006).
Oligodendrocytes (OLs) form myelin in the CNS through an elaboration of their plasma membranes, which wrap around axons to form multi-lamellar sheaths, resulting in a dramatic increase in nerve conduction rate and efficiency. Inadequate myelination or damage to the myelin sheaths, as in multiple sclerosis, leads to severe neurological deficits (Trapp and Kidd, 2004). OL and myelin pathology also has been observed in schizophrenia (Davis and Haroutunian, 2003).
OL development from progenitors to myelinating differentiated cells progresses along a developmental lineage that is well characterized both in vivo and in vitro (Pfeiffer et al., 1993; Warrington and Pfeiffer, 1992; Miller and Reynolds, 2004). FGFs and their receptors are important regulators of OL development (reviewed in Bansal, 2002). Multiple FGFRs are expressed in a regulated manner during OL lineage progression (Bansal et al., 1996; Bansal, 2002). FGFR1 is expressed throughout the lineage; FGFR3, maximally expressed in Late Progenitors, is downregulated upon OL differentiation; FGFR2 is reciprocally upregulated; and FGFR4 is not expressed by OL lineage cells. FGF2 activates all FGF receptors and mediates multiple responses that vary markedly as a function of the stage of OL lineage in vitro. For example, FGF2, when it is combined with PDGF, supports long-term proliferation and migration of Early Progenitors, and reversibly blocks terminal differentiation of Late Progenitors (McKinnon et al., 1990; Bansal, 2002). FGF2 also induces multiple responses in mature OLs, including OL process elongation, re-entry into the cell cycle, and downregulation of major myelin proteins and FGFR2 (Bansal and Pfeiffer, 1997). These multiple responses of OL lineage cells to FGF2 are due to the activation of a developmentally regulated changing repertoire of specific FGFRs, each of which contributes a subset of the overall phenotype at each stage of the lineage (Fortin et al., 2005).
FGFR2 is significant since it is highly expressed in vitro by mature OLs (but not progenitors) concurrently with major myelin proteins (Bansal et al., 1996; Cohen et al., 2000; Yim et al., 2001; Fortin et al., 2005, Dugas et al., 2006). Furthermore, FGFR2 is expressed in vivo by OLs in myelinated fiber tracts of adult rodent brain, spinal cord, optic nerve, and purified myelin, but it is low or absent in astrocytes and neurons (Yazaki et al., 1994; Messersmith et al., 2000; Miyake et al., 1996; Bansal et al., 2003). In contrast to FGFR2, FGFR3 and FGFR4 proteins are absent in mature OLs, and FGFR1 is present at low levels, thereby making FGFR2 the major FGFR in OLs (Bansal et al., 1996; Yazaki et al., 1994; Miyake et al., 1996; Oh et al., 2003). Stimulation of OL process outgrowth and myelin-like membrane formation occurs with selective activation of FGFR2 in vitro, whereas inhibition of FGFR2 with blocking antibodies leads to attenuation of these responses (Fortin et al., 2005). Conditional knockout mice that lack FGFR2 and the myelin protein CNP display dopamine-related hyperactive behavior (Kaga et al., 2006). Interestingly, downregulation of both CNP and FGFR2 mRNA expression occurs in schizophrenia, a condition that also is intimately related to the brain dopamine system (Peirce et al., 2006 and personal communications, Dr. V. Haroutunian, Bronx V.A., N.Y.).
Specific cellular responses are produced by activation of signal transduction pathways that emanate from FGFRs (Dailey et al., 2005). Fibroblast growth factor substrate 2 (FRS2), a lipid-anchored docking protein that is phosphorylated upon activation of FGFR, is critical for recruitment of downstream signaling molecules and links the FGFRs to the Ras/Mek/Erk pathway and the PI3-Kinase/Akt pathway (Hadari et al., 2001). Enhancement of signaling cascades can be achieved by compartmentalization of growth factor receptors into specialized microdomains in the cell membrane that are called “lipid rafts”, which are enriched in cholesterol and glycosphingolipids (Simons and Toomre, 2000; Zajchowski and Robbins, 2002; Baron et al., 2003). For example, concentration of the receptor tyrosine kinase TrkA into lipid raft microdomains increases its NGF-mediated autophosphorylation (Tsui-Pierchala et al., 2002). Proteins that are associated with lipid raft microdomains routinely are identified biochemically as low-density, insoluble complexes that are formed at 4°C in non-ionic detergents, such as 1% TX-100 (Simons and Toomre, 2000; Taylor et al., 2002). Several components of the FGF signaling system have been observed in lipid rafts, including the ligand FGF2, the co-receptors HSPGs, and several downstream signaling molecules (e.g., FRS2, Fyn, Lyn, and Src; Fuki et al, 2000; Tkachenko et al, 2002; Chu et al., 2004; Kramer et al, 1999; Ridyard and Robbins, 2003). However, the FGFRs themselves, which are the main component of the FGF signaling system, had not been identified in these microdomains (Davy et al, 2000).
Here, we show that 25% of FGFR2, but not FGFR1, was associated with lipid raft microdomains in OLs and 50% in myelin, but none in astrocytes. FGFR2 phosphorylated the downstream signaling molecule FRS2. Disruption of these microdomains led to a significant loss of phosphorylated FRS2 from lipid rafts, which was coupled with the loss of Akt but not of Mek or Erk1/2 phosphorylation. This suggests that the segregation of FGFR2 that is achieved by these microdomains allows for activation of distinct signaling pathways in and out of lipid raft microdomains—i.e., P13-Kinase/Akt or Ras/Mek/Erk pathways, respectively. In addition, we identified a highly tyrosine-phosphorylated protein in OLs as the three-Ig-like isoform of FGFR2, which was phosphorylated even in the absence of FGF2 stimulation, suggesting an FGF-independent function for this isoform. Analysis of FGFR2-deficient mice revealed the presence of a novel low molecular weight isoform of FGFR2 in OLs and myelin, but not in astrocytes. A better understanding of the unique characteristics of FGFR2 in OLs will provide further insights into its role in normal and pathological conditions.
MATERIALS AND METHODS
Cell Culture
Purified populations of OLs were prepared and their purity and phenotype characterized by immuno-labeling cells with a panel of antibodies, as described previously (Bansal et al., 1996). Briefly, progenitors were obtained from mixed primary cultures of postnatal (P1-2) rat telencephalon by overnight shaking method. OL progenitors were expanded in serum-free defined medium and PDGF for two days and then allowed to differentiate into OLs for 6 days after removing PDGF.
Astrocytes isolated from monolayer cultures after releasing OL progenitors from mixed primary cultures (above) were 99% positive for the astrocyte marker Glial Fibrillary Acidic Protein (GFAP).
Myelin Purification
Myelin was purified from control and our FGFR2 conditional knock-out (Fgfr2 -/-) mice (Kaga et al., 2006) according to the procedure described previously (Menon et al., 2003). Compact myelin (main band) was used for all analyses.
Immunoblotting
Cells were harvested on ice in lysis buffer (10mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 1% deoxycholate and 1% NP40, pH 7.4) with protease and phosphatase inhibitors (2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 50mM NaF, 10mM NaP2O7, and 1mMNa o-Vanadate) and cup sonicated (3×30 sec; 4°C). The homogenates were then incubated (15 min, on ice) and centrifuged (15,000Xg, 10 min, 4°C). Protein concentration was assayed with the DC Protein Assay Kit (Bio-Rad, Hercules, CA). Aliquots of equal amounts of total protein from different experimental conditions or purified myelin fractions were electrophoresed on 12% SDS polyacrylamide gels or 8-16% precast Tris-Glycine gels (Invitrogen, EC60485) and transferred onto PVDF membranes. The membranes were blocked for 1 h in either milk or BSA, depending on the antibody, (Tris buffered saline, 0.2% Tween 20 and 5% powder milk or 5% BSA), incubated for either 1 h at room temperature or overnight at 4°C in primary antibodies; p-MAPK (1:10,000), pan-MAPK (1:5000) from Sigma, St Louis, MO; FGFR1 and FGFR2 against the C-terminal (1:500), Caveolin-1 (1:1000), Grb2 (1:1000), Shp2 (1:1000) from Santa Cruz Biotech, Santa Cruz, CA; Flotillin 2 (1:2000), Fyn (1:1000) from BD Biosciences, Palo Alto, CA; p-FRS2 (1:500), p-Akt (1:1000) pan Akt (1:1000), p-MEK1/2 (1:1000) from Cell Signaling Technology, Beverly, MA; p-FAK (1:1000), Src pY418 (1:1000), Src pY529, (1:1000) from Biosource, Camarillo, CA; pan FRS2 (1:1000, R&D Systems, Minneapolis, MN); Ab6 (FGFR N-terminal)(1:1000, Maher, PA, The Scripps Research Institute, La Jolla, CA); phosphotyrosine (pY) (4G10, 1:2000, Upstate Biotechnology, Charlottesville, VA). The membranes were then incubated for 30min in either anti-rabbit IgG (1:5,000; Santa Cruz Biotech, Santa Cruz, CA) or anti-mouse Ig (1:3000, BD Biosciences, Palo Alto, CA) both conjugated to horseradish peroxidase. The membranes were developed using the ECL Plus kit (GE Healthcare, Piscataway, NJ). In some cases, blots were stripped with 100mM Glycine, pH 2.0 for 20 minutes, washed with TBS with 0.2% Tween 20 for 3 × 5 minutes and reprobed with another antibody. Similar immunoblotting was performed on purified myelin.
Detergent Extraction and Sucrose Gradient Ultracentrifugation
Isolation of lipid raft from OLs and myelin by extraction with 1% TX100 at 4C and sucrose gradient centrifugation has been described previously (Marta et al., 2005 Taylor et al., 2002). Detailed procedures are presented in Supplementary Materials.
Cholesterol Extraction
To disrupt lipid rafts, cholesterol was depleted from OL membranes and purified myelin by use of the detergent, saponin, or the cholesterol-extracting agent, Methyl-β-cyclodextran (βMCD) as described previously (Marta et al., 2005) and in Supplementary Materials.
Disruption of cholesterol from live cells was carried out by treatment of OLs in culture with 5mM βMCD for 25-30min at 37°C, harvested and extracted in 1% TX-100 at 4°C as described previously. In some cases, after βMCD treatment, cells were washed 2x with culture media, then treated with FGF2 or FGF9 (10ng/ml) for 15 minutes before harvest and extraction.
Two-Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE)
2D-PAGE of protein from cultured OLs or myelin was carried out by a procedure described previously (Marta et al.; 2005; Taylor et al., 2004) and in Supplementary Materials.
Deglycosylation
Mature oligodendrocytes were harvested in PBS containing 1mM PMSF, 10μg/ml leupeptin/aprotinin, 50mM NaF, 10μg/ml NaP2O7 and 1mM Na o-vanadate. Protein deglycosylation was carried out using the PNGase F kit (New England Biolabs, cat# P0704S) according to the manufacturer’s protocol with slight modifications. In brief, 14μg of total protein was denatured in 1x glycoprotein denaturing buffer for 10min at 100°C. Samples were centrifuged at 14,000g for 15 minutes at 4°C. G7 reaction buffer and NP-40 were added to the supernatant to a final concentration of 1x and 1% respectively. 2μl of PNGase F was added to the reaction mixture and incubated overnight at 37°C. Sample buffer was next added to the reaction mixture and proteins were separated by SDS PAGE followed by immunoblotting with anti-FGFR2 (as above). Identical samples, without PNGase F, were run in parallel as control.
RNA Isolation and Northern Blotting
Total cellular RNA was isolated by acid guanindium thiocyanate-phenol-chloroform extraction. For northern blot analysis, radioactive probes were prepared by random priming (Prime-It II: Stratagene), and hybridized in the presence of salmon sperm DNA. Membranes were washed twice with 2x SSC/0.1% SDS (5 min, room temperature); 2x SSC/1%SDS (30min 62°C) and 0.1x SSC (30 min, room temperature). Blots were stripped (0.1x SSC, 1% SDS, 100°C, 15 min) and reprobed for GADPH to normalize for RNA loading. The cDNA probe used was mouse FGFR2 and was described previously (1.2-kb Pvu II fragment; Mansukhani et al., 1992).
RNA Polymerase Chain Reaction
First-strand cDNA was synthesized from 5ug total RNA (cDNA synthesis kit and oligo(dT) primers from Stratagene). The 5’ primer (nt 113-136) was located in the untranslated region and the 3’ primer (nt 1196-1217) in the Ig-like domain III (Yamaguchi et al., 1994; Bansal et al., 1996). RT-PCR was performed using the PCR kit (GenAmp, Perkin Elmer) (Yamaguchi et al., 1994). RT-PCR products were separated on 1.5% agarose gels and the band positions were aligned with molecular weight markers. Each sample was assayed at least three times resulting in the amplification of the same fragments.
RESULTS
The High Molecular Weight Form of FGF-Receptor 2 is the Most Abundantly Tyrosine Phosphorylated Protein of OLs
Mature OLs were treated with FGF2 (30 min) and analyzed for protein phosphorylation by 2D SDS-PAGE immunoblotting (Fig.1A-C). The profile of total silver-stained proteins was essentially identical between the control and treated samples, showing equal protein loading and similar separation of proteins (Fig. 1A). Immunoblotting with anti-phosphotyrosine (pY) detected a number of phosphoproteins (Fig. 1B). Three of these proteins, Fyn, β-tubulin, and Erk1/2, were identified by a comparison of their pI/MW coordinates to previously prepared 2D maps (Marta et al., 2005). Upon FGF2 stimulation, Erk1/2 became hyperphosphorylated, as expected, whereas Fyn and β-Tubulin remained unchanged. A fourth protein, the most abundantly tyrosine-phosphorylated, had a pI and MW that were consistent with those that have been reported for FGFR2. Serial immunolabeling of 2D blots with anti-pY and anti-FGFR2 confirmed the identity of the phosphorylated120-kd protein as FGFR2 (Fig. 1C). This conclusion was drawn taking into account the following considerations: the signal that was observed upon re-probe of the anti-pY blot with anti-FGFR2 was not a residual phosphotyrosine signal because anti-pY and anti-FGFR2 are mouse monoclonal and rabbit polyclonal antibodies, respectively; the rabbit secondary antibody alone produced no signal following stripping of anti-pY; and two identical blots, immunolabeled with anti-FGFR2 or anti-pY, labeled the same spot.
Fig. 1. The 120-kd form of FGFR2 is the most abundant tyrosine phosphorylated protein in OLs.
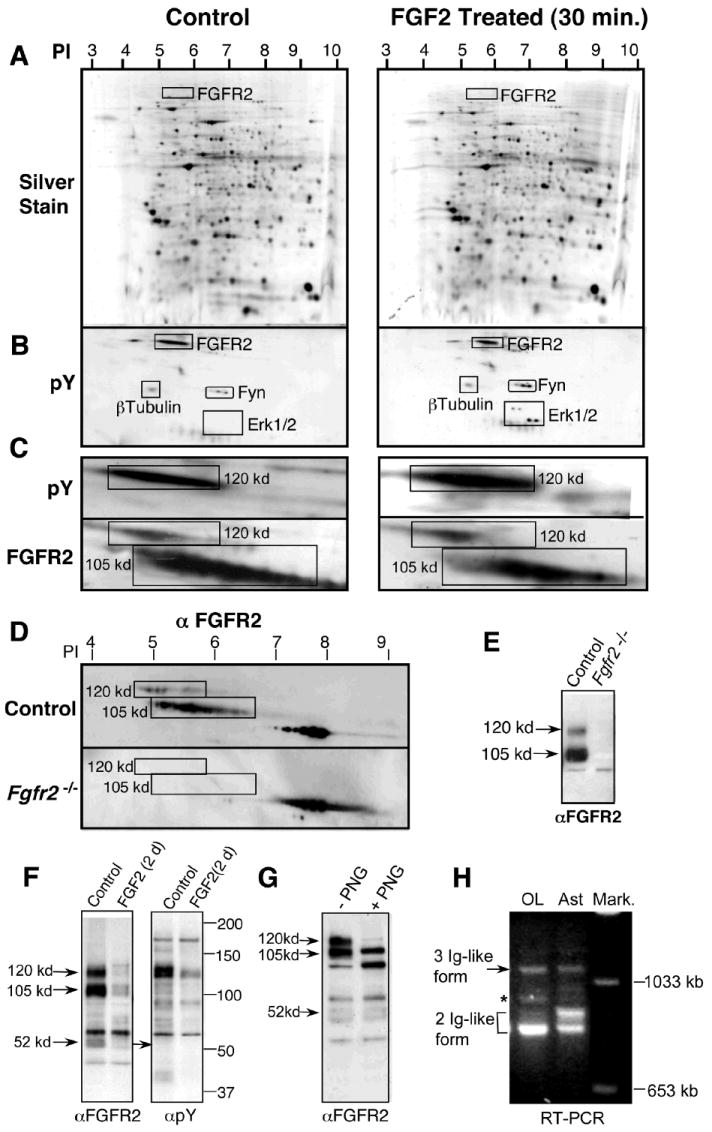
Mature OLs treated with FGF2 (30 min) were analyzed by 2D PAGE (A-C). Equal amounts (350 ug) of total protein separated on each gel were silver stained (A) or immunoblotted with anti-phosphotyrosine (pY) (B, C) and re-probed with anti-FGFR2 (C), showing that the tyrosine-phosphorylated 120-kd band is a form of FGFR2 and that it is phosphorylated even in the absence of FGF2. β-Tubulin, Fyn, and MAPK also are identified as landmarks. Myelin from control and Fgfr2 -/- mice was analyzed by 2D (D) and 1D (E) PAGE and immunolabeled with anti-FGFR2. The 120-kd and105-kd proteins that correspond to the two elongated spots in OLs (C) are present in controls, but not in Fgfr2 -/- myelin, confirming that these are forms of FGFR2. Spot (pI, 7-9) present in both mutant and controls is nonspecific. (F) 1D PAGE of OLs untreated or treated with FGF2 for two days (2d), immunoblotted with anti-FGFR2 and anti-pY, shows that concomitant to the expected downregulation of FGFR2 by 2-d FGF2 treatment, the tyrosine-phosphorylated 120-kd band also is downregulated, further confirming its identity as a form of FGFR2. (G) OL proteins were deglycosylated with PNGase F (PNG) and immunoblotted with anti-FGFR2, showing that both the 105- and 120-kd bands are N-glycosylated equally, evident by similar decreases in their sizes following PNGase treatment compared with untreated. (H) Total RNA samples from OLs or astrocytes (Ast) analyzed by RT-PCR showed amplification of specific DNA fragments indicative of the presence of both the two- and three-Ig-like domain isoforms of FGFR2. Representative experiments of four or more are shown.
To confirm that the 120- and 105-kd proteins in OLs were FGFR2, we examined myelin that was purified from either control or our conditional knockout mice that had a disruption of FGFR2 in OLs (Fgfr2 -/-; Kaga et al., 2006). Immunoblotting with anti-FGFR2 of 2D blots (Fig. 1D) showed that in the control, the two elongated spots between ~5-7 pI corresponded to the 120-kd and 105-kd forms of FGFR2, observed in OLs (Fig. 1C), and that these spots were absent in Fgfr2 -/- myelin (the large nonspecific spot between pI 7-8 served as a landmark). One-dimensional PAGE immunoblot analysis also showed loss of the 120-kd and 105-kd bands in Fgfr2 -/- mice (Fig. 1E). Thus, we concluded that both the 105- and 120-kd bands were FGFR2. To further confirm that the 120-kd FGFR2 form was tyrosine-phosphorylated, we showed that concomitant to the downregulation of FGFR2 bands by 2-day exposure to FGF2 (as shown previously in Fortin et al., 2005), the tyrosine-phosphorylated 120-kd band also was downregulated (Fig. 1F). Note that the 120-kd band appears to resolve into two bands that run very close together, both of which are downregulated by FGF2 exposure. It is likely that these two protein bands represent two slightly different splice variant forms of 120-kd FGFR2. Consistent with this idea, we observed a second novel PCR fragment in OLs that ran close to the known three-Ig-like isoform fragment (Fig 1H, asterisk, Bansal et al., 1996).
The phosphorylation of FGFR2 in OLs, in the absence or presence of FGF2, also has been observed in our previous studies (Fortin et al., 2005). Specifically, when OL lysates were immunoprecipitated with anti-FGFR2 and immunoblotted with anti-pY, FGFR2 was found to be phosphorylated in both control and FGF2-treated conditions (Fortin et al., 2005, Fig.4). However, in that study, the 105-kd and 120-kd bands were not resolved as separate bands on the gel. It also should be noted that further phosphorylation of FGFR2 occurs over the base level upon acute stimulation with FGF2 (Fig. 1C-pY and Fortin et al., 2005, Fig. 4).
Fig. 4. Phosphorylated FGFR2, FRS2, and several downstream signaling molecules are present in lipid rafts.
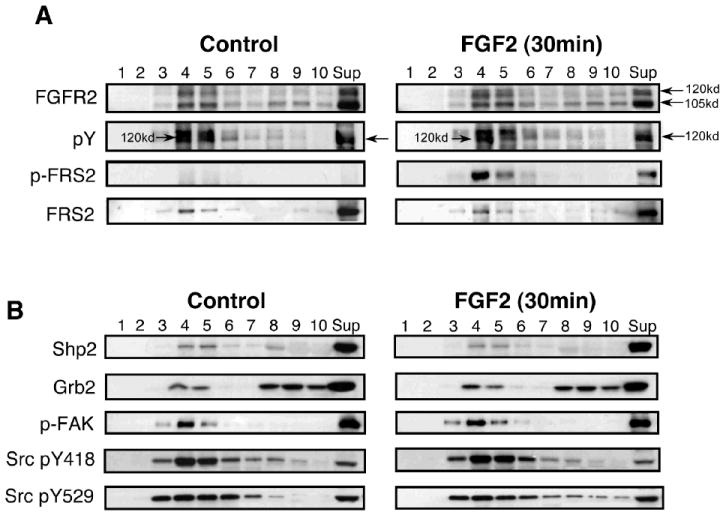
Control and FGF2 (10 ng/ml, 30 min)- treated OLs were extracted with 1% TX-100 (4°C), and the insoluble pellets were fractionated by sucrose density gradient centrifugation. (A) The fractions were analyzed by immunoblotting with αFGFR2, anti-phosphotyrosine (pY), anti-phospho-FRS2, or anti-pan FRS2, showing that the phosphorylated 120-kd isoform of FGFR2 is present in the lipid raft fractions 4-5, as is FRS2, which becomes phosphorylated upon FGF2 stimulation. (B) Downstream signaling molecules, including Shp2, Grb2, phospho-FAK, phospho-Src418, and phospho-Src529, also are partially associated with lipid rafts. Like FGFR2, FAK and Srcs are phosphorylated in both controls and FGF2-treated conditions, while FRS2 is phosphorylated only upon FGF2 stimulation.
Since, of the two FGFR2 bands, tyrosine phosphorylation occurred primarily of the 120-kd band, and since N-glycosylation of FGFR2 is implicated in ligand-independent autophosphorylation (Steinberger et al., 1996; Mangasarian et al., 1997), we further investigated the nature of these two bands of FGFR2. We asked if the two bands of FGFR2 represented differential glycosylation of the same protein and if the constitutive phosphorylation of the 120-kd band was related to its differential glycosylation. To test this possibility, protein lysates from OLs were deglycosylated with PNGase F, before separation by SDS-PAGE and immunoblotting with anti-FGFR2 (Fig. 1G). Because the molecular weights of both bands reduced correspondingly upon deglycosylation, we concluded that both bands were N-glycosylated and that they did not represent a single protein that was differentially glycosylated. This suggested that the two FGFR2 bands were two distinct variant isoforms, containing either two or all three Ig-like extracellular domains of FGFR2, which possibly had arisen from alternative splicing of mRNA transcripts. To confirm this, we extracted RNA from OLs or astrocytes and performed nonquantitative RT-PCR using appropriate primers (Fig. 1H). As described previously (Bansal et al., 1996), specific DNA fragments that were indicative of the presence of both the three- (1100 bp) and two- (800/900bp) Ig-like domain isoforms of FGFR2 were amplified in OLs and astrocytes. A novel fragment of ~950 bp also was identified in OLs (Fig. 1H asterisk), perhaps corresponding to another novel three-Ig-like isoform of FGFR2.
We conclude that in OLs, the 105- and 120-kd bands of FGFR2 originate from two separate transcripts, and while both forms are N-glycosylated, only the complete form, which has three Ig-like domains, is the most abundantly tyrosine-phosphorylated protein in OLs.
FGFR2 but not FGFR1 is Present in Detergent-Insoluble Lipid Raft Microdomains in OLs and Myelin
Tyrosine kinase receptor signaling specificity often is governed by protein-protein interactions that occur in cholesterol-glycosphingolipid-enriched lipid raft microdomains. We therefore asked if the 120-kd phosphorylated isoform of FGFR2 selectively localized to lipid rafts. As a biochemical assay for lipid rafts (Kim and Pfeiffer, 1999; Taylor et al., 2002), we examined the detergent solubility of FGFR2 (Figs. 2,3). OLs (Fig. 2A), myelin (Fig. 2B), and astrocytes (Fig. 2C) were extracted with 1% TX-100 at 4°C, separated into soluble supernatant (S) and insoluble pellet (P) fractions, and analyzed by immunoblotting with anti-FGFR2 and with known protein markers of lipid rafts (Fyn, Flotillin 2, caveolin 1), (Fig. 2A). The distributions of proteins in the detergent-insoluble and -soluble fractions were quantified by densitometric scanning of the protein bands on immunoblots (Fig. 2A,B). Both the 120- and 105-kd isoforms of FGFR2 consistently partitioned into detergent-insoluble fractions at 4°C at a proportion of about ~25% of the total FGFR2 in OLs and ~50% in myelin. By comparison, FGFR1 was detergent-soluble in OLs (Fig. 2A). Unlike OLs and myelin, the two bands of FGFR2 in astrocytes ran at slightly higher molecular weights and did not partition into the pellet fraction (Fig. 2C).
Fig. 2. FGFR2 but not FGFR1 partitions into detergent-insoluble lipid rafts from OL and myelin but not from astrocytes.
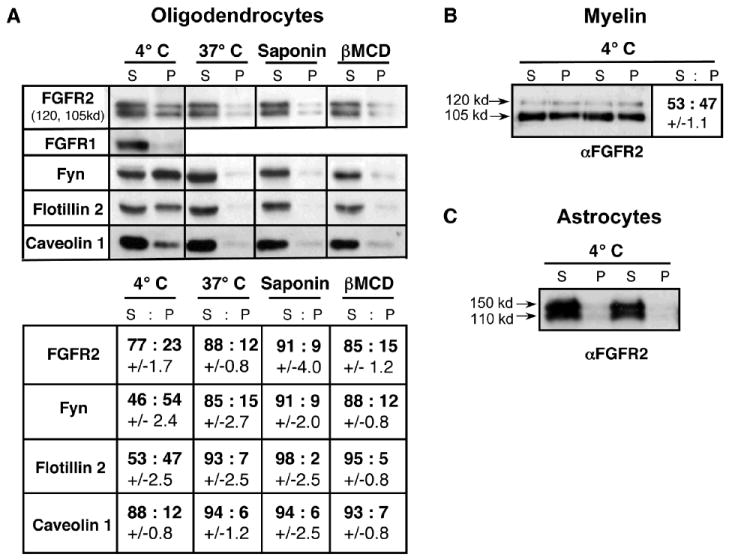
(A) OL were extracted with 1% TX-100 at 4°C, 37°C, or after saponin or βMCD treatment, separated into soluble supernatant (S) and insoluble pellet (P) fractions and analyzed by immunoblotting with anti-FGFR2, anti-FGFR1, and other markers of lipid rafts (Fyn, Flotillin2, Caveolin1). Upon disruption of lipid rafts by various treatments (37°C, saponin, βMCD), a decrease of FGFR2 in the pellet was observed. FGFR1 in OLs did not partition into the insoluble fraction. The relative distribution of FGFR2 and other proteins in detergent-insoluble and -soluble fractions was quantified by densitometric scanning of the protein bands. (B) Purified myelin also was extracted with 1% TX-100 at 4°C and analyzed similarly for the relative distribution of FGFR2 into (S) and (P) fractions. Duplicate samples are shown. Both 120-kd and 105-kd isoforms of FGFR2 partitioned into detergent-insoluble lipid raft fractions, the proportions being ~25% in OLs and ~50% in myelin (A and B). (C) FGFR2 in astrocytes did not partition into the insoluble fraction. Duplicate samples are shown. Standard error, N=3-6.
Fig. 3. Both FGFR2 isoforms are found in ‘lipid raft fractions’ of sucrose density gradients from OLs and myelin.
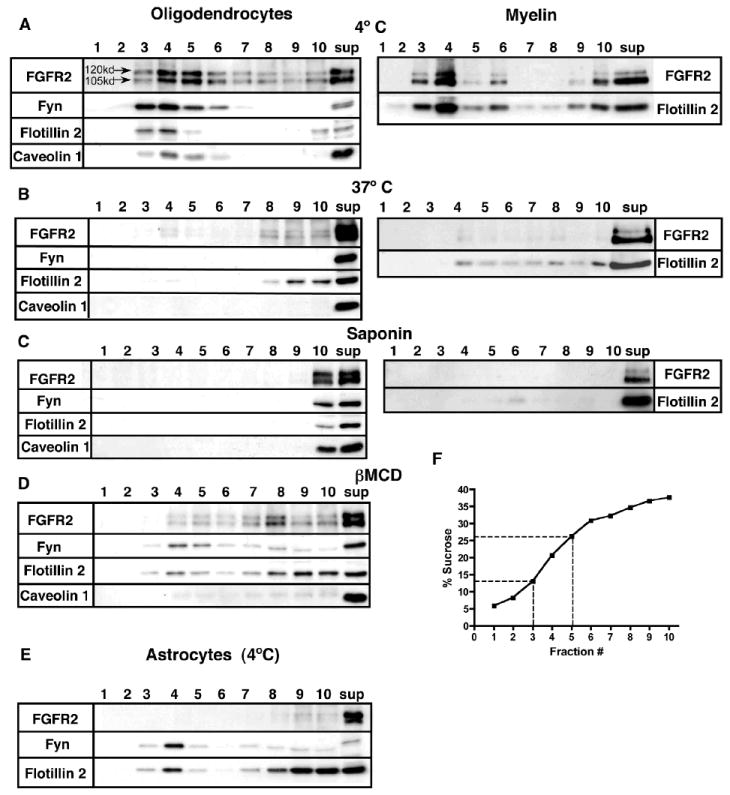
(A) OL lysates and myelin were extracted at 4°C with 1% TX-100, and the insoluble pellet was fractionated by sucrose density gradient centrifugation. The total proteins in each fraction (1-10) and a portion of proteins in the soluble fraction (sup) were immunoblotted for FGFR2 and for the raft markers Fyn, Flotillin2, and Caveolin1. Both forms of FGFR2, along with other raft associated proteins, float in fractions 3-5 of the gradient, corresponding to a sucrose density of 13-26% (F). Disruption of lipid rafts by extraction of OLs and myelin at 37°C (B), pretreatment of protein lysates with 0.2% saponin (C), or pretreatment of cultured OLs with methyl-βcyclodextran (βMCD) (D), prior to a 4°C 1% TX-100 extraction, results in depletion of FGFR2 and other raft associated protein from fractions 3-5. (E) FGFR2 in astrocytes that were analyzed identically did not float in raft fractions. N=4.
To further study the apparent raft association of FGFR2, we asked if FGFR2 was solubilized upon treatments that are known to disrupt rafts (Kim and Pfeiffer, 1999; Taylor et al., 2002); i.e., 1% TX-100 extraction at 37°C, or at 4°C following depletion/sequestration of cholesterol with saponin or methyl-β-cyclodextrin (βMCD). In all of these conditions, we found that the FGFR2 from OLs was solubilized by 1% TX-100 (Fig. 2A), further indicating an association of a fraction of FGFR2 with lipid rafts. Other lipid raft markers (Fyn, Flotillin2, Caveolin1) followed a similar pattern, demonstrating the effectiveness of these treatments.
Because some receptors can transiently partition in or out of lipid rafts upon ligand stimulation (Zajchowski and Robbins, 2002), we examined this possibility by stimulating OLs with FGF2 for 5-30 min prior to detergent extraction. The proportion of FGFR2 that was present in the detergent-insoluble fraction remained constant irrespective of FGF2 exposure (data not shown), suggesting that a subfraction of FGFR2 remained constitutively lipid raft-associated in OLs.
Finally, we employed sucrose density gradient centrifugation as another criterion of raft association for FGFR2. The insoluble pellet from 1% TX-100 extracts of OLs and myelin was fractionated by sucrose density gradient centrifugation (Fig. 3). Proteins that were recovered in the 13-26% sucrose density fractions 3-5 (Fig. 3F) of the gradient were classified as raft-associated (Kim and Pfeiffer, 1999; Taylor et al., 2002), consistent with the presence in these fractions of the known raft proteins Fyn, Flotillin2, and Caveolin1 (Fig. 3A). The majority of the detergent-insoluble portion of FGFR2 was found in fractions 3-5 for both OLs and myelin (Fig. 3A). The raft association of FGFR2 was once again tested by applying additional criteria as in Fig. 2; i.e., 1% TX-100 extraction at 37°C, and prior disruption of cholesterol with saponin or βMCD. Again, under these conditions, FGFR2 and the raft marker proteins were solubilized by 1% TX-100 (Fig. 3B-D), indicating that the majority of the 1% TX-100-insoluble fraction of FGFR2 was associated with lipid rafts. In contrast to OLs and myelin, astrocytes that were subjected to identical sucrose gradient analyses at 4°C did not show the presence of FGFR2 in the raft fractions (Fig. 3E).
We concluded that both isoforms of FGFR2, but not FGFR1, associated with lipid raft microdomains in OLs (but not in astrocytes), showing specificity of this association.
Phosphorylated FGFR2, FRS2, and Several Downstream Signaling Molecules are Present in Lipid Rafts
Having found that a subfraction of FGFR2 isoforms partitioned into lipid rafts in OLs, we next investigated if they could signal in lipid rafts. We therefore first asked if the phosphorylated form of FGFR2 and its downstream signaling molecules also were present in lipid raft microdomains (Fig. 4). Control and FGF2-treated (10 ng/ml, 30 min) cultures were extracted with 1% TX-100 (4°C), and the insoluble pellets were analyzed after sucrose density gradient centrifugation by immunoblotting with anti-FGFR2, anti-pY, anti-phospho-FRS2, and anti-pan-FRS2 (Fig. 4A). The 120-kd tyrosine-phosphorylated isoform of FGFR2 was found in lipid rafts. In addition, FRS2 was present in lipid rafts and became highly phosphorylated upon FGF2 stimulation. Several other signaling molecules that are known to be involved in FGFR signaling also were present in lipid rafts (Fig. 4B). Specifically, Shp2, which became phosphorylated in OLs in response to FGF2 (data not shown), was present in lipid rafts. Grb2, phosphorylated FAK, and phosphorylated Src (both activating and inhibiting forms) also were present in the lipid raft fractions; however, the extent of their phosphorylation did not change upon FGF2 treatment. Further, the amount of signaling molecules in lipid rafts, e.g. FRS2, Shp2 and Grb2, did not change upon FGF2 stimulation. Although many signaling molecules are known to partition in and out of lipid rafts after stimulation, this is apparently not an absolute requirement for their activation (Zajchowski and Robbins, 2002; Navratil et al., 2003). Therefore, considering that a subfraction of phosphorylated FGFR2, FRS2, and many downstream targets are constitutively present in lipid rafts, it is conceivable that FGFR2 signals, at least in part, in lipid rafts.
FGFR2-Mediated Phosphorylation of Lipid Raft-Associated FRS2, is Coupled with Activation of the PI3-Kinase/Akt Pathway but not the Ras/Mek/Erk Pathway
In order to evaluate the biological significance of raft association of a given protein, an approach is used to ask if specific biological effects are lost upon disruption of lipid raft integrity in live cells by βMCD treatment (Pike and Miller, 1998). We examined the effect of βMCD treatment on FRS2, the primary docking protein, phosphorylated by FGF2 linking FGFRs to downstream signaling pathways (Fig. 5). OLs in culture were treated with βMCD (5mM, 25 min), then exposed to FGF2 for 15 min, and extracted with 1% TX-100 at 4°C, and the supernatant (S) and pellet (P) fractions were analyzed by immunoblot with anti-phospho-FRS2. We found that in the absence of βMCD, phosphorylated FRS2 was found in both the supernatant (non-raft) and pellet (raft) fractions after FGF2 treatment. Following βMCD treatment, phosphorylated FRS2 was significantly lost from the lipid raft pellet fraction but not from the supernatant, confirming that a subfraction of phosphorylated FRS2 is associated with lipid rafts.
Fig. 5. FGFR2-Mediated Phosphorylation of Lipid Raft-Associated FRS2, is Coupled with Activation of the PI3-Kinase/Akt Pathway but not the Ras/Mek/Erk Pathway.
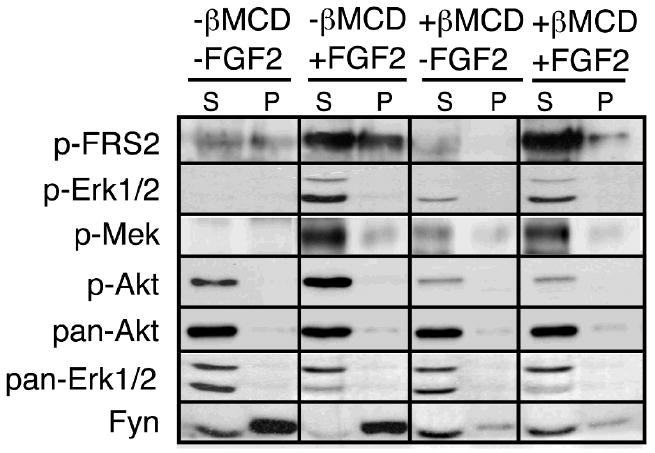
OLs in culture were treated with βMCD (5mM, 25 min) to disrupt lipid rafts and then exposed to FGF2 for 15 min before extraction with 1% TX-100 at 4°C and analysis of supernatant (S) and pellet (P) fractions by immunoblot analysis with phospho-FRS2, phospho-Akt, phospho-Erk1/2, phospho-Mek1/2, pan Akt, pan-Erk1/2, and Fyn. Following FGF2 treatment, phosphorylated FRS2 was found both in supernatant and pellet fractions of OLs that were not treated with βMCD. Disruption of lipid rafts by βMCD treatment resulted in loss of FRS2 in the pellet fraction. Akt, Mek, and Erk1/2, the signaling molecules that are downstream of FRS2, also were phosphorylated by exposure to FGF2; however, βMCD treatment only abolished the phosphorylation of Akt but not of Mek or Erk1/2. Blots were reprobed with pan-Akt, pan-Erk1/2 and Fyn. A representative experiment out of three is shown.
Classically, FRS2 can signal through the Ras/Mek/Erk pathway and the PI3-kinase/Akt pathway. Therefore, we asked if perturbing lipid raft integrity would adversely affect activation of these pathways by FGF2 in mature OLs. We first examined the phosphorylation of Erk1/2 and Mek as indicators of Ras signaling in the Ras/Mek/Erk pathway. We observed that both Mek and Erk1/2 were phosphorylated in response to FGF2 exposure; however, this phosphorylation was not abolished by βMCD treatment. This suggests that activation of Mek and Erk1/2 must occur by a non-raft-associated mechanism, perhaps by the non-raft-associated subfraction of FRS2. Curiously, βMCD treatment by itself caused low-level phosphorylation of Erk1/2, Mek and FRS2 in the soluble fraction, even in the absence of FGF2, perhaps as a stress response.
Next, we examined phosphorylation of Akt as an indicator of signaling by the PI3-kinase/Akt pathway. We found that Akt was hyper-phosphorylated by FGF2 in mature OLs. In contrast to Mek and Erk1/2, this phosphorylation was abolished upon disruption of rafts by βMCD, suggesting that lipid raft integrity is required for Akt phosphorylation. Further, because Akt itself partitioned into the 1% TX-100 soluble fraction, the loss of Akt phosphorylation upon raft disruption indicated that signaling upstream of Akt must be initiated through a raft-dependent mechanism, most likely through the raft-associated subfraction of FRS2. Reprobe of the blots with pan-Akt, pan-Erk1/2 and Fyn antibodies are shown as controls for equal loading and for the effectiveness of βMCD.
βMCD perturbation experiments also were carried out using FGF9 instead of FGF2 for stimulation of OLs (data not shown). This is because although FGF2 and FGF9 both activate FGFR2 in OLs, FGF9 is more selective for FGFR2, based on data described by Fortin et al. (2005). Both FGF2 and FGF9 gave similar results, confirming that FGFR2 activation was involved in the signaling that is described above.
We conclude that FGFR2 signals both in and out of lipid raft microdomains, activating different downstream signaling molecules.
A Novel Low Molecular Weight, Lipid Raft-Associated Isoform of FGFR2 is Found in OLs and Myelin
Myelin from control and Fgfr2 -/- mice that was separated by 2D (Fig. 6A) or 1D (Fig. 6B) PAGE and immunoblotted with anti-FGFR2 revealed an additional ~52-kd protein that was present in controls but missing from Fgfr2 -/-, suggesting that this is a form of FGFR2. This form also was present in OLs but not in astrocytes (Fig. 6C). Further, similar to the 105- and 120-kd bands, the 52-kd form of FGFR2 was downregulated by treatment of OLs for 2 d with FGF2 (Fig. 1F). But, unlike these forms, it was not phosphorylated or glycosylated (Fig. 1F,G). Similar to the high molecular weight forms, a subfraction of the 52-kd form of FGFR2 was lipid raft-associated in OLs and myelin, as it partitioned into the insoluble pellet (P) fraction after 1% TX-100 extraction at 4°C and floated in fractions 3 and 4 of the sucrose density gradient (Fig. 6D).
Fig. 6. A novel low molecular weight, lipid raft-associated isoform of FGFR2 is present in OLs and myelin but not in astrocytes.
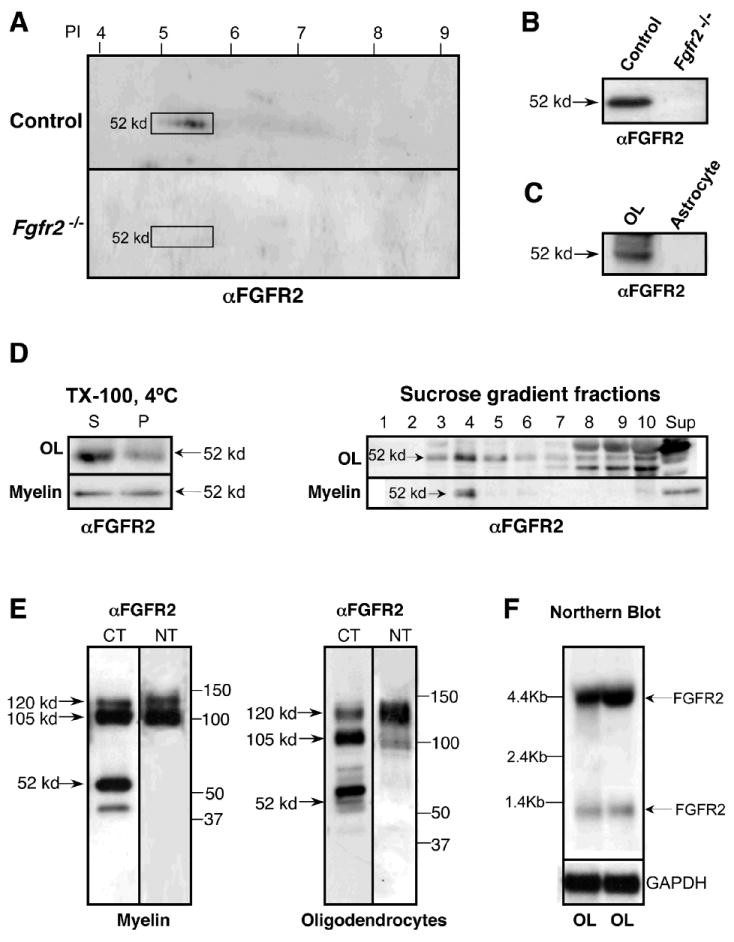
Myelin from control and Fgfr2 -/- mice was analyzed by 2D (A) and 1D (B) PAGE and immunolabeled with anti-FGFR2. A 52-kd protein was detected in controls, but not in Fgfr2 -/- myelin, showing that this is a form of FGFR2. (C) OL or astrocyte lysates immunoblotted with anti-FGFR2 show that the 52-kd band also is present in OLs but not in astrocytes. (D) OL or myelin extracted with 1% TX-100 at 4°C, separated into soluble (S) and insoluble (P) fractions or further fractionated into 10 fractions by sucrose gradient centrifugation, were immunoblotted with anti-FGFR2. A subfraction of the 52-kd isoform of FGFR2 partitions into the pellet fraction and floats in fractions 3-5 of the gradient, showing its lipid raft association. (E) Myelin or OLs, immunoblotted with antibodies that recognize either intracellular (CT) or extracellular (NT) domains of FGFR2, show that the 52-kd protein is recognized only by the CT-specific anti-FGFR2. (F) Northern blot analysis of total RNA from OLs (20 ug/lane) with a specific probe for FGFR2 identified the expected 4.4-kb transcript and a second novel transcript of ~1 kb, a likely message for the 52-kd FGFR2 isoform. Blots reprobed for GADPH show that the RNA was not degraded.
We next investigated the nature of the 52-kd form. Because the anti-FGFR2 antibody that identified this form was against the cytoplasmic region of FGFR2, we hypothesized that the 52-kd band was a truncated intracellular form of the receptor, noting that the predicted size of the intracellular plus transmembrane domains of FGFR2 was 52 kd. To test this hypothesis, we carried out immunoblot analyses of myelin and OLs (Fig. 6E) with anti-FGFR2 against the intracellular C-terminus (CT; Santa Cruz cat. # SC-122) or anti-FGFR against the extracellular region (NT; monoclonal antibody Ab6; Hanneken et al., 1995; note that since the Ab6 antibody recognizes not only the extracellular 120-kd FGFR2, but also 125-kd FGFR1, which is present in OLs but not in myelin, the relative intensities of the 105- and 120/125-kd bands between OLs and myelin appear different). The extracellular anti-FGFR failed to detect the 52-kd band in either myelin or OLs, suggesting that this band represents the intracellular plus transmembrane region of FGFR2. This form is distinct from any other low molecular weight isoforms that have been reported previously, which comprise the extracellular region of the receptor (Hanneken, 2001; Kiefer et al., 1991).
The extracellular isoforms are known to be generated either from alternative splicing of mRNA (Johnson et al., 1991) or, as for FGFR1, through cleavage of the receptor protein by metalloproteases (Levi et al., 1996). To investigate the origin of the 52-kd FGFR2 protein, we explored these possibilities. Sequence alignment of FGFR1 and FGFR2 revealed that FGFR2 did not contain the necessary cleavage site; thus, it was unlikely that the 52-kd form was generated by this mechanism. To test the second possibility, we performed northern blot analysis of total RNA from OLs and found that in addition to the expected major band at 4.4 kb, a second smaller RNA band of ~1 kb was detected in OLs (Fig. 6F; Bansal et al., 1996), suggesting that in principle, this smaller mRNA could be a transcript for the 52-kd form of FGFR2. This mRNA band was not present in astrocytes (Bansal et al., 1996).
Thus, we conclude that the 52-kd protein is a lipid raft-associated, unphosphorylated, unglycosylated intracellular novel isoform of FGFR2, present in OLs and myelin but not in astrocytes, suggesting some yet unknown function of this isoform in OLs and myelin.
DISCUSSION
Multiple lines of evidence predict an important role for FGFR2 in OL development and myelin formation. In this study, we determined that three isoforms of FGFR2, including a novel 52-kd form, were distinctively regulated in mature OLs. One of the isoforms was constitutively tyrosine-phosphorylated and two of the three were glycosylated, and all three isoforms were associated with lipid raft microdomains in OLs and myelin but not in astrocytes. In contrast, FGFR1 was not present in lipid rafts. Furthermore, we determined the importance of lipid rafts in segregating alternate signaling pathways that stemmed from FGFR2.
Signaling by FGF receptors is initiated upon ligand binding, when the FGF receptors dimerize and autophosphorylate up to seven tyrosine residues that are located in the intracellular catalytic domain of the receptor, subsequently linking it to downstream signaling cascades (Eswarakumar et al., 2005). Intriguingly, we found that the 120-kd (three-Ig-like form) FGFR2 was the most abundant tyrosine-phosphorylated protein in OLs. The physiological consequence of this high level of phosphorylation is not clear. In some proteins, phosphorylation of one tyrosine residue is sufficient to elicit a response, while in other proteins, phosphorylation of multiple residues is needed to achieve a threshold level of phosphorylation for the proteins to function appropriately (Patwardhan and Miller, 2007). Interestingly, the 120-kd FGFR2 was phosphorylated even in the absence of externally supplied FGF2, posing the possibility that FGFR2 may be constitutively phosphorylated in OLs and may thus have a ligand-independent function. However, the possibility remains that a ligand that is sufficient to activate FGFR2 could be provided within the culture, either by contaminant astrocytes that make FGF2 or by cultured OLs themselves, which could potentially be a source of FGF9 (another ligand of FGFR2), as in vivo (Nakamura et al., 1999; Fortin et al., 2005; Dhandapani et al., 2007). Because no FGF2 or FGF9 protein was detected in the cultures (data not shown), this possibility is unlikely.
The FGF-independent phosphorylation of FGFR2 could have been initiated by alternate ligands. For example, cell adhesion molecules (CAMs) such as NCAM, L1, and N-cadherin, known to activate FGFRs in neurons (Williams et al., 1994), could be involved. Specifically, CAMs directly bind to the extracellular region of FGFR1 (Kiselyov et al., 2003; Kulahin et al., 2008) and FGFR2 (Christensen et al., 2006) by specific sequences, thereby co-clustering the FGFRs and leading to their FGF-independent phosphorylation (Kiselyov et al., 2005; Kulahin et al., 2008). NCAM-120, expressed in mature OLs and myelin, could conceivably serve this purpose (Trotter et al., 1989). Alternatively, ligands may not be necessary at all to dimerize and phosphorylate FGFR2, because a high concentration of receptors in the cell membrane (further enriched in raft micrdomains) may result in self-clustering and autophosphorylation by direct receptor-receptor interaction (Plotnikov et al., 1999), as has been demonstrated in cells that overexpress FGFR1 (Hinsby et al., 2003). Other likely candidates for mediating or enhancing FGFR signaling are integrins. Integrin-FGF interactions lead to biological responses in endothelial cells and certain cell lines (Rusnati et al., 1997; Toledo et al., 2005). For example, activation of α3β1 and α5β1 integrins results in FGF-independent phosphorylation of FGFR and stimulation of proliferation (Toledo et al., 2005). Further, FGFR is known to colocalize with integrins and their common intracellular transducers, including pp125FAK in focal adhesions (Plopper et al., 1995), thereby providing ample opportunity for enhancement of signals by integrin-FGFR interactions.
It is interesting to note that ligand-independent autophosphorylation that leads to constitutive activation of FGFR2, due to mutations in the N-glycosylation site of the receptor, occurs in several human diseases with craniofacial pathology, demonstrating a biological relevance of N-glycosylation and autophosphorylation of FGFR2 (Steinberger et al., 1996; Mangasarian et al., 1997). However, constitutive phosphorylation of the 120-kd isoform in OLs is unlikely to be related to differential N-glycosylation, because both the 105- and 120-kd isoforms are equally N-glycosylated. Nevertheless, N-glycosylation of FGFR2 in OLs is significant because heterogeneity of N-glycosylation may produce variants of FGFRs that have different activities (Duchesne et al., 2006).
Although the functional significance of expression of the two- and three-Ig-like isoforms of FGFR2 in OLs and the constitutive phosphorylation of only one of these isoforms remains unclear, several other cell types also express these isoforms. A switch in the expression profile of the isoforms has been implicated with progression of malignancy in gliomas (Yamaguchi et al., 1994), suggesting that a change in alternate RNA splicing can provide cells with altered signaling potential. Interestingly, the three-Ig-like isoform (120-kd) of FGFR2 was downregulated in myelin compared with OLs, perhaps signifying a developmentally regulated alteration in the signaling potential of this isoform. Similar developmental regulation of alternative splicing also is known to occur for other myelin proteins, such as myelin-associated glycoprotein, myelin basic protein, and proteolipid protein (Lazzarini, 2004).
Partitioning of several signaling proteins, including growth factor receptors, into lipid raft microdomains provides an important mechanism for the compartmentalization and enhancement of intracellular signal transduction by segregating and concentrating signaling molecules in these microdomains (Simons and Toomre, 2000; Zajchowski and Robbins, 2002). Previous studies in neuroblastoma cells have failed to detect the presence of the FGFR itself in these TX-100-insoluble lipid microdomains (Davy et al., 2000). Our findings that a significant subfraction of FGFR2, but not FGFR1, resides within lipid rafts in OLs and myelin but not in astrocytes suggest that raft association of FGFRs may be specific for cell and receptor types. The differential compartmentalization of FGFR1 and FGFR2 in distinct membrane microdomains potentially is significant for OL function, because the two receptors initiate different cellular responses in differentiated OLs (Fortin et al., 2005). Specifically, FGFR2 activation leads to the regulation of normal OL functions such as process elongation and myelin-like membrane formation, while FGFR1 activation leads to pathogenic responses such as downregulation of myelin genes and aberrant re-entry into the cell cycle. It is likely that this segregation of the two receptors into distinct microdomains promotes their coupling with different sets of proteins within these compartments and thus provides a mechanism for eliciting different cellular responses. For example, FGFR2-mediated OL process elongation could be mediated through its interactions with proteins that either are themselves raft-associated, such as Fyn, the activation of which leads to process growth (Kramer et al., 1999; Osterhaut et al., 1999), or are downstream of raft-associated proteins, such as Akt, the constitutive activation of which leads enhanced myelination (Hadari et al., 2001; Flores et al., 2008). Consistent with this idea, we observed increased partitioning of FGFR2 in myelin (50%) relative to OLs (25%), suggestive of its role in myelin maturation from the immature myelin-like membrane of OLs to compact mature myelin. Other molecules that are involved in adhesion and process growth, such as NCAM-120, integrins, and phospho-FAK, also are present in lipid rafts (Taylor et al., 2002; Baron et al., 2003, present study), and their co-clustering and activation of FGFR2 could be further increased within these microdomains, thus enhancing their mutual signaling potential, as has been seen for PDGFR-integrin interactions (Baron et al., 2003; Decker et al., 2004).
To determine the signaling pathways that emerge from FGFR2 in lipid rafts, we examined the effect of raft disruption on phosphorylated FRS2 and its associated downstream signaling molecules Mek, Erk1/2, and Akt. Our finding that the loss of phosphorylated FRS2 in lipid rafts was coupled to the loss of Akt phosphorylation, but not to Mek or Erk1/2 phosphorylation, suggests that FGFR2-FRS2 signaling in lipid rafts operates via the PI3-kinase/Akt pathway rather than the Ras/Mek/Erk pathway. This shows the specificity of FGFR2 signaling, whereby lipid rafts provide a mechanism for a single FGF receptor to independently control different signaling pathways in and out of lipid rafts. Differential signaling and activation of different sets of proteins in or out of lipid rafts also has been shown for the GDNF receptor c-ret (Saarma, 2001). These studies together emphasize the importance of microenvironments within the cell membrane and provide an additional mechanism for generating diversity in FGF-mediated signaling in OLs.
In summary, the extent of the modifications to each of the isoforms of FGFR2 in OLs and its selective association with lipid raft microdomains in OLs illustrate the importance of customizing a protein for operation within a specific signaling system, providing the potential for eliciting multiple cellular responses.
Supplementary Material
Acknowledgments
We thank Dr. R. McKinnon (UNDMJ, Piscataway, NJ) for critical review of the manuscript and S. Fewou for technical help with a gel. This work was supported by grants from the National Institute of Health, NS038878 and NS41078. We dedicate this manuscript to the memory of Dr. S. E. Pfeiffer who was a source of inspiration for this work and will continue to be remembered for his pioneering contributions in the field of lipid rafts in myelin biology.
References
- Bansal R. Fibroblast growth factors and their receptors in oligodendrocyte development: Implications for demyelination and remyelination. Dev Neurosci. 2002;24:35–46. doi: 10.1159/000064944. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. doi: 10.1002/(SICI)1097-4547(19971015)50:2<215::AID-JNR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bansal R, Lakhina V, Remedios R, Tole S. Expression of FGF receptors 1, 2, 3 in the embryonic and postnatal mouse brain compared with Pdgfralpha, Olig2 and Plp/dm20: implications for oligodendrocyte development. Dev Neurosci. 2003;25:83–95. doi: 10.1159/000072258. [DOI] [PubMed] [Google Scholar]
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE. Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci. 1996;7:263–275. doi: 10.1006/mcne.1996.0020. [DOI] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- Christensen C, Lauridsen JB, Berezin V, Bock E, Kiselyov VV. The neural cell adhesion molecule binds to fibroblast growth factor receptor 2. FEBS Lett. 2006;580:3386–3390. doi: 10.1016/j.febslet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Chu CL, Buczek-Thomas JA, Nugent MA. Heparan sulphate proteoglycans modulate fibroblast growth factor-2 binding through a lipid raft-mediated mechanism. Biochem J. 2004;379:331–341. doi: 10.1042/BJ20031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Chandross KJ. Fibroblast growth factor-9 modulates the expression of myelin related proteins and multiple fibroblast growth factor receptors in developing oligodendrocytes. J Neurosci Res. 2000;61:273–287. doi: 10.1002/1097-4547(20000801)61:3<273::AID-JNR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Davis KL, Haroutunian V. Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:758. doi: 10.1016/S0140-6736(03)14297-3. [DOI] [PubMed] [Google Scholar]
- Davy A, Feuerstein C, Robbins SM. Signaling within a caveolae-like membrane microdomain in human neuroblastoma cells in response to fibroblast growth factor. J Neurochem. 2000;74:676–683. doi: 10.1046/j.1471-4159.2000.740676.x. [DOI] [PubMed] [Google Scholar]
- Decker L, Baron W, Ffrench-Constant C. Lipid rafts: microenvironments for integrin-growth factor interactions in neural development. Biochem Soc Trans. 2004;32:426–430. doi: 10.1042/BST0320426. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Khan MM, Wade MF, Wakade C, Mahesh VB, Brann DW. Induction of transforming growth factor-β1 by basic fibroblast growth factor in rat C6 glioma cells and astrocytes is mediated by MEK/ERK signaling and AP-1 activation. J Neurosci Res. 2007;85:1033–1045. doi: 10.1002/jnr.21182. [DOI] [PubMed] [Google Scholar]
- Duchesne L, Tissot B, Rudd TR, Dell A, Fernig DG. N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J Biol Chem. 2006;281:27178–27189. doi: 10.1074/jbc.M601248200. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Flores AI, Priyadarshini Narayanan S, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Perriss M, Abud H, Murphy M. Fibroblast growth factors in the developing central nervous system. Clin Exper Pharmaco Physiol. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J Neurosci. 2005;25:7470–7479. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki IV, Meyer ME, Williams KJ. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem J. 2000;351(Pt 3):607–612. [PMC free article] [PubMed] [Google Scholar]
- Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2α in FGF recetor-mediated signal transduction pathways. Proc Natl Acad Sci. 2001;98:8578–8583. doi: 10.1073/pnas.161259898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneken A. Structural characterization of the circulating soluble FGF receptors reveals multiple isoforms generated by secretion and ectodomain shedding. FEBS Lett. 2001;489:176–181. doi: 10.1016/s0014-5793(00)02409-1. [DOI] [PubMed] [Google Scholar]
- Hanneken A, Maher PA, Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells--implications for the modulation of FGF-2. J Cell Biol. 1995;128:1221–1228. doi: 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsby AM, Olsen JV, Bennett KL, Mann M. Signaling initiated by overexpression of the fibroblast growth factor receptor-1 investigated by mass spectrometry. Mol Cell Proteomics. 2003;2:29–36. doi: 10.1074/mcp.m200075-mcp200. [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Hrstka SC, Mohammadi M, Linhardt RJ. Kinetic model for FGF, FGFR, and proteoglycan signal transduction complex assembly. Biochemistry. 2004;43:4724–4730. doi: 10.1021/bi0352320. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lee PL, Lu J, Williams LT. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol. 1990;10:4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga Y, Shoemaker WJ, Furusho M, Bryant M, Rosenbluth J, Pfeiffer SE, Oh L, Rasband M, Lappe-Siefke C, Yu K, Ornitz DM, Nave KA, Bansal R. Mice with conditional inactivation of fibroblast growth factor receptor-2 signaling in oligodendrocytes have normal myelin but display dramatic hyperactivity when combined with Cnp1 inactivation. J Neurosci. 2006;26:12339–12350. doi: 10.1523/JNEUROSCI.3573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer MC, Baird A, Nguyen T, George-Nascimento C, Mason OB, Boley LJ, Valenzuela P, Barr PJ. Molecular cloning of a human basic fibroblast growth factor receptor cDNA and expression of a biologically active extracellular domain in a baculovirus system. Growth Factors. 1991;5:115–127. doi: 10.3109/08977199109000276. [DOI] [PubMed] [Google Scholar]
- Kim T, Pfeiffer SE. Myelin glycosphingolipid/cholesterol-enriched microdomains selectively sequester the non-compact myelin proteins CNP and MOG. J Neurocytol. 1999;28:281–293. doi: 10.1023/a:1007001427597. [DOI] [PubMed] [Google Scholar]
- Kiselyov VV, Soroka V, Berezin V, Bock E. Structural biology of NCAM homophilic binding and activation of FGFR. J Neurochem. 2005;94:1169–1179. doi: 10.1111/j.1471-4159.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- Kiselyov VV, Skladchikova G, Hinsby AM, Jensen PH, Kulahin N, Soroka V, Pedersen N, Tsetlin V, Poulsen FM, Berezin V, Bock E. Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure. 2003;11:691–701. doi: 10.1016/s0969-2126(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Kulahin N, Li S, Hinsby A, Kiselyov V, Berezin V, Bock E. Fibronectin type III (FN3) modules of the neuronal cell adhesion molecule L1 interact directly with the fibroblast growth factor (FGF) receptor. Mol Cell Neurosci. 2008;37(3):528–536. doi: 10.1016/j.mcn.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. The myelin genes and products. In: Lazzarini, editor. Myelin biology and disorders. San Diego, CA: Elsevier Academic Press; 2004. pp. 387–491. [Google Scholar]
- Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangasarian K, Li Y, Mansukhani A, Basilico C. Mutation associated with Crouzon syndrome causes ligand-independent dimerization and activation of FGF receptor-2. J Cell Physiol. 1997;172:117–125. doi: 10.1002/(SICI)1097-4652(199707)172:1<117::AID-JCP13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Mansukhani A, Dell’Era P, Moscatelli D, Kornbluth S, Hanafusa H, Basilico C. Characterization of the murine BEK fibroblast growth factor (FGF) receptor: activation by three members of the FGF family and requirement for heparin. Proc Natl Acad Sci U S A. 1992;89:3305–3309. doi: 10.1073/pnas.89.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta CB, Montano MB, Taylor CM, Taylor AL, Bansal R, Pfeiffer SE. Signaling cascades activated upon antibody cross-linking of myelin oligodendrocyte glycoprotein: potential implications for multiple sclerosis. J Biol Chem. 2005;280:8985–8993. doi: 10.1074/jbc.M413174200. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- Menon K, Rasband MN, Taylor CM, Brophy P, Bansal R, Pfeiffer SE. The myelin-axolemmal complex: biochemical dissection and the role of galactosphingolipids. J Neurochem. 2003;87:995–1009. doi: 10.1046/j.1471-4159.2003.02075.x. [DOI] [PubMed] [Google Scholar]
- Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res. 2000;62:241–256. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Reynolds R. Oligodendroglial Lineage. In: Lazzarini RA, editor. Myelin Biology and Disorders. New York: Elsevier Academic; 2004. pp. 289–298. [Google Scholar]
- Miyake A, Hattori Y, Ohta M, Itoh N. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and -3 mRNAs. J Neurosci Res. 1996;45:534–541. doi: 10.1002/(SICI)1097-4547(19960901)45:5<534::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Todo T, Motoi Y, Haga S, Aizawa T, Ueki A, Ikeda K. Glial expression of fibroblast growth factor-9 in rat central nervous system. Glia. 1999;28:53–65. [PubMed] [Google Scholar]
- Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonodotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- Oh Y, Denninger A, Colvin JS, Vyas A, Tole S, Ornitz DM, Bansal R. Fibroblast growth factor receptor-3 signaling regulates the onset of oligodendrocyte terminal differentiation. J Neurosci. 2003;23:883–894. doi: 10.1523/JNEUROSCI.23-03-00883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan P, Miller WT. Processive phosphorylation: mechanism and biological importance. Cell Signal. 2007;19:2218–2226. doi: 10.1016/j.cellsig.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, Haroutunian V, Buxbaum JD, Owen MJ, O’Donovan MC. Convergent evidence for 2’,3’-cyclic nucleotide 3’-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18–24. doi: 10.1001/archpsyc.63.1.18. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanski K, Ingber DE. Convergence of integrin and growth factor receptor signailing pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–65. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Ridyard MS, Robbins SM. Fibroblast growth factor-2-induced signaling through lipid raft-associated fibroblast growth factor receptor substrate 2 (FRS2) J Biol Chem. 2003;278:13803–13809. doi: 10.1074/jbc.M210245200. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Tanghetti E, Dell’Era P, Gualandris A, Presta M. alphavbeta3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarma M. GDNF recruits the signaling crew into lipid rafts. Trends Neurosci. 2001;24:427–429. doi: 10.1016/s0166-2236(00)01864-6. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews/Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Steinberger D, Mulliken JB, Muller U. Crouzon syndrome: previously unrecognized deletion, duplication, and point mutation within FGFR2 gene. Hum Mutat. 1996;8:386–390. doi: 10.1002/(SICI)1098-1004(1996)8:4<386::AID-HUMU18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Coetzee T, Pfeiffer SE. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J Neurochem. 2002;81:993–1004. doi: 10.1046/j.1471-4159.2002.00884.x. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Marta CB, Claycomb RJ, Han DK, Rasband MN, Coetzee T, Pfeiffer SE. Proteomic mapping provides powerful insights into functional myelin biology. Proc Natl Acad Sci U S A. 2004;101:4643–4648. doi: 10.1073/pnas.0400922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko E, Simons M. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J Biol Chem. 2002;277:19946–19951. doi: 10.1074/jbc.M200841200. [DOI] [PubMed] [Google Scholar]
- Toledo MS, Suzuki E, Handa K, Hakomori S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J Biol Chem. 2005;280:16227–16234. doi: 10.1074/jbc.M413713200. [DOI] [PubMed] [Google Scholar]
- Trapp B, Kidd G. Structure of the Myelinated Axon. In: Lazzarini RA, editor. Myelin Biology and Disorders. New York: Elsevier Academic; 2004. pp. 3–27. [Google Scholar]
- Trotter J, Bitter-Suermann D, Schachner M. Differentiation-regulated loss of the polysialylated embryonic form and expression of the different polypeptides of the neural cell adhesion molecule by cultured oligodendrocytes and myelin. J Neurosci Res. 1989;22:369–383. doi: 10.1002/jnr.490220402. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- Unsicker SBaK. Functions of fibroblast growth factors(FGFs) in the nervous system. In: Bell C, editor. Chemical Factors in Neural Growth, Degeneration and Repair. New York: Elsevier; 1996. [Google Scholar]
- Warrington AE, Pfeiffer SE. Proliferation and differentiation of O4+ oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. J Neurosci Res. 1992;33:338–353. doi: 10.1002/jnr.490330218. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci U S A. 1994;91:484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki N, Hosoi Y, Kawabata K, Miyake A, Minami M, Satoh M, Ohta M, Kawasaki T, Itoh N. Differential expression patterns of mRNAs for members of the fibroblast growth factor receptor family, FGFR-1-FGFR-4, in rat brain. J Neurosci Res. 1994;37:445–452. doi: 10.1002/jnr.490370403. [DOI] [PubMed] [Google Scholar]
- Yim SH, Hammer JA, Quarles RH. Differences in signal transduction pathways by which platelet-derived and fibroblast growth factors activate extracellular signal-regulated kinase in differentiating oligodendrocytes. J Neurochem. 2001;76:1925–1934. doi: 10.1046/j.1471-4159.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


