Abstract
Microglial activation is an important pathogenic component of neurodegenerative disease processes. This state of increased inflammation is associated not only with neurotoxic consequences but also neuroprotective effects, e.g., phagocytosis and clearance of amyloid in Alzheimer’s disease. In addition, activation of microglia appears to be one of the major mechanisms of amyloid clearance following active or passive immunotherapy. Imaging techniques may provide a minimally invasive tool to elucidate the complexities and dynamics of microglial function and dysfunction in aging and neurodegenerative diseases. Imaging microglia in vivo in live subjects by confocal or two/multiphoton microscopy offers the advantage of studying these cells over time in their native environment. Imaging microglia in human subjects by positron emission tomography scanning with translocator protein-18 kDa ligands can offer a measure of the inflammatory process and a means of detecting progression of disease and efficacy of therapeutics over time.
Keywords: Keywords microglia, neuroinflammation, Alzheimer’s disease, immunization, neurodegeneration, imaging, microscopy, positron emission tomography, translocator protein-18 Kda
Introduction
Microglial cells were first described by Del Rio Hortega (1932) and represent approximately 5% of the total cell population of the brain. They are considered the resident macrophages of the brain, capable of phagocytosis and antigen presentation (Gehrmann et al. 1995; Kreutzberg 1996; Minghetti and Levi 1998). Under physiologic conditions, they exhibit a ramified morphology and weak expression of molecules associated with macrophage function (Schwartz et al. 2006; Hanisch and Kettenmann 2007), a state traditionally termed “resting microglia”.
A variety of signals that pose a potential threat to central nervous system homeostasis, including bacterial, viral, and fungal structures, abnormal endogenous proteins, complement factors, antibodies, cytokines, and chemokines and many more, are sensed by microglial receptors and subsequently induce microglial activation (van Rossum and Hanisch 2004; Hanisch and Kettenmann 2007). In addition to this “on-signaling”, microglial cells are also under the influence of calming “off-signaling”, e.g., CD200–CD200R receptor–ligand interaction or fractalkine CX3CL1–CS3CR1 interaction (Frank et al. 2006; Hanisch and Kettenmann 2007; Wang et al. 2007). Interruption of this constitutive signaling by impairment of neuronal integrity can also lead to microglial activation. The state of activation is associated with increased proliferation of residential microglia as well as recruitment of bone-marrow-derived monocytes; however, the relative importance of these processes is a matter of controversy. While studies using total body irradiation followed by bone marrow transplantation suggested that there is a significant contribution of bone-marrow-derived cells to the microglial pool (Simard and Rivest 2004), more recent studies using different experimental approaches found no evidence of microglia progenitor recruitment from the circulation under physiological conditions or in models of denervation or neurodegeneration. These studies suggest that microgliosis is dependent on local expansion and self-renewal, whereas recruitment of precursors from the blood stream only occurs under certain host conditions (Ajami et al. 2007; Mildner et al. 2007) and suggest that the origin of activated microglia may vary based upon the specific disease process. These findings suggest that the origin of activated in microglia may vary based upon the specific disease process. In addition to proliferation, microglial cells exhibit morphologic changes upon activation with adoption of a more amoeboid phenotype, altered expression of cell surface markers such as upregulation of major histocompatibility complex II (MHC II) antigens or complement receptor 3 and release of a variety of cytokines and growth factors (van Rossum and Hanisch 2004; Li et al. 2007; Streit et al. 2008).
In the past, activation of microglia has been associated predominantly with neurotoxic and pro-inflammatory downstream effects. More recently, it has become evident that microglial activation is a phenotypically and functionally diverse process, which is dependent on stimulus type, stimulus intensity, and context and is not necessarily detrimental but may have neuroprotective and anti-inflammatory potential (Schwartz et al. 2006; Hanisch and Kettenmann 2007). Similar to peripheral macrophages, microglia can be induced into a classical (pro-inflammatory) activation state, but are also capable of entering an alternative (anti-inflammatory) activation state involved in tissue repair and extracellular matrix remodeling (Colton et al. 2006; Ponomarev et al. 2007; Maier et al. 2008). The balance between neurotoxic versus protective and anti- versus pro-inflammatory microglial factors might determine the role of microglia in a given disease or injury condition (Li et al. 2007).
In the following sections, we review some recent findings as well as controversies regarding microglial activation in aging and neurodegeneration followed by a discussion on current imaging of microglia in vivo in live subjects in the setting of neurological disorders.
Neuroinflammation in aging and neurodegeneration
Microglial senescence in normal and pathological aging
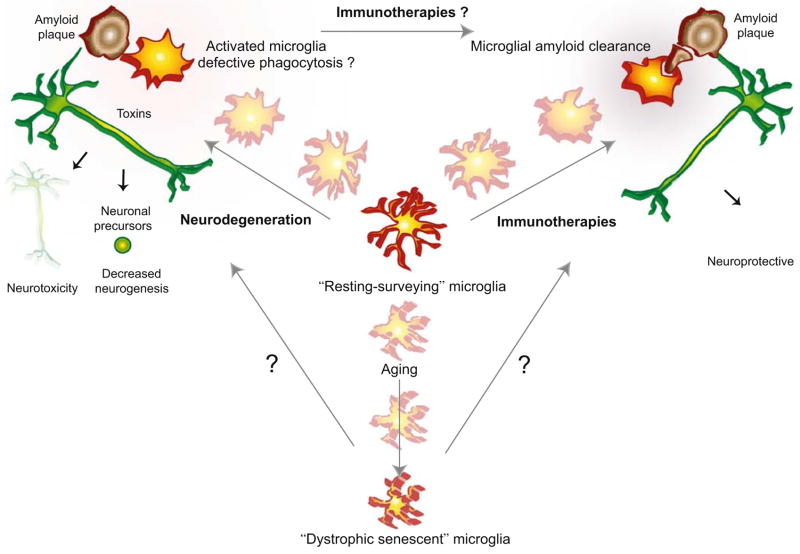
Normal aging is associated with a decline in both innate and adaptive immune functions, a process termed immunosenescence (Richartz et al. 2005). Microglia, as the resident immune cells of the brain, are likely to undergo similar age-related changes. The presence of “dystrophic” microglial cells in the aged human brain has been described as cells characterized by non-ramified, atrophic, fragmented, or unusually tortuous processes (Fig. 1; Streit et al. 2004; Flanary et al. 2007). Unfortunately, as of now no markers other than morphologic criteria are available to distinguish activated from dystrophic microglia, making an unequivocal assessment challenging. Microglial cells also exhibit telomere shortening and decreased telomerase activity with aging, indicating that they may undergo replicative senescence (Flanary and Streit 2004; Flanary et al. 2007). Overall, normal brain aging is associated with a shift towards a pro-inflammatory microenvironment with increased expression of pro-inflammatory mediators including MHC II, MHC II transcriptional activator (CIITA), CD86 and IFN-γ, and downregulation of anti-inflammatory IL-10 (Sobel and Ames 1988; Sheffield and Berman 1998; Frank et al 2006). In addition, downregulation of CD200 has been demonstrated in the brain with aging, suggesting that neuronal inhibition of microglial activation may attenuate with age (Frank et al. 2006).
Fig. 1.
Complex dynamics of microglial activation in aging, Alzheimer’s disease, and immunotherapies. Resting microglia constantly survey their environment and are thus termed “surveying microglia”. With aging microglia cells become “dystrophic” characterized by morphological and functional changes. In disease states such as Alzheimer’s disease, microglia undergo activation causing release of toxins that are detrimental to neurons and neuronal precursor cells promoting neurodegeneration. Immunotherapies result in increased microglial phagocytosis of amyloid plaques and promote amyloid clearance
Alzheimer’s disease (AD) is associated with a further impairment of both innate and adaptive arms of the immune system compared to normal aging, affecting peripheral monocytes and lymphocytes as well as microglia (Richartz et al. 2005). Postmortem studies of human brains showed a significantly higher number of dystrophic microglia in AD patients compared to age-matched controls. Preliminary data also indicate that microglial cells isolated from brains of AD patients have shorter telomeres than microglia from non-demented patients (Flanary et al. 2007). These findings suggest that by activating microglia, β-amyloid (Aβ) may also promote microglial deterioration and accelerate senescence (Streit 2004). Studies in PS1-APP transgenic mice demonstrated that with advanced age the microglia of these animals become dysfunctional with a significant reduction in expression of Aβ-binding receptors (scavenger receptor A, CD36, receptor of advanced glycation endproducts [RAGE]) and Aβ-degrading enzymes (neprilysin, insulin-degrading enzyme, metalloproteinase 9) but maintained the ability to produce pro-inflammatory cytokines (Hickman et al. 2008).
These findings indicate that premature immunosenescence and microglial senescence in particular may contribute to the development or progression of neurodegenerative diseases as senescent microglia appears to be functionally impaired with decreased ability to produce neurotrophic factors, impaired phagocytosis and amyloid clearance, and increased neurotoxicity (Richartz et al. 2005; Streit et al. 2008).
Microglial activation in neurodegeneration—neuroprotective or neurotoxic?
AD is associated with an innate immune response consisting of activated microglial cells in the vicinity of plaques and tangles, activated astrocytes, activation of the classical and alternative complement pathways, and increased secretion of various pro-inflammatory cytokines (Fig. 1; Akiyama et al. 2000; Cooper et al. 2000; Raine 2000; Richartz et al. 2005; Weiner and Frenkel 2006). Aβ can interact directly with various microglial receptors including RAGE, scavenger receptors, CD36, or formyl peptide receptor or indirectly via complement activation (Rogers et al. 2002; Heneka and O’Banion 2007; Hickman et al. 2008). Activation of microglia by Aβ leads to secretion of soluble factors, microglial proliferation, and chemotaxis, amyloid clearance by phagocytosis and release of amyloid-degrading enzymes (Rogers et al. 2002; Weiner and Frenkel 2006).
This inflammatory state in AD has been traditionally viewed as detrimental, a concept that was originally supported by epidemiological studies which showed a reduced prevalence, delayed onset and progression of AD among chronic users of non-steroidal anti-inflammatory drugs (NSAID; McGeer et al. 1996; Weiner and Frenkel 2006; Heneka and O’Banion 2007; Szekely et al. 2008). A variable reduction in the degree of plaque-associated inflammation was found in some postmortem studies of AD patients with long-term NSAID therapy compared to untreated control patients (Mackenzie and Munoz 1998; Alafuzoff et al. 2000), while others found no decrease in the number of activated microglia despite an improvement on neuropsychological test scores (Halliday et al. 2000). Interestingly, no difference was seen in the number of amyloid plaques or neurofibrillary tangles with NSAID treatment in any of these neuropathological studies. Contrary to epidemiological studies, randomized clinical prevention or treatment trials with selective or non-selective cyclo-oxygenase inhibitors have been disappointing so far (Aisen et al. 2003; Thal et al. 2005; Group et al. 2007), suggesting that the beneficial effects of NSAIDs are not due to their anti-inflammatory actions (Heneka and O’Banion 2007; Szekely et al. 2008). NSAIDs have also been shown to protect against Aβ aggregation, to modify γ-secretase-mediated amyloid precursor protein processing and to activate peroxisome proliferator-activated receptor gamma (Mackenzie 2001; Gasparini et al. 2004a; Gasparini et al. 2004b). It has been postulated that one or more of these mechanisms may be more critical for the observed beneficial effects of these drugs in AD than their anti-inflammatory activity (for review, Heneka and O’Banion 2007). In a recently published analysis of six pooled cohort studies, however, no advantage in AD risk reduction was found for the subset of NSAIDs that selectively lower Aβ-42 via modification of γ-secretase cleavage (Szekely et al. 2008), whereas in a phase II trial of tarenflurbil, a selective Aβ-42 lowering agent without COX inhibitory activity, a dose-dependent effect on functional abilities was seen in patients with mild, but not moderate AD (Wilcock et al. 2008). As of now, the discrepant results between observational and randomized clinical trials have not been resolved. Other factors such as timing and duration of exposure, brain penetration, or differences in study participants may play a role (Gasparini et al. 2004b).
As indicated above, microglia are capable of amyloid phagocytosis. Microglial activation may, therefore, be beneficial in AD by promoting amyloid clearance, which is supported by findings from various human and animal studies (Morgan et al. 2005). For example, microglial activation following intrahippocampal lipopolysaccharide (LPS) administration has been shown to result in improved amyloid clearance in APP-PS1 double transgenic mice (DiCarlo et al. 2001). Inhibition of complement activation in APP transgenic mice was accompanied by increased Aβ deposition but reduced microglial activation, suggesting that complement-mediated Aβ phagocytosis by microglia is an important mechanism of amyloid clearance (Wyss-Coray et al. 2002). Deficiency of the CC-chemokine receptor 2 blocked migration of microglia/macrophages to sites of Aβ deposition and led to increased mortality and Aβ levels (El Khoury et al. 2007). Induction of experimental autoimmune encephalitis (EAE) in APP transgenic mice resulted in a marked decrease of Aβ deposition in the brain (Frenkel et al. 2005). Similarly, in patients with ischemic infarcts, a striking clearance of amyloid plaques from the vicinity of the ischemic lesions was observed (Wisniewski et al. 1991; Akiyama et al. 1996; Akiyama and McGeer 2004).
Taken together, these findings suggest that the phagocytic capacity of microglia depends on the type and strength of the activating stimulus, and that in AD, microglia-mediated clearing mechanisms are insufficient or too slow to counteract the deposition of amyloid in the brain (Rogers et al. 2002; Boche and Nicoll 2008). Other stimuli in addition to Aβ may be required for efficient clearance of amyloid plaques by activated microglia. Microglia also appear unable to completely digest phagocytosed material, which may further stimulate the secretion of inflammatory mediators (Paresce et al. 1997). Pro-inflammatory cytokines such as TNF-α have been shown to induce a downregulation of Aβ receptors, which may start a vicious cycle, by leading to a further compromise in the phagocytic capacity of microglia (Hickman et al. 2008).
Further complicating the interpretation of the role of microglial activation in AD, recent studies found increased expression of macrophage alternative activation genes (arginase I, mannose receptor MRC1, chitinase-3 like 1 and 2) in mouse models of AD and in brain samples of human AD patients in addition to increased expression of some genes commonly associated with classical activation (Colton et al. 2006). As immunohistochemical studies are lacking, it is not known if more than one phenotypically different microglial population exists in AD brains or if individual cells can display a hybrid activation state. As alternative activation is commonly associated with anti-inflammatory effects, tissue repair, and extracellular matrix remodeling, further studies are necessary to determine the contribution of these mechanisms to the pathogenesis of AD and to explore if they may represent a novel therapeutic target.
In summary, it is now hypothesized that in early stages of AD, microglia are protective by promoting amyloid clearance but that the cells become increasingly dysfunctional at later disease stages and contribute to disease progression (Fig. 1; Hickman et al. 2008). This dichotomous role of microglial activation has to be taken into therapeutic consideration as anti-inflammatory interventions likely have different effects at different disease stages (Group et al. 2007). Drugs or interventions that selectively alter microglial functions in such a way as to promote phagocytosis while at the same time decreasing the release of pro-inflammatory mediators may have more therapeutic success than non-discriminating anti-inflammatory agents (Hickman et al. 2008).
In addition to its important functions as part of the immune system, microglia have also emerged as important regulators of adult neurogenesis. Activated microglia have been demonstrated to promote neurogenesis in the dentate gyrus and subventricular zone of the adult brain via production of soluble factors (Battista et al. 2006; Walton et al. 2006; Choi et al. 2008). Adult neurogenesis is important for learning and memory and has been shown to decline with age. Studies on the effects of AD on neurogenesis have yielded mixed results depending on experimental design, but overall it appears that the most severely affected step is differentiation of immature cells into mature neurons due to a hostile microenvironment in the AD brain (Waldau and Shetty 2008). A potentially critical role for microglia in regulation of neurogenesis in AD has been indicated in a recent report showing that mice carrying a presenilin 1 (PS1) mutation have impaired neurogenesis accompanied by decreased numbers of activated microglia in the hippocampus. Follow-up in vitro studies demonstrated that microglia expressing PS1 variants markedly inhibited the proliferation and neuronal lineage commitment of cultured neuronal precursor cells from wild-type mice. As wild-type and PS1 mutant microglia exhibited considerable differences in the expression profiles of several secreted molecules, it appears that soluble factors released from microglia contribute to the impaired neurogenesis seen in PS1 mutant mice (Fig. 1; Choi et al. 2008). Further investigation will be necessary to test which of these secreted molecules mediate(s) the described effects of microglia on neurogenesis, how they are affected in sporadic AD, and whether they represent a potential new therapeutic target.
The role of microglia in immunotherapy for AD
Based on the amyloid hypothesis, which implicates the accumulation of Aβ peptides as central factor in the pathogenesis of AD, new therapeutic approaches aim, therefore, to reduce Aβ in the brain either by decreased production or increased clearance. Immunotherapy via active or passive immunization against Aβ peptides has been shown to be very successful in reducing the number of amyloid plaques in AD transgenic mouse models (Schenk et al. 1999; Bard et al. 2000; Janus et al. 2000; DeMattos et al. 2001; Wilcock et al. 2004b; Buttini et al. 2005) and non-human primates (Lemere et al. 2004). Although the first phase II active immunization trial in humans had to be aborted because of the development of meningoencephalitis in 6% of the study population (Orgogozo et al. 2003; Gilman et al. 2005), postmortem analysis of a limited number of study participants who have died since, showed patchy clearance of amyloid plaques from the brain (Nicoll et al. 2003, 2006; Masliah et al. 2005). These areas of clearing were accompanied by abundant Aβ-immunoreactive microglial cells similar to the findings in animal models and consistent with the hypothesis that Aβ-specific antibodies may lead to phagocytosis of Aβ by microglial cells (Fig. 1; Bard et al. 2000). The involvement of microglia in the clearance of amyloid after immunization was elegantly demonstrated by two-photon confocal microscopy. After direct application of an Aβ-specific antibody to the brain tissue of PDAPP transgenic mice, a substantial decrease in amyloid deposits was seen in association with a marked microglial response (Bacskai et al. 2001). Supportive evidence for a central role of microglia was also provided by experimental downregulation of microglial activation either through the use of F(ab) fragments or various anti-inflammatory drugs which severely reduced the clearance of fibrillar but not diffuse plaques (Wilcock et al. 2004a). In addition, microglial activation following immunotherapy may be associated with a shift in the phenotypic state as different microglial markers exhibited a different temporal expression profile after passive immunization in mice. Based on these observations, it is postulated that activated microglia may transition from a condition associated with inflammation and ineffective clearing of Aβ deposits to one with reduced inflammation and capable of clearing deposited amyloid (Fig. 1; Morgan et al. 2005).
Microglia-independent mechanisms also appear to be involved in plaque clearance as amyloid clearance is also seen in Fc-receptor knockout mice (Das et al. 2003), and with modified anti-amyloid specific antibodies or F(ab′)2 fragments which show no or reduced binding to Fc receptors (Bacskai et al. 2002; Carty et al. 2006). First, there may be a direct effect of antibody on Aβ leading to dissolution of amyloid fibrils or neutralization of Aβ oligomers (Solomon et al. 1997; Klyubin et al. 2008). A second mechanism, termed the peripheral sink hypothesis (DeMattos et al. 2001), postulates that administration of antibodies to the circulation results in a net efflux of Aβ from the brain to the plasma. By modulating microglial activity, it was shown that stimulation of microglia with IFN-γ increased amyloid plaque clearance but not to the extent of antibody-mediated clearance. On the other hand, when microglia were eliminated or inhibited by immunotoxin or minocyclin, a partial reduction in the effectiveness of plaque removal by passive immunotherapy was observed (Garcia-Alloza et al. 2007). Overall, a combination of microglia-dependent and -independent mechanisms is likely involved in the prevention and clearance of amyloid plaques following immunotherapy (Wilcock et al. 2003, 2004b; Weiner and Frenkel 2006; Boche and Nicoll 2008).
Despite early termination, results from the human active immunization trial suggest a beneficial effect in some clinical outcome measures with slowing of cognitive performance decline in antibody responders (Hock et al. 2003; Gilman et al. 2005). In animal studies, Aβ immunization was shown to reduce behavioral impairment (Janus et al. 2000) and prevent synaptic degeneration (Buttini et al. 2005; Klyubin et al. 2008). The adverse occurrence of meningoencephalitis in a subset of humans has been attributed mainly to an unwanted activation of T cells (Gelinas et al. 2004; Weiner and Frenkel 2006). Other mechanisms that have been suggested include an over-exuberant microglial activation prompted by opsonization of Aβ and alterations in fluid balance in the brain triggered by interaction of antibody–Aβ immune complexes and the cerebral vasculature (Boche and Nicoll 2008). New vaccination strategies are currently being tested in human clinical trials using passive and active immunization approaches with newly developed vaccines that were specifically designed to minimize the risk of unwanted T-cell activation.
In a different approach, it may be possible to achieve beneficial immunotherapeutic effects without the use of Aβ-specific antibodies. After immunization with glatiramer and protollin, a reduced Aβ burden has been observed in a transgenic mouse model. This effect was associated with microglial activation and increased expression of IFN-γ and macrophage-colony stimulating factor by microglial cells. Glatiramer, which has been shown to suppress EAE, is a copolymer that induces specific T cells that accumulate in the brain due to cross-reactivity with myelin basic protein. Secretion of cytokines by these T cells may lead to microglial activation and subsequent amyloid clearance. Protollin is a proteosome-based adjuvant composed of outer membrane proteins of Neisseria meningitidis and LPS. Protollin may function by stimulating microglial cells both by LPS through TLR4 and by porB, which makes up 70% of the proteosome protein and is known to activate antigen-presenting cells through TLR2. Administration of protollin alone was also effective in clearing amyloid although to a lesser degree than in combination with glatiramer (Frenkel et al. 2005). A glatiramer-based vaccine is successfully being used in clinical patients with the relapsing–remitting form of multiple sclerosis (Sela 2006). It remains to be seen if this immunomodulatory approach will also be a successful strategy in AD.
Imaging microglia in vivo in live subjects
Why and how do we image microglia?
In the first section, we provided a short overview about the diverse functions of microglia in aging and neurodegeneration. As highlighted by this review, it is evident now more than ever that microglial activation is a very dynamic and context-dependent process which is still incompletely understood. This gap in our knowledge is in part due to limitations in studying the dynamics of these cells in vivo. The vast majority of studies have focused on cell culture systems supplemented by immunohistochemical approaches in brain tissues derived from animal models and human subjects. These data have offered many breakthroughs and insights into the structure and function of microglia and indeed form the foundation of hypotheses that implicates microglia in the pathogenesis of several neurological disorders. However, immunolabeling of cells in brain tissues offers only a snap shot at a solitary time point of complex dynamic processes. Cell culture systems offer many insights but cannot model complex cell-cell in vivo interactions. Imaging microglia in vivo in live subjects offers the advantages of studying these cells over time in their native environment providing a better understanding of their function in the normal central nervous system (CNS) and pathologic states. Imaging microglia in human subjects can offer a measure of the inflammatory process and a means of detecting progression of disease and efficacy of therapeutics over time.
Imaging microglial cells in vivo in live subjects is a challenging and growing field utilizing multiple, technically complex approaches ranging from confocal microscopy in zebra fish embryos, two/multiphoton microscopic imaging in transgenic mice, to positron emission tomography in larger animal models and humans (Fig. 2). Each technology has advantages and limitations and more detailed analyses of these techniques are beyond the scope of this review. Magnetic resonance imaging (MRI) has been applied to studying macrophage infiltration into the brain. This review focuses on imaging microglial cells in the brain. For a more detailed discussion on imaging CNS-infiltrating macrophages, the reader is referred to a recent review (Stoll and Bendszus 2008). In the following paragraphs, we review microglial in vivo imaging studies in the context of how imaging has provided insight into the physiology and functions of microglia in the setting of neurological disorders.
Fig. 2.
In vivo imaging of microglia in living subjects by microscopic techniques and positron emission tomography (PET). a PET imaging of microglial cells in vivo involves utilizing ligands labeled with radioisotopes such as [11C](R)-PK11195 that bind translocator protein-18 kDa (TSPO, also called the peripheral benzodiazepine receptor (PBR)) expressed on the outer mitochondrial membrane in microglia. The radioisotope undergoes positron emission decay resulting in the emission of a positron, which annihilates with an electron producing a pair of gamma particles that are emitted 180° apart and are detected by the PET scanner. b Microglia are labeled with green florescent protein under control of genetic loci specific to microglia such as CXC3R1 and Iba-1. In such transgenic mice, the skulls of the animals are thinned and animals are imaged with two/multiphoton microscopy. Deep visual penetration and optical sectioning of the tissue enable time-lapse, real-time, 3D imaging of microglia in vivo in live animals
Microscopic imaging of microglia—visualization of microglia in vivo in live transgenic animal models
Transgenic technologies in mice and zebra fish combined with microscopy have revolutionized this field by direct, high-resolution visualization of microglia in the non-pathologic state and in disease models. These genetic techniques have revolved around labeling microglia with green florescent protein under control of different genetic loci specific to microglia. Transgenic animals can then be directly imaged by microscopy (Fig. 2). The small size of zebra fish embryonic brain enables direct visualization using confocal microscopy in intact animal (Peri and Nusslein-Volhard 2008). In transgenic mice, the skulls of the animals are thinned or a small craniotomy is made before the animals are imaged with two/multiphoton microscopy. Deep visual penetration and optical sectioning of the tissue enable time-lapse, real-time, 3D imaging in vivo in live animals to a depth of 5 mm (Skoch et al. 2006). Further, by labeling blood vessels with Texas red dextran, fiduciary vascular markers can be established that enable imaging and analyzing the same brain area over a period of time such as a month (Bolmont et al. 2008; Meyer-Luehmann et al. 2008). This enables longitudinal assessment of dynamic processes such as microglial recruitment to a specific area of interest over time. Moreover, labeling of surrounding cells such as neurons with yellow florescent protein permits studying microglial–neuronal interaction in vivo (Meyer-Luehmann et al. 2008).
Microscopic imaging of normal microglial physiology
Seminal studies by Nimmerjahn et al. (2005) and Davalos et al. (2005) have thrown light on normal microglial physiology. Both groups utilized transgenic mice that express enhanced green florescent protein in the locus encoding the chemokine-fractalkine receptor CX3CR1 to study the behavior of microglia using time-lapse two-photon microscopic imaging. Remarkably, “resting” microglia, previously thought to be quiescent and non-motile are extremely active cells, constantly sampling their surrounding environment. While the cell bodies of microglia remained relatively stable, microglial processes underwent repeated cycles of extension and withdrawal. The cells were organized into defined regions spanning cell–cell distances of 50–60 μm. The cell processes showed filopodia-like motile appendages that formed and withdrew constantly. Following focused laser-induced injury, ramified processes showed rapid movement into the site of injury with responses depending on the severity of the injury. Microglial processes fused to form an area of containment separating healthy and injured tissues within about 30 s, suggesting that microglia may represent a first line of defense in CNS injury. Davalos et al. further showed that adenosine triphosphate (ATP) regulated rapid microglial response to injury as suggested by similar responses to local application of ATP, adenosine diphosphate (ADP), and UDP and blockade by apyrase, an enzyme that degrades bi-phosphate bonds. Inhibitors of connexin hemi-channels expressed highly in astrocytes inhibited microglial responses suggesting that ATP in these conditions may be derived from astrocytes. These studies have prompted reexamining the term “resting” microglia leading to Kettenmann and colleagues to propose “surveying” microglia to reflect the more dynamic nature of these cells in the normal brain (Hanisch and Kettenmann 2007).
By expressing green fluorescent protein under the Apolipoprotein-E locus Peri et al. imaged microglia in zebra fish embryos using time-lapse confocal microscopy (Peri and Nusslein-Volhard 2008). These studies confirmed the highly dynamic nature of microglia and helped elucidate the less understood phagocytic functions of microglia in vivo. Phagocytosis of apoptotic neurons during development occurred in compartments mediated by fusion of phagosomes with lysosomes. Elegant knockdown experiments suggest that the v0-ATPase a1 subunit, a part of the v-ATPase complex that regulates acidifications of subcellular compartments, mediated phagocytosis. It remains to be seen if this mechanism is conserved in mammalian systems during development and disease. A better understanding of the subcellular events mediating phagocytosis in microglia is essential to understand how these pathways are altered in Alzheimer’s disease and can be targeted for therapeutics.
In vivo microscopic imaging of microglia in disease models
Multiphoton microscopy has been used to study the relationship between amyloid deposition and microglial activation in AD in live animals. PDAPP mice expressing enhanced green fluorescent protein in the Cx3cr1 locus (to label microglia) were injected with Methoxy-OX4 to detect amyloid plaques and imaged in vivo with multiphoton microscopy at ages when plaques begin to form. Plaques appeared surprisingly rapidly, with microglial responses occurring within a day (Meyer-Luehmann et al. 2008).
Similar experiments were conducted by Bolmont et al. using APP/PS1 mice expressing green fluorescent protein at the Iba-1 (a calcium binding protein expressed in microglia) locus (Bolmont et al. 2008). In vivo multiphoton imaging confirmed that microglia respond rapidly to plaque formation by extending processes and migrating towards plaques. The number of microglia associated with each plaque increased at a rate of three per month independent of the size of the plaque. Interestingly, the size of the plaque influenced the volume of each microglial cell with larger plaques associated with larger microglial cells. Microglia that are defective in phagocytosis assume a larger morphology in the zebra fish model (Peri and Nusslein-Volhard 2008) raising the possibility that large microglia surrounding plaques may represent cells with defective or saturated phagocytic capacity. Further, these authors observed that the amyloid binding dye often exhibited a punctate pattern within plaque-associated microglia in vivo that co-localized in postmortem tissues with Aβ and microglial lysozymes. The authors suggest that this may represent an ability of microglia to ingest Aβ either as an active or passive process (Bolmont et al. 2008).
High-resolution in vivo time lapse imaging of microglia now provides a tool to examine several questions such as the role of microglia in promoting neurodegeneration, immunotherapies, and amyloid clearance. It is possible that microscopic imaging of microglia in vivo can be adapted to other diseases such as Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease by generating appropriate transgenic animals. While this technology cannot be directly applied to large animals or human subjects, these studies have expanded our knowledge on microglial function in both the normal and diseased state.
Positron emission tomography imaging of microglia in vivo
Positron emission tomography (PET) imaging has been used extensively in both animal models and human subjects to image activated microglia in the brain. Pioneered by Dr. Richard Banati from the Hammersmith Hospital UK, this technique takes advantage of radioisotope labeled (such as carbon-11 [C11]) ligands that bind specifically to the translocator protein-18 (TSPO, also called the peripheral benzodiazepine receptor (PBR)) expressed in microglia (Fig. 2; Banati 2002). A simplified description of the procedure begins with radiolabeling in a cyclotron a ligand (such as PK11195) that binds TSPO. The radiolabeled ligand is then injected intravenously into the subject. The lipophilic nature of the ligand enables entry across the blood–brain barrier into the brain and binding to TSPO. The tracer emission is measured over a time period dependent upon the half-life (~20 min for C11) of the radioisotope. The radioisotope undergoes positron emission decay resulting in the emission of a positron, which annihilates with an electron producing a pair of gamma particles that are emitted 180° apart and are detected by the PET scanner (Fig. 2). Multiple such events are recorded over time (Aine 1995). These data are analyzed using mathematical models that then can be translated into time activity curves (activity of the ligand measured in a specific brain region over time) or images that represent the spatial and temporal distribution of radioactivity in terms of absolute units of radioactivity concentration (for a more detailed review of mathematical algorithms see, Venneti et al. 2006). The attractiveness of PET imaging includes the relatively non-invasive nature of the technology, direct translation to human studies, and the ability to extract quantitative information regarding physiologic parameters that are relevant to a disease process. Key factors that need to be taken into consideration when evaluating a TSPO ligand are its ability to cross the blood–brain barrier, in vivo specific binding kinetics to TSPO, in vivo non-specific binding, specific activity, regional delivery, the rate of metabolism, potential toxicity of the ligand or metabolites, and sensitivity and specificity of the ligand in detecting microglia.
Translocator protein-18 kDa as a molecular target for PET imaging of activated microglia
TSPO is expressed at very low levels in the resting brain and is thought to be mainly in astrocytes and microglia. Several studies indicate that TSPO ligand binding increases in activated microglia in response to CNS insults (discussed below). This increase in TSPO ligand binding can be taken advantage of as a molecular target for PET imaging. While characteristics of TSPO that facilitate imaging activated microglia are discussed below, the function and regulation of TSPO in activated microglia are not known. TSPO was first discovered as a benzodiazepine binding site distinct from the central GABAergic receptor-binding site and was named peripheral benzodiazepine receptor (Braestrup et al. 1977). The nomenclature is confusing, as this receptor does not mediate effects of benzodiazepines. This led a recent focus group to rename it “translocator protein-18 kDa” (TSPO) to reflect the main function of the receptor in cholesterol translocation in steroid synthesizing cells and the molecular weight of the protein (Papadopoulos et al. 2006b). TSPO is an 18-kDa protein localized to the outer mitochondrial membrane as a part of a hetero-oligomeric complex comprised of the voltage-dependent anion channel and an adenine nucleotide carrier forming the putative mitochondrial permeability transition pore (McEnery et al. 1992). Although the functions of TSPO are not completely known in the brain, the most extensively characterized function of TSPO is in steroid synthesis (Papadopoulos et al. 1997). While it is not known if microglia have the ability to synthesize physiologic steroids, TSPO is thought to be involved in neurosteroid synthesis (Papadopoulos et al. 2006a). As a constituent of the mitochondrial permeability transition pore, it is thought to regulate mitochondrial respiration (Hirsch et al. 1989) and cell death directly (McEnery et al. 1992) or indirectly by influencing interactions of mitochondrial permeability transition pore proteins with factors that regulate apoptosis including Bcl-2, Bcl-Xl, and Bax (Castedo et al. 2002). Several other functions of TSPO in microglia have been speculated and require further elucidation.
PET imaging using the TSPO (PBR) ligand PK11195
Several ligands that bind specifically to TSPO have been synthesized. Of these ligands, an isoquinoline carboxamide derivative PK11195 (1-(2-chlorophenyl)-N-methyl-N-(1methylpropyl)-3-isoquinolinecarboxamide), the R-enantiomer of which has a high affinity for TSPO (Shah et al. 1994) has been extensively characterized. PK11195 is a pharmacologic antagonist of TSPO but has no pharmacologic effects in concentrations used in PET imaging studies. PK11195 is the prototype TSPO ligand since it fits several of the criteria (discussed earlier). PK11195 labeled with [3H] and [11C] has enabled the study of microglial activation extensively in a number of animal models including experimental autoimmune encephalitis (Vowinckel et al. 1997; Banati et al. 2000), stroke (Myers et al. 1991; Stephenson et al. 1995; Rojas et al. 2007), brain trauma (Raghavendra Rao et al. 2000; Venneti et al. 2007a), facial nerve transaction (Banati et al. 1997; Gehlert et al. 1997), hepatic encephalopathy (Desjardins et al. 1997), SIV encephalitis (Mankowski et al. 2003; Venneti et al. 2004, 2008d), hippocampal nerve transection (Pedersen et al. 2006), Parkinson’s disease (Cumming et al. 2001; Cicchetti et al. 2002; Venneti et al. 2007b; Chen et al. 2008), injection of neurotoxins such as cuprizone and trimethyltin (Kuhlmann and Guilarte 2000; Chen et al. 2004; Chen and Guilarte 2006), and alcohol-induced striatal injury (Toyama et al. 2008).
These studies have been effectively translated to human studies. Imaging studies in human subjects have covered a wide spectrum of neurological disorders including Rasmussen’s encephalitis (Banati et al. 1999), ischemic stroke (Gerhard et al. 2000, 2005; Price et al. 2006), multiple sclerosis (Banati et al. 2000; Debruyne et al. 2003; Versijpt et al. 2005), herpes encephalitis (Cagnin et al. 2001a), cerebral vasculitis (Goerres et al. 2001), AD (Cagnin et al. 2001b), amyotrophic lateral sclerosis (Turner et al. 2004), corticobasal degeneration (Gerhard et al. 2004; Henkel et al. 2004), frontotemporal dementia (Cagnin et al. 2004), Parkinson’s disease (Ouchi et al. 2005; Gerhard et al. 2006), upper motor neuron syndromes (Turner et al. 2005), HIV infection (Hammoud et al. 2005; Wiley et al. 2006), hepatic encephalopathy (Cagnin et al. 2006b; Iversen et al. 2006), Huntington’s disease (Pavese et al. 2006), pre-symptomatic Huntington’s disease (in carriers; Tai et al. 2007), intractable epilepsy (Kumar et al. 2008), methamphetamine abuse (Sekine et al. 2008), and early onset schizophrenia (van Berckel et al. 2008). While the utility of PK11195 as a PET ligand is beyond doubt, several issues dampen enthusiasm regarding its investigative utility and are discussed below.
PK11195 binding—reactive astrocytes versus activated microglia
A central question in the field has been the relative contributions to PK11195 binding of reactive astrocytes and activated microglia. PK11195 binding to TSPO in the non-pathologic brain is thought to be mainly in “resting” astrocytes and microglia. Support for expression in “resting” astrocytes and microglia is largely derived from cell culture studies (Itzhak et al. 1993, 1995; Park et al. 1996; Wilms et al. 2003) and remains to be confirmed in brain tissues. In animal models, increased [3H]-PK11195 binding corresponding to activated microglia in brain tissues is reported in rodent models of stroke (Myers et al. 1991), ischemia (Stephenson et al. 1995), experimental autoimmune encephalitis (Vowinckel et al. 1997), multiple sclerosis (Vowinckel et al. 1997; Banati et al. 2000), facial nerve axotomy (Banati et al. 1997), brain trauma (Raghavendra Rao et al. 2000), hippocampal axonal lesions (Pedersen et al. 2006), transgenic mouse models of Alzheimer’s disease (Venneti et al. 2008c), and SIV encephalitis in macaques (Mankowski et al. 2003; Venneti et al. 2004, 2008d). Some of these studies also report a stronger correlation of [3H]-PK11195 binding with microglia versus astrocytes in brain tissues (Raghavendra Rao et al. 2000; Mankowski et al. 2003; Venneti et al. 2004, 2008c). However, some reports indicate TSPO immunostaining and [3H]-PK11195 binding corresponding to reactive astrocytes following an initial increase in activated microglia in rodents treated with the neurotoxin trimethyltin (Kuhlmann and Guilarte 2000) and cuprizone (Chen et al. 2004). Others have shown that TSPO immunostaining co-localizes with activated microglia in rats injected intracerebrally with ethanol (Maeda et al. 2007a) and in rat models of stroke (Rojas et al. 2007). In our hands, TSPO ligand binding mainly correlates with activated microglia (as determined by CD68 labeling (a lysozomal marker of activated microglia)) in regions of pathology in human brain tissues derived from patients with multiple sclerosis, Alzheimer’s disease, amyotrophic lateral sclerosis, cerebrovascular infarction, and frontotemporal dementia (Venneti et al. 2008b).
These discrepancies perhaps reflect the specific animal model in question as well as the technical challenges in localization of PK11195 to a specific cell type. The cellular processes of reactive astrocytes are intimately associated with activated microglia as demonstrated by immunohistochemistry and in vivo two-photon microscopy and are difficult to tease apart in representative brain sections. The approach of combining immunohistochemical markers for reactive astrocytes and activated microglia with PK11195 high-resolution emulsion autoradiography has to be performed on frozen brain sections where cellular morphology may not be well preserved. While an alternative approach is performing double-label immunohistochemistry in paraffin-embedded sections (where cellular morphology is better preserved) using antibodies against TSPO and glial markers, these data should be interpreted with caution, as the binding sites of TSPO antibodies and PK11195 may be different (Cagnin et al. 2006a). These approaches are also limited by the antibodies used to detect specific cell types, as well as by sampling biases restricted to the brain sections used in the analyses. Further, PET imaging in vivo adds additional complexities that make it difficult to extrapolate these studies. While the relative contributions of either cell type is still subject to debate, perhaps alternative approaches such as in vivo two-photon microscopy with fluorescently labeled PK11195 in mice transgenically labeled for either glial cell marker will help resolve these issues.
PET imaging of activated microglia—the need for more sensitive and specific TSPO ligands
While [11C]-PK11195 has been extensively used in several diseases, issues regarding the sensitivity and specificity should be considered. [11C]-PK11195 retention in the brain is often noted in regions traditionally not associated with disease pathology. Patients with Alzheimer’s disease not only showed [11C]-PK11195 retention in all regions associated with Alzheimer’s disease, but also showed increased [11C]-PK11195 retention in the brainstem (Cagnin et al. 2001b). Similarly, a recent study using [11C]-PK11195 to image activated microglia in amyotrophic lateral sclerosis (ALS) patients showed high binding in the occipital cortex and thalamus, areas that are not traditionally implicated in ALS pathology (Turner et al. 2004). While these findings may represent a form of microglial activation due to “synaptic stripping” in regions connected to areas of primary pathology, it is not possible to confirm the histological presence of activated microglia in these regions in human studies. Alternatively, these increases may reflect regional variations in the constitutive TSPO population that are independent of the disease pathology or a non-uniform element of non-specific [11C](R)-PK11195 binding.
Another consideration regarding the sensitivity is that the specific binding signal with [11C](R)-PK11195 is generally very low and challenging to quantify with typical PET image noise levels (Petit-Taboue et al. 1991; Groom et al. 1995; Banati et al. 2000; Pappata et al. 2000). Further, [11C](R)-PK11195 may not be able to detect mild forms of neuroinflammation. For instance, no differences were seen in [11C](R)-PK11195 brain retention in HIV-infected individuals with or without neurological deficits (Hammoud et al. 2005; Wiley et al. 2006). Similarly, [11C](R)-PK11195 retention in the brain did not differ in normal subjects when compared with patients with minor cognitive impairment (Schuitemaker et al. 2006) and moderate Alzheimer’s disease (Wiley et al. 2008). These concerns highlight the necessity of developing new ligands that bind with more specificity and sensitivity to activated microglia for PET imaging in neurological diseases.
Several newer ligands have been synthesized in the past couple of years that bind TSPO. Some of these ligands exhibit properties that may render them suitable for PET imaging. DAA1106 [N-(2,5-dimethoxybenzyl)-N-(4-fluoro-2-phenoxyphenyl) acetamide], an aryloxyanilide derivative, shows a higher affinity and selective binding to TSPO compared to PK11195 suggested by a significantly lower dissociation constant (Chaki et al. 1999; Okuyama et al. 1999). [3H]DAA1106 labeled activated microglia and showed higher binding affinity compared to [3H]-PK11195 in animal models of HIV encephalitis (Venneti et al. 2008a), brain trauma (Venneti et al. 2007a), Parkinson’s disease (Venneti et al. 2007b), and rats injected with LPS (Venneti et al. 2007b). [11C]DAA1106 also showed retention at the lesioned site in vivo in rats injected with LPS or 6-OHDA (Venneti et al. 2007b) and rat models of stroke (Maeda et al. 2007a). Further, the fluorinated derivative [18F]-FEDAA1106 showed higher brain retention compared to controls in animal models of Alzheimer’s disease (Maeda et al. 2007b). While data from animal models showed significant promise, in vivo data from ten human subjects with mild to moderate Alzheimer’s disease are less clear (Yasuno et al. 2008). Increased [11C]DAA1106 retention was seen in all measured regions such as the dorsal and medial prefrontal cortex, lateral temporal cortex, parietal cortex, occipital cortex, anterior cingulate cortex, including some regions not typically associated with Alzheimer’s disease pathology such as the striatum and cerebellum (Yasuno et al. 2008). While the authors consider various possibilities, it is conceivable that the higher binding affinity of DAA1106 compared with PK11195 may compromise specificity. However, further studies in human subjects and human postmortem tissues are required to completely characterize DAA1106.
The past few years have seen an explosion of promising compounds that are in various preclinical stages of development. These ligands include the high affinity quinoline-carboxamides ([11C]-VC195) and halogenated 2-quinolinecarboxamides that are structurally similar to PK11195 (Belloli et al. 2004; Cappelli et al. 2006), [11C]-vinpocetine (Gulyas et al. 2005), the pyrazolopyrimidines [11C]-DPA-713 (James et al. 2005) and DPA-714 (James et al. 2008), [18F]imidazo[1,2-a]pyridines and [18F]pyrazolo [1,5-a]pyrimidines (Fookes et al. 2008), [(125)I]-CLINDE (6-chloro-2-(4′iodophenyl)-3-(N,N-diethyl)-imidazo[1,2-a] pyridine-3-acetamide) (Arlicot et al. 2008), N-[11C]methylated imidazopyridineacetamides (Sekimata et al. 2008), 11C-AC-5216 (Yanamoto et al. 2007), and [(11)C] CLINME (Boutin et al. 2007). While the utility of all of these novel ligands for in vivo PET imaging of neuroinflammation remains to be demonstrated, they represent several new classes of ligands that are potent and selective for TSPO.
Summary
Activation of microglia is seen in several neurological disorders and constitutes a significant component of the underlying pathology, although the pathogenesis of these various diseases differs enormously. In this review, we discussed some of the current literature implicating activated microglia not only as perpetrators of disease, but also as cells with neuroprotective potential. Understanding the common cellular pathways that are involved in activation of microglia and associated neuronal injury may help in the design and implementation of more effective therapeutics.
The clinical diagnosis of neurodegenerative disorders requires the presence of irreversible clinical symptoms and signs. Traditional imaging studies such as CT and MRI have been of limited assistance in the diagnosis of dementia in general (Das et al. 2003). Current methodologies, such as structural MRI assessment of brain volume, are insensitive late measures of neurological damage and the diagnosis by neurocognitive tests is possible only after irreversible neuronal damage has occurred. Effective therapy requires early intervention at the onset of neuronal injury prior to the appearance of structural atrophy and irreversible signs and symptoms. In vivo imaging of activated microglia in neurological disorders may enable early diagnosis and therapeutic interventions. The recent years have revolutionized in vivo imaging of activated microglia in live subjects. Microscopic imaging in transgenic animal models has produced a better understanding of the physiology and pathology of microglia. Attempts to develop therapies targeting neuroinflammation will require some means of monitoring the inflammatory pathogenic process. Imaging activated microglia may provide a better index of disease progression during treatment with immunomodulatory or other potentially neuroprotective drugs than clinical neuropsychological testing. Imaging of activated microglia may be able to help in the early assessment of neuroinflammation, monitor the severity and progression of the disease, and help evaluate the effectiveness of CNS therapies aimed at modulating neuroinflammation.
Contributor Information
Clayton A. Wiley, Email: wileyca@upmc.edu.
Julia Kofler, Email: koflerjk@upmc.edu.
References
- Aine CJ. A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/FMRI and PET. Crit Rev Neurobiol. 1995;9:229–309. [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akiyama H, McGeer PL. Specificity of mechanisms for plaque removal after A beta immunotherapy for Alzheimer disease. Nat Med. 2004;10:117–118. doi: 10.1038/nm0204-117. author reply 118–119. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Schwab C, Kondo H, Mori H, Kametani F, Ikeda K, McGeer PL. Granules in glial cells of patients with Alzheimer’s disease are immunopositive for C-terminal sequences of beta-amyloid protein. Neurosci Lett. 1996;206:169–172. doi: 10.1016/S0304-3940(96)12474-5. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Overmyer M, Helisalmi S, Soininen H. Lower counts of astroglia and activated microglia in patients with Alzheimer’s disease with regular use of non-steroidal anti-inflammatory drugs. J Alzheimers Dis. 2000;2:37–46. doi: 10.3233/jad-2000-2105. [DOI] [PubMed] [Google Scholar]
- Arlicot N, Katsifis A, Garreau L, Mattner F, Vergote J, Duval S, Bodard S, Guilloteau D, Chalon S. Evaluation of CLINDE as potent translocator protein (18 kDa) SPECT radiotracer reflecting the degree of neuroinflammation in a rat model of microglial activation. Eur J Nucl Med Mol Imaging. 2008 doi: 10.1007/s00259-008-0834-x. (in press) [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)-binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H] PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. doi: 10.1023/A:1018567510105. [DOI] [PubMed] [Google Scholar]
- Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, Brooks DJ, Jones T, Duncan JS. [11C] (R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology. 1999;53:2199–2203. doi: 10.1212/wnl.53.9.2199. [DOI] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Belloli S, Moresco RM, Matarrese M, Biella G, Sanvito F, Simonelli P, Turolla E, Olivieri S, Cappelli A, Vomero S, Galli-Kienle M, Fazio F. Evaluation of three quinoline-carboxamide derivatives as potential radioligands for the in vivo pet imaging of neurodegeneration. Neurochem Int. 2004;44:433–440. doi: 10.1016/j. neuint.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Boche D, Nicoll JA. The role of the immune system in clearance of Abeta from the brain. Brain Pathol. 2008;18:267–278. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H, Chauveau F, Thominiaux C, Kuhnast B, Gregoire MC, Jan S, Trebossen R, Dolle F, Tavitian B, Mattner F, Katsifis A. In vivo imaging of brain lesions with [(11)C]CLINME, a new PET radioligand of peripheral benzodiazepine receptors. Glia. 2007;55:1459–1468. doi: 10.1002/glia.20562. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, Khan K, Seubert P, Freedman S, Schenk D, Games D. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Myers R, Gunn RN, Lawrence AD, Stevens T, Kreutzberg GW, Jones T, Banati RB. In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain. 2001a;124:2014–2027. doi: 10.1093/brain/124.10.2014. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001b;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Rossor M, Sampson EL, Mackinnon T, Banati RB. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol. 2004;56:894–897. doi: 10.1002/ana.20332. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand Suppl. 2006a;185:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Taylor-Robinson SD, Forton DM, Banati RB. In vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathy. Gut. 2006b;55:547–553. doi: 10.1136/gut.2005.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli A, Matarrese M, Moresco RM, Valenti S, Anzini M, Vomero S, Turolla EA, Belloli S, Simonelli P, Filannino MA, Lecchi M, Fazio F. Synthesis, labeling, and biological evaluation of halogenated 2-quinolinecarboxamides as potential radioligands for the visualization of peripheral benzodiazepine receptors. Bioorg Med Chem. 2006;14:4055–4066. doi: 10.1016/j.bmc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Carty NC, Wilcock DM, Rosenthal A, Grimm J, Pons J, Ronan V, Gottschall PE, Gordon MN, Morgan D. Intracranial administration of deglycosylated C-terminal-specific anti-Abeta antibody efficiently clears amyloid plaques without activating microglia in amyloid-depositing transgenic mice. J Neuroinflammation. 2006;3:11. doi: 10.1186/1742-2094-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Kroemer G. Mitochondrial apoptosis and the peripheral benzodiazepine receptor: a novel target for viral and pharmacological manipulation. J Exp Med. 2002;196:1121–1125. doi: 10.1084/jem.20021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Yoshikawa R, Okuyama S, Okubo T, Nakazato A, Nagamine M, Tomisawa K. Binding characteristics of [3H]DAA1106, a novel and selective ligand for peripheral benzodiazepine receptors. Eur J Pharmacol. 1999;371:197–204. doi: 10.1016/S0014-2999(99)00118-1. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol Sci. 2006;91:532–539. doi: 10.1093/toxsci/kfj172. [DOI] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, Guilarte TR. Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain. 2004;127:1379–1392. doi: 10.1093/brain/awh161. [DOI] [PubMed] [Google Scholar]
- Chen MK, Kuwabara H, Zhou Y, Adams RJ, Brasic JR, McGlothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte TR. VMAT2 and dopamine neuron loss in a primate model of Parkinson’s disease. J Neurochem. 2008;105:78–90. doi: 10.1111/j.1471-4159.2007.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Kalaria RN, McGeer PL, Rogers J. Key issues in Alzheimer’s disease inflammation. Neurobiol Aging. 2000;21:451–453. doi: 10.1016/S0197-4580(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Cumming P, Danielsen EH, Vafaee M, Falborg L, Steffensen E, Sorensen JC, Gillings N, Bender D, Marthi K, Andersen F, Munk O, Smith D, Moller A, Gjedde A. Normalization of markers for dopamine innervation in striatum of MPTP-lesioned miniature pigs with intrastriatal grafts. Acta Neurol Scand. 2001;103:309–315. doi: 10.1034/j.1600-0404.2001.103005309.x. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–264. doi: 10.1046/j.1468-1331.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- Del Rio Hortega P. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. Hoeber; New York: 1932. pp. 482–534. [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P, Bandeira P, Raghavendra Rao VL, Ledoux S, Butterworth RF. Increased expression of the peripheral-type benzodiazepine receptor-isoquinoline carboxamide binding protein mRNA in brain following portacaval anastomosis. Brain Res. 1997;758:255–258. doi: 10.1016/S0006-8993(97)00339-9. [DOI] [PubMed] [Google Scholar]
- DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–1012. doi: 10.1016/S0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Fookes CJ, Pham TQ, Mattner F, Greguric I, Loc’h C, Liu X, Berghofer P, Shepherd R, Gregoire MC, Katsifis A. Synthesis and biological evaluation of substituted [18F]imidazo[1,2-a]pyridines and [18F]pyrazolo[1,5-a]pyrimidines for the study of the peripheral benzodiazepine receptor using positron emission tomography. J Med Chem. 2008;51:3700–3712. doi: 10.1021/jm7014556. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Ferrara BJ, Dodwell SA, Hickey GA, Hyman BT, Bacskai BJ. A limited role for microglia in antibody mediated plaque clearance in APP mice. Neurobiol Dis. 2007;28:286–292. doi: 10.1016/j.nbd.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J Neurochem. 2004a;91:521–536. doi: 10.1111/j.1471-4159.2004. 02743.x. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004b;88:337–348. doi: 10.1111/j.1471-4159.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Stephenson DT, Schober DA, Rash K, Clemens JA. Increased expression of peripheral benzodiazepine receptors in the facial nucleus following motor neuron axotomy. Neurochem Int. 1997;31:705–713. doi: 10.1016/S0197-0186(97)00007-7. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-H. [DOI] [PubMed] [Google Scholar]
- Gelinas DS, DaSilva K, Fenili D, St George-Hyslop P, McLaurin J. Immunotherapy for Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14657–14662. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Neumaier B, Elitok E, Glatting G, Ries V, Tomczak R, Ludolph AC, Reske SN. In vivo imaging of activated microglia using [11C]PK11195 and positron emission tomography in patients after ischemic stroke. Neuroreport. 2000;11:2957–2960. doi: 10.1097/00001756-200009110-00025. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Watts J, Trender-Gerhard I, Turkheimer F, Banati RB, Bhatia K, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in corticobasal degeneration. Mov Disord. 2004;19:1221–1226. doi: 10.1002/mds.20162. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C] (R)-PK11195 PET study. Neuroimage. 2005;24:591–595. doi: 10.1016/j. neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Goerres GW, Revesz T, Duncan J, Banati RB. Imaging cerebral vasculitis in refractory epilepsy using [(11)C](R)-PK11195 positron emission tomography. AJR Am J Roentgenol. 2001;176:1016–1018. doi: 10.2214/ajr.176.4.1761016. [DOI] [PubMed] [Google Scholar]
- Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE. PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer’s disease. J Nucl Med. 1995;36:2207–2210. [PubMed] [Google Scholar]
- Group AR, Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, Piantadosi S, Sabbagh M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Halldin C, Vas A, Banati RB, Shchukin E, Finnema S, Tarkainen J, Tihanyi K, Szilagyi G, Farde L. [11C] vinpocetine: a prospective peripheral benzodiazepine receptor ligand for primate PET studies. J Neurol Sci. 2005;229–230:219–223. doi: 10.1016/j.jns.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Shepherd CE, McCann H, Reid WG, Grayson DA, Broe GA, Kril JJ. Effect of anti-inflammatory medications on neuropathological findings in Alzheimer disease. Arch Neurol. 2000;57:831–836. doi: 10.1001/archneur.57.6.831. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, McArthur JC, Pomper MG. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol. 2005;11:346–355. doi: 10.1080/13550280500187351. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j. jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Henkel K, Karitzky J, Schmid M, Mader I, Glatting G, Unger JW, Neumaier B, Ludolph AC, Reske SN, Landwehrmeyer GB. Imaging of activated microglia with PET and [11C]PK 11195 in corticobasal degeneration. Mov Disord. 2004;19:817–821. doi: 10.1002/mds.20040. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JD, Beyer CF, Malkowitz L, Beer B, Blume AJ. Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol. 1989;35:157–163. [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/S0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Baker L, Norenberg MD. Characterization of the peripheral-type benzodiazepine receptors in cultured astrocytes: evidence for multiplicity. Glia. 1993;9:211–218. doi: 10.1002/glia.440 090306. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Roig-Cantisano A, Norenberg MD. Ontogeny of peripheral-type benzodiazepine receptors in cultured astrocytes and brain from rat. Brain Res Dev Brain Res. 1995;84:62–66. doi: 10.1016/0165-3806(94)00163-T. [DOI] [PubMed] [Google Scholar]
- Iversen P, Hansen DA, Bender D, Rodell A, Munk OL, Cumming P, Keiding S. Peripheral benzodiazepine receptors in the brain of cirrhosis patients with manifest hepatic encephalopathy. Eur J Nucl Med Mol Imaging. 2006;33:810–816. doi: 10.1007/s00259-005-0052-8. [DOI] [PubMed] [Google Scholar]
- James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg Med Chem. 2005;13:6188–6194. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- James ML, Fulton RR, Vercoullie J, Henderson DJ, Garreau L, Chalon S, Dolle F, Costa B, Guilloteau D, Kassiou M. DPA-714, a new translocator protein-specific ligand: synthesis, radiofluorination, and pharmacologic characterization. J Nucl Med. 2008;49:814–822. doi: 10.2967/jnumed.107.046151. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236 (96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem. 2000;74:1694–1704. doi: 10.1046/j.1471-4159.2000. 0741694.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Chugani HT, Luat A, Asano E, Sood S. Epilepsy surgery in a case of encephalitis: use of 11C-PK11195 positron emission tomography. Pediatr Neurol. 2008;38:439–442. doi: 10.1016/j. pediatrneurol.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lu J, Tay SS, Moochhala SM, He BP. The function of microglia, either neuroprotection or neurotoxicity, is determined by the equilibrium among factors released from activated microglia in vitro. Brain Res. 2007;1159:8–17. doi: 10.1016/j.brainres.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR. Postmortem studies of the effect of anti-inflammatory drugs on Alzheimer-type pathology and associated inflammation. Neurobiol Aging. 2001;22:819–822. doi: 10.1016/S0197-4580(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang MR, Suzuki K, Suhara T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res. 2007a;1157:100–111. doi: 10.1016/j.brainres.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Maeda J, Ji B, Irie T, Tomiyama T, Maruyama M, Okauchi T, Staufenbiel M, Iwata N, Ono M, Saido TC, Suzuki K, Mori H, Higuchi M, Suhara T. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J Neurosci. 2007b;27:10957–10968. doi: 10.1523/JNEUROSCI.0673-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PJ, Adams RJ, Guilarte TR. Elevated peripheral benzodiazepine receptor expression in simian immunodeficiency virus encephalitis. J Neurovirol. 2003;9:94–100. doi: 10.1080/713831342. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/S0301-0082(97)00052-X. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01. jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Yoshikawa R, Ogawa S, Suzuki Y, Okubo T, Nakazato A, Nagamine M, Tomisawa K. Neuropharmacological profile of peripheral benzodiazepine receptor agonists, DAA1097 and DAA1106. Life Sci. 1999;64:1455–1464. doi: 10.1016/S0024-3205(99)00079-X. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006a;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006b;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C] PK1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Park CH, Carboni E, Wood PL, Gee KW. Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996;16:65–70. doi: 10.1002/(SICI)1098-1136. (199601)16:1<65::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Pedersen MD, Minuzzi L, Wirenfeldt M, Meldgaard M, Slidsborg C, Cumming P, Finsen B. Up-regulation of PK11195 binding in areas of axonal degeneration coincides with early microglial activation in mouse brain. Eur J Neurosci. 2006;24:991–1000. doi: 10.1111/j.1460-9568.2006.04975.x. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j. cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Baron JC, Barre L, Travere JM, Speckel D, Camsonne R, MacKenzie ET. Brain kinetics and specific binding of [11C]PK 11195 to omega 3 sites in baboons: positron emission tomography study. Eur J Pharmacol. 1991;200:347–351. doi: 10.1016/0014-2999(91)90594-G. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wang D, Menon DK, Guadagno JV, Cleij M, Fryer T, Aigbirhio F, Baron JC, Warburton EA. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke. 2006;37:1749–1753. doi: 10.1161/01.STR.00 00226980.95389.0b. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol. 2000;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Raine CS. Inflammation in Alzheimer’s disease: a view from the periphery. Neurobiol Aging. 2000;21:437–440. doi: 10.1016/S0197-4580(00)00138-X. discussion 451–433. [DOI] [PubMed] [Google Scholar]
- Richartz E, Stransky E, Batra A, Simon P, Lewczuk P, Buchkremer G, Bartels M, Schott K. Decline of immune responsiveness: a pathogenetic factor in Alzheimer’s disease. J Psychiatr Res. 2005;39:535–543. doi: 10.1016/j.jpsychires.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, Llop J, Gomez V, Gispert JD, Millan O, Chamorro A, Planas AM. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab. 2007;27:1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, Van Berckel BN, Boellaard R, Kropholler M, Boellaard R, Jonker C, Lubberlink M, Scheltens P, Lammertsma AA. Assessment of microglial activation in mild cognitive impairment using [11C](R)-PK11195 and PET. Neuroimage. 2006;31:T159. [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible. Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sekimata K, Hatano K, Ogawa M, Abe J, Magata Y, Biggio G, Serra M, Laquintana V, Denora N, Latrofa A, Trapani G, Liso G, Ito K. Radiosynthesis and in vivo evaluation of N-[11C] methylated imidazopyridineacetamides as PET tracers for peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:327–334. doi: 10.1016/j.nucmedbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. Immunomodulatory vaccines against autoimmune diseases. Rejuvenation Res. 2006;9:126–133. doi: 10.1089/rej.2006.9.126. [DOI] [PubMed] [Google Scholar]
- Shah F, Hume SP, Pike VW, Ashworth S, McDermott J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviours as radioligands for PK binding sites in rats. Nucl Med Biol. 1994;21:573–581. doi: 10.1016/0969-8051(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Skoch J, Hyman BT, Bacskai BJ. Preclinical characterization of amyloid imaging probes with multiphoton microscopy. J Alzheimers Dis. 2006;9:401–407. doi: 10.3233/jad-2006-9s345. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Ames MB. Major histocompatibility complex molecule expression in the human central nervous system: immunohistochemical analysis of 40 patients. J Neuropathol Exp Neurol. 1988;47:19–28. doi: 10.1097/00005072-198801000-00003. [DOI] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. Imaging of inflammation in the peripheral and central nervous system by magnetic resonance imaging. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.045. (in press) [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain—bad news for neurons. Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Szekely CA, Green RC, Breitner JC, Ostbye T, Beiser AS, Corrada MM, Dodge HH, Ganguli M, Kawas CH, Kuller LH, Psaty BM, Resnick SM, Wolf PA, Zonderman AB, Welsh-Bohmer KA, Zandi PP. No advantage of A beta 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–2298. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain. 2007;130:1759–1766. doi: 10.1093/brain/awm044. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, Reines SA. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- Toyama H, Hatano K, Suzuki H, Ichise M, Momosaki S, Kudo G, Ito F, Kato T, Yamaguchi H, Katada K, Sawada M, Ito K. In vivo imaging of microglial activation using a peripheral benzodiazepine receptor ligand: [11C]PK-11195 and animal PET following ethanol injury in rat striatum. Ann Nucl Med. 2008;22:417–424. doi: 10.1007/s12149-008-0136-1. [DOI] [PubMed] [Google Scholar]
- Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–609. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Turner MR, Gerhard A, Al-Chalabi A, Shaw CE, Hughes RA, Banati RB, Brooks DJ, Leigh PN. Mills’ and other isolated upper motor neurone syndromes: in vivo study with 11C-(R)-PK11195 PET. J Neurol Neurosurg Psychiatry. 2005;76:871–874. doi: 10.1136/jnnp.2004.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[(11)C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S, 3rd, Kress GJ, Davis DK, Ruszkiewicz J, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113:981–989. doi: 10.1172/JCI20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–322. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wagner AK, Wang G, Slagel SL, Chen X, Lopresti BJ, Mathis CA, Wiley CA. The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: implications for PET imaging. Exp Neurol. 2007a;207:118–127. doi: 10.1016/j.expneurol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Slagel SL, Mason NS, Mathis CA, Fischer ML, Larsen NJ, Mortimer AD, Hastings TG, Smith AD, Zigmond MJ, Suhara T, Higuchi M, Wiley CA. A comparison of the high-affinity peripheral benzodiazepine receptor ligands DAA1106 and (R)-PK11195 in rat models of neuroinflammation: implications for PET imaging of microglial activation. J Neurochem. 2007b;102:2118–2131. doi: 10.1111/j.1471-4159.2007.04690.x. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wang G, Wiley CA. The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds to activated and infected brain macrophages in areas of synaptic degeneration: implications for PET imaging of neuroinflammation in lentiviral encephalitis. Neurobiol Dis. 2008a;29:232–241. doi: 10.1016/j.nbd.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wang G, Nguyen J, Wiley CA. The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol. 2008b;67:1001–1010. doi: 10.1097/NEN.0b013e318188b204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Hamilton RL, Mathis CA, Klunk WE, Apte UM, Wiley CA. PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol Aging. 2008c doi: 10.1016/j.neurobiolaging.2007.11.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Bonneh-Barkay D, Lopresti BJ, Bissel SJ, Wang G, Mathis CA, Piatak M, Jr, Lifson JD, Nyaundi JO, Murphey-Corb M, Wiley CA. Longitudinal in vivo positron emission tomography imaging of infected and activated brain macrophages in a macaque model of human immunodeficiency virus encephalitis correlates with central and peripheral markers of encephalitis and areas of synaptic degeneration. Am J Pathol. 2008d;172:1603–1616. doi: 10.2353/ajpath.2008.070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versijpt J, Debruyne JC, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler. 2005;11:127–134. doi: 10.1191/1352458505ms1140oa. [DOI] [PubMed] [Google Scholar]
- Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, Antel JP. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res. 1997;50:345–353. doi: 10.1002/(SICI)1097-4547(19971015)50:2<345::AID-JNR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Waldau B, Shetty AK. Behavior of neural stem cells in the Alzheimer brain. Cell Mol Life Sci. 2008;65:2372–2384. doi: 10.1007/s00018-008-8053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Ye M, Zhang YH, Chen SD. CD200–CD200R regulation of microglia activation in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2007;2:259–264. doi: 10.1007/s11481-007-9075-1. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis. 2004a;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004b;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: a randomised phase II trial. Lancet Neurol. 2008;7:483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, Meltzer CC, Wisniewski SR, Mathis CA. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol. 2006;12:262–271. doi: 10.1080/13550280600873868. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Lopresti BJ, Venneti S, Price CJ, Mathis CA, Klunk WE, DeKosky ST, Mathis CA. [11C]PIB and [11C](R)-PK11195 PET imaging in Alzheimer’s disease. Arch Neurol. 2008 doi: 10.1001/archneurol.2008.511. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Claasen J, Rohl C, Sievers J, Deuschl G, Lucius R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol Dis. 2003;14:417–424. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta Neuropathol. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamoto K, Zhang MR, Kumata K, Hatori A, Okada M, Suzuki K. In vitro and ex vivo autoradiography studies on peripheral-type benzodiazepine receptor binding using [11C]AC-5216 in normal and kainic acid-lesioned rats. Neurosci Lett. 2007;428:59–63. doi: 10.1016/j.neulet.2007.09.050. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [(11)C]DAA1106. Biol Psychiatry. 2008;64(10):835–841. doi: 10.1016/j.biopsych.2008.04.021. [DOI] [PubMed] [Google Scholar]