Abstract
Background
We test the hypothesis that in older persons higher plasma levels of inflammatory markers predict the development of depressive symptoms during a 6-year follow-up.
Method
This study is part of the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study, a prospective population-based study of older persons. The sample consisted of 991 participants, ages 65 years and older. Serum levels of C-reactive protein, interleukin (IL)-1β, IL-1 receptor antagonist (ra), tumor necrosis factor-α, IL-6, IL-6 receptor, and IL-18 were measured. Depressive symptoms were assessed at baseline and at the 3- and 6-year follow-ups with the Center for Epidemiological Studies-Depression Scale (CES-D). Depressed mood was defined as CES-D > 20. Potential confounders were baseline variables related to sociodemographic, somatic health, and functional status.
Results
At baseline, IL-1ra levels were significantly higher (p =.004) in depressed compared with nondepressed participants. After adjustment for confounders, among subjects free of depression at baseline, those in the third and fourth IL-1ra quartiles compared with those in the lowest quartile had, respectively, a 2.32-fold (95% confidence interval: 1.21–4.42, p =.01) and 2.78-fold (95% confidence interval: 1.47–5.26, p =.002) higher risk of developing depressed mood during a 6-year follow-up.
Conclusions
In old age, persons with high plasma levels of IL1-ra had a higher risk of developing depressive symptoms over time. These findings suggest a potential causal role for inflammation in the development of depressive symptoms in older persons.
Keywords: Aging, cytokines, depression, inflammation
The “cytokine hypothesis of depression” (1–6) assumes that inflammatory mediators play a key role in the pathophysiology of depressive disorders. This hypothesis was initially based on clinical findings that depression is accompanied by direct and indirect evidence of upregulated inflammatory response, such as an increased production of pro-inflammatory cytokines (interleukin [IL]-1, IL-6) and an acute phase response indicated by the release of C-reactive protein (CRP) or other acute phase reactive proteins (1–3,7–10). Administration of cytokines as treatment produces symptoms such as dysphoria, fatigue, psychomotor retardation and impaired cognitive function that might be alleviated by withdrawing the cytokines administration or by antidepressant treatment (11,12). In a study (13) of healthy young men, experimental endotoxemia caused both increasing levels of inflammatory markers and depressive symptoms. In spite of this evidence, epidemiological studies have generated contradictory results, and some of them have confirmed and some of them rejected the hypothesis of a connection between inflammatory markers and depressive symptoms (14–17).
Older persons are often affected by a chronic “low-grade proinflammatory state” (18) and have a high prevalence of chronic syndromes of depression (19), and cross-sectional studies have found that high levels of inflammatory markers are associated with depression (17,20,21). However, because most of the available data on the relationship between inflammation and depression are cross-sectional, it is unclear whether cytokine abnormalities precede or follow the onset of depressive symptoms. In a recent study (22) higher production of IL-1β and IL-1 receptor antagonist (ra), determined by ex vivo whole blood stimulation with bacterial lipopolysaccharide (LPS), was identified as an independent risk factor for the development of depressive symptoms in older persons. In the present study we test the hypothesis that in older persons higher plasma levels of inflammatory markers predict the development of depressive mood during a 6-year follow-up.
Methods and Materials
Study Population
Participants were part of the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study, a prospective population-based study of older persons in Tuscany, Italy, designed to investigate factors contributing to decline in mobility function in later life. A description of the study rationale, design, and method is given elsewhere (23). Briefly, in 1998–1999 the sample was randomly selected from two sites, Greve in Chianti and Bagno a Ripoli, with a multistage stratified sampling method. Data collection included: 1) a home interview concerning demographic data, health-related behaviors, functional status, and cognitive function; 2) a medical examination including several performance-based tests of physical function conducted in the study clinic; 3) 24-hour urine collection and blood-drawing. Participants were seen again for a 3-year follow-up visit (2001–2003) and 6-year follow-up visit (2004–2006). All respondents signed an informed consent, and the Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol.
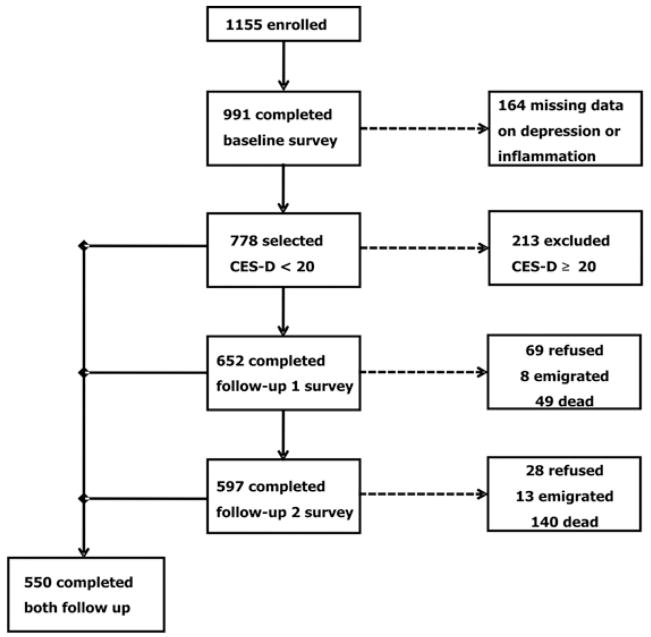
Of the 1155 participants age ≥ 65 enrolled in the study, we excluded 164 participants because of missing data on the Center for Epidemiological Studies–Depression Scale (CES-D) or inflammatory markers. In cross-sectional analyses we included all 991 remaining participants. For the longitudinal analyses we also excluded 213 participants with depressed mood at baseline. Among the remaining 778 subjects, 652 completed the first follow-up (69 refused, 8 emigrated, 49 dead), 597 the completed second follow-up (28 refused, 13 emigrated, 140 dead), and 550 completed both follow-up. The study population selection is summarized in Figure 1.
Figure 1.
Flow chart of study design. CES-D, Center for Epidemiological Studies-Depression Scale.
Depressive Symptoms
Depressive symptoms were assessed at baseline and at the 3-and 6-year follow-up with the CES-D (24). The CES-D is a 20-item self-report scale, ranging from 0 to 60. The CES-D has been shown to have good psychometrics properties in assessing depressive symptoms, in older population-based studies (25) as well as in an Italian sample (26). A score ≥ 20 was operationally defined as clinically relevant “depressed mood.” Whereas a cut-off of 16 is generally considered to represent relevant depression, we selected a cut-off of 20 that has been shown to avoid overestimation in older subjects (27).
Inflammatory Markers
Measures for the cytokines were obtained from frozen plasma samples originally collected at baseline. Morning fasting blood samples were collected after a 15-min rest. Aliquots of serum were stored at −80°C and never thawed before analysis. Serum levels of IL-6, soluble IL-6 receptor (sIL-6r) (80 kDa), IL-1β, IL-1 receptor antagonist (IL-1ra), tumor necrosis factor (TNF)-α( kits from BIOSOURCE International, Camarillo, California), and IL-18 (kits from Quantikine HS, R&D Systems, Minneapolis, Minnesota) were measured by enzyme linked immuno-absorbent assays (ELISAs). Serum CRP (high-sensitivity) was measured in duplicate with an ELISA and colorimetric competitive immuno-assay. The lowest detectable concentration was.1 pg/mL for IL-6, 8 pg/mL for sIL-6r,.09 pg/mL for TNF-α,.01 pg/mL for IL-1β, 4 pg/mL for IL1ra,.7 pg/mL for IL-18, and.03 mg/L for CRP. The inter-assay coefficient of variation was 4.5% for IL-1ra, 5% for CRP, and 7% for other inflammatory markers.
Other Variables
The following variables were also selected. Age, gender, site, education (years), smoking habit (current/former vs. non smoker), alcohol use (< 30 g/day vs. ≥ 30 g/day), Mini Mental State Examination (MMSE) score, body mass index, number of drugs, use of nonsteroidal anti-inflammatory drugs (NSAIDs) and use of antidepressant drugs coded according to Anatomical Therapeutic Chemical codes. Major chronic diseases ascertained according to previously validated algorithms (28) with information on self-reported history, pharmacological treatments, medical exam data, and hospital discharge records included: congestive heart failure, coronary heart disease including angina and myocardial infarction, stroke, chronic obstructive lung disease, hypertension, diabetes, cancer, and hip arthritis. Number of activity of daily living (ADL) and instrumental activity of daily living disabilities was defined as self-report of inability or needing personal help in performing any basic or instrumental activities of daily living (29). Level of physical activity in the previous 12 months, on the basis of response to multiple questions, was classified as sedentary/light/moderate-high. The Short Physical Performance Battery (SPPB) was used to assess lower extremity function with a standard protocol as described elsewhere (30).
Statistical Analyses
Variables were reported as percentage, means ± SD, or median and interquartile range as appropriate. Because plasma levels of inflammatory markers were non-normally distributed, log-transformed values were used in the analyses. Differences in baseline characteristics according to depressed mood were analyzed with χ2 and t test statistics as appropriate. Pearson’s correlation tests were used to evaluate correlations between inflammatory markers. To explore the functional form of the association between inflammatory markers and depressive symptoms, we divided the CES-D scores into four levels: the highest level corresponded to the depressed mood category, and the remaining CES-D scores were divided into three groups of equal size. Levels of inflammatory markers across CES-D subgroups were compared with gender/age adjusted analysis of covariance. Linear regression analyses were used to estimate regression coefficients/SD increase in (log) plasma inflammatory markers associated with baseline CES-D score after adjusting for multiple confounders. The RRs (relative risks) of developing depression at 3-year follow-up, 6-year follow-up, and at 3- or 6-year follow-up according to baseline quartiles of cytokines were calculated. Recent articles (31) pointed out that when the outcome event occurs in more than 10% of the participants it is desirable to estimate RRs directly instead of the odds ratios approximation. Therefore, we estimated RRs and confidence intervals (CIs) with the “modified Poisson” approach proposed by Zou (32). These analyses were restricted to subjects free of depression at baseline and were adjusted for confounders in parsimonious models that only included variables with a p value <.1.
All analyses were performed with the SAS statistical package, version 8.2 (SAS Institute, Cary, North Carolina) with a significance level set at p <.05.
Results
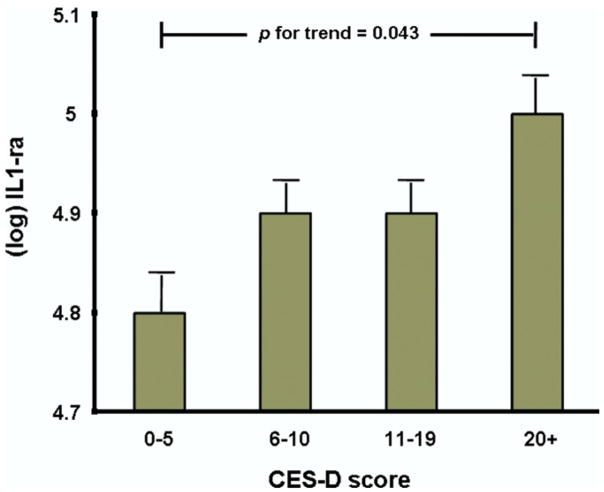
The mean age of the study sample was 75 ± 7 years, and 55.9% were women; 21.5% had depressed mood at baseline. As shown in Table 1, depressed persons were older; more often women; less likely to be smokers or heavy alcohol drinkers; took more drugs, antidepressant and NSAID; had a lower MMSE score; and were more likely to have hypertension, congestive heart failure, and hip arthritis. Furthermore persons with depressed mood were more likely to be disabled and sedentary and had lower SPPB scores. Among inflammatory markers, only levels of IL-1ra were significantly higher in depressed participants. Pearson’s correlations between inflammatory markers are shown in Table 2. Figure 2 shows age- and gender-adjusted means ± SE of (log) IL-1ra levels across different CES-D scores subgroups, calculated with analysis of covariance. Adjusted levels of IL-1ra significantly increased from 4.88 ± .04 in the CES-D < 6 group to 4.99 ± .04 in the CES-D ≥ 20 group. Adjusted regression coefficients for the association of inflammatory markers with baseline CES-D scores are shown in Table 3. Higher levels of IL-1ra were associated with higher CES-D scores.
Table 1.
Characteristics of the Study Population at Baseline
| Population at Baseline (n = 991) |
|||
|---|---|---|---|
| Not Depressed CES-D < 20 (n = 778) | Depressed CES-D ≥ 20 (n = 213) | p | |
| Age (yrs) (mean ± SD) | 74.3 ± 6.8 | 77.5 ± 7.1 | <.0001 |
| Gender, Female (%) | 50.6 | 75.1 | <.0001 |
| Site (%) | |||
| Greve in Chianti | 48.6 | 44.6 | |
| Bagno a Ripoli | 51.4 | 55.4 | .3 |
| Education (yrs) (mean ± SD) | 6.9 ± 35.8 | 5.1 ± 3.5 | .17 |
| Smoking Status (%) | 15 | 11.3 | |
| Nonsmoker | 54.4 | 73.7 | |
| Former smoker | 30.6 | 15 | |
| Current smoker | 15 | 11.3 | <.0001 |
| Alcohol Use (≥ 3 drinks/day) (%) | 17 | 8.45 | .0021 |
| BMI (mean ± SD) | 27.5 ± 4 | 27.4 ± 4.4 | .71 |
| MMSE Scores (mean ± SD) | 25.5 ± 3.2 | 24.4 ± 3.4 | <.0001 |
| Number of Drugs (mean ± SD) | 2 ± 1.9 | 3.2 ± 2.2 | <.0001 |
| Antidepressant Drug Use (%) | 2.7 | 11.3 | <.0001 |
| NSAID Use (%) | 6.8 | 12.1 | .01 |
| Hypertension (%) | 33.3 | 41.8 | .0214 |
| Angina/MI (%) | 7.5 | 8.9 | .48 |
| Stroke (%) | 6 | 7 | .59 |
| CHF (%) | 3.7 | 8 | .0089 |
| Cancer (%) | 5.9 | 7.5 | .39 |
| COPD (%) | 8.1 | 6.6 | .46 |
| Diabetes (%) | 11.1 | 9.4 | .49 |
| Hip Arthritis (%) | 4.1 | 9.86 | .001 |
| ADL Disabilities, n (mean ± SD) | .1 ±.4 | .3 ±.9 | .0002 |
| IADL Disabilities, n (mean ± SD) | .4 ± 1.3 | 1.4 ± 2.1 | <.0001 |
| SPPB Scores (mean ± SD) | 10.5 ± 2.4 | 8.0 ± 3.5 | <.0001 |
| Physical Activity (%) | |||
| Low | 13.8 | 40.9 | |
| Medium | 80.5 | 55.4 | |
| High | 5.8 | 3.8 | <.0001 |
| CRP (μg/mL) (median and IQR) | 2.8 (1.3–5.4) | 2.6 (1.4–6.6) | .09 |
| Il-1β (pg/mL) (median and IQR) | .0 (.0–.8) | .0 (.0–.7) | .7 |
| IL-1ra (pg/mL) (median and IQR) | 130.9 (94.7–180.8) | 136.8 (99.8–194.8) | .0041 |
| TNF-α (pg/mL) (median and IQR) | 2 (1.5–3.4) | 2 (1.5–2.9) | .41 |
| IL-6 (pg/mL) (median and IQR) | 1.4 (.9–2.2) | 1.4 (.8–2.2) | .82 |
| IL-6r (ng/mL) (median and IQR) | 92.7 (67.7–129.1) | 97.8 (71.7–122.2) | .71 |
| IL-18 (μg/mL) (median and IQR) | 381.8 (299.6–484.9) | 381.8 (308.8–461.5) | .91 |
Based on χ2 for dichotomous variables and independent t test for continuous variables. Variables with a skewed distribution are presented as value and interquartile range (IQR) and were rank-transformed for the analysis.
CES-D, Center for Epidemiological Studies-Depression Scale; BMI, body mass index; MMSE, Mini Mental State Examination; NSAID, nonsteroidal anti-inflammatory drugs; MI, myocardial infarction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ADL, activity of daily living; IADL, instrumental activity of daily living; SPPB, Short Physical Performance Battery; CRP, C-reactive protein; TNF, tumor necrosis factor; IL, interleukin; IL-1ra, interleukin-1 receptor antagonist; IL-6r, interleukin-6 receptor.
Table 2.
Pearson’s Correlation Between (log) Inflammatory Markers
Figure 2.
Age- and gender-adjusted levels of interleukin-1 receptor antagonist (IL-1ra) (mean ± SEM) across Center for Epidemiological Studies-Depression Scale (CES-D) score subgroups.
Table 3.
Adjusted Regression Coefficients/SD Increase in (log) Plasma Inflammatory Markers in Relation to CES-D Scores
| Baseline CES-D Scores |
||||
|---|---|---|---|---|
| Adjusted for Sociodemographic Variablesa |
Fully Adjustedb |
|||
| Standardized Regression Coefficients | p | Standardized Regression Coefficients | p | |
| CRP | .041 | .17 | .014 | .65 |
| Il-1β | .0002 | .99 | .012 | .69 |
| IL-1ra | .089 | .0027 | .064 | .0352 |
| TNF-α | −.02 | .51 | −.025 | .40 |
| IL-6 | .023 | .46 | −.007 | .83 |
| IL-6r | −.015 | .62 | −.029 | .35 |
| IL-18 | .024 | .45 | .001 | .97 |
Abbreviations as in Table 1.
Adjusted for age, gender, site, education, alcohol use, smoking status, MMSE.
Adjusted for age, gender, site, education, alcohol use, smoking status, MMSE, BMI, number of drugs, use of antidepressant, use of NSAID, hypertension, angina/MI, CHF, stroke, cancer, diabetes, COPD, hip arthritis, ADL, IADL, SPPB, and physical activity.
Subjects not-depressed at baseline (778) were selected for longitudinal analysis. Subjects who did not complete one or both follow-up had at baseline significantly higher mean levels of IL-6, CRP, TNF-α, IL-18, and IL-1ra compared with those who completed both follow-up.
We calculated the RRs for the development of depressed mood during the follow-up according to baseline quartiles of inflammatory markers. The parsimonious multivariate models shown in Table 4 were adjusted for potential confounders that were independently associated with the outcome with a p level of < .1. Among the tested inflammatory markers, only levels of IL-1ra predicted the development of depressed mood over 6 years’ follow-up. However, this effect of IL-1ra was not detectable after the first 3 years. Among the 550 subjects who completed both follow-up interviews, 22% developed depressed mood at 3 years’ or 6 years’ follow-up. Estimated RR for persons in the third and fourth IL-1ra quartiles compared with those in the lowest quartile were, respectively, 1.7 (95% CI:1.07–2.68, p =.03) and 1.7 (95% CI:1.05–2.64, p =.02) after adjustment for age, gender, hypertension, chronic obstructive pulmonary disease, diabetes, ADL disabilities, and use of antidepressant drugs and NSAIDs. Among the 597 subjects that completed the interview after 6 years, 14.2% developed depressed mood. Estimated RR for subjects in the third and fourth IL-1ra quartile compared with those in the lowest quartile were, respectively, 2.32 (95% CI: 1.21–4.42, p =.01) and 2.78 (95% CI:1.47–5.26, p =.002), after adjustment for age, gender, chronic obstructive pulmonary disease, diabetes, ADL disabilities, hip arthritis, and use of antide-pressant drugs. We repeated the analysis also with logistic regression, and the results were substantially the same.
Table 4.
Adjusted Relative Risks for Depressed Mood Associated with Quartiles of IL-1ra
| Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | RR | 95% CI | p | |
| Risk of Depressed Mood (CES-D ≥ 20) at 3-Yr Follow-Up (n = 652) | ||||||
| IL-1ra | ||||||
| Quartile 1 | 1 | 1 | ||||
| Quartile 2 | .88 | (.45–1.72) | .71 | 1.00 | (.51–1.96) | 1.00 |
| Quartile 3 | 1.18 | (.63–2.21) | .61 | 1.08 | (.58–2.03) | .80 |
| Quartile 4 | .96 | (.49–1.90) | .92 | .77 | (.38–1.59) | .49 |
| Model 1a |
Model 2c |
|||||
| Risk of Depressed Mood (CES-D ≥ 20) at 3- or 6-Yr Follow-Up (n = 550) | ||||||
| IL-1ra | ||||||
| Quartile 1 | 1 | 1 | ||||
| Quartile 2 | 1.24 | (.76–2.03) | .40 | 1.18 | (.73–1.91) | .49 |
| Quartile 3 | 1.72 | (1.08–2.74) | .0235 | 1.70 | (1.07–2.68) | .0235 |
| Quartile 4 | 1.77 | (1.10–2.83) | .0179 | 1.66 | (1.05–2.64) | .0310 |
| Model 1a |
Model 2d |
|||||
| Risk of Depressed Mood (CES-D ≥ 20) at 6-Yr Follow-Up (n = 597) | ||||||
| IL-1ra | ||||||
| Quartile 1 | 1 | 1 | ||||
| Quartile 2 | 1.72 | (.85–3.49) | .13 | 1.59 | (.78–3.22) | .20 |
| Quartile 3 | 2.34 | (1.20–4.57) | .0124 | 2.32 | (1.21–4.42) | .0108 |
| Quartile 4 | 3.12 | (1.63–5.95) | .0006 | 2.78 | (1.47–5.26) | .0017 |
Relative risks (RRs) were estimated with a modified Poisson regression approach.
CI, confidence interval; other abbreviations as in Table 1.
Adjusted for age and gender.
Adjusted for age, gender, years of education, BMI, and use of NSAID.
Adjusted for age, gender, hypertension, COPD, diabetes, ADL disabilities, use of antidepressant drugs, and use of NSAID.
Adjusted for age, gender, COPD, diabetes, ADL disabilities, hip arthritis, and use of antidepressant drugs.
We performed the same analyses with the cut-off of 16 for the CES-D, and we obtained the same results: only IL-ra predicted the development of depressed mood at 3 or 6 years’ follow-up and at 6 years’ follow-up.
Discussion
With data from a population-based study in older persons, we examined the relationship between plasma inflammatory markers and symptoms of depression. We found evidence of a cross-sectional and prospective independent association between IL-1ra and depressive symptoms assessed by CES-D. Several cross-sectional studies (1,2,10) found a significant association between depression and high serum IL-1ra. In an experimental study in healthy young men (13) positive correlations were found between IL-1ra levels and depressed mood in people with endotoxemia. In a previous prospective study of older individuals (22) elevated levels of IL-1ra preceded the onset of depressed mood. In our study, subjects in the two highest quartiles of IL-1ra at baseline, compared with those in the lowest quartile, had a 2.32- and 2.78-fold higher risk of developing depressed mood after 6 years.
Interleukin-1ra, the pure antagonist of IL-1α and IL-1β, is a reliable marker of immune system activation. There is evidence that IL-1ra is an acute phase protein (33). As a member of the IL-1 gene family, IL-1ra production increases under the same inflammatory conditions that stimulate IL-1α and IL-1β. However, although these molecules are produced locally, rapidly metabolized, and their serum concentrations are often below the detectable limits with standard methods, IL-1ra is produced by the liver in larger quantities and remains in the circulation for long time (34–36). Therefore, IL-1ra is considered a marker of inflammation even more reliable than IL-1. Regardless of the mechanism, our findings suggest that serum IL-1ra might capture aspects on inflammation that are most relevant to the development of depressive mood. If these findings are confirmed, IL1ra might someday become a valuable clinical tool for risk assessment.
Interestingly, in our study baseline plasma levels of IL-1ra were not predictive of depressed mood at 3-year follow-up. This could partly be explained by the small mean increase in CES-D scores after 3 years (2.6 points). We could hypothesize that the influence of inflammation on the development of depressive symptoms is a slow process that takes several years to cross the threshold of clinical manifestation.
Previous epidemiological studies on the association between inflammation and depression have produced discrepant results (14–17). This discrepancy between studies is probably attributable to differences in the study populations, assessments of depression, and measures of cytokines (clinical vs. population-based samples, questionnaire vs. DSM diagnosis, and choice of the inflammatory markers/technical limitations of assay). Our findings are consistent with the “cytokine hypothesis of depression” (1–6). The IL-1 network molecules could communicate with the brain directly crossing the blood brain barrier (37,38) or indirectly via the afferent projections of the vagus nerve (39). This central action might account for neurochemical and neu-roendocrine features of depressive disorders (40–44). Cytokines have been found to induce serotonin depletion by lowering the availability of tryptophan through activation of tryptophan-metabolizing enzyme (indoleamine 2,3-dioxygenase [IDO]) (42–44).
To date the exact mechanisms through which inflammation plays a role in the pathophysiology of depression are still unclear. Further research in this area is needed.
One limitation of this study is the loss of participants to follow-up. Those lost had high levels of inflammatory markers at baseline. Therefore, censoring of these participants probably led to an underestimation of the relationship between inflammation and depression. Another limitation is that depressive symptoms were evaluated by the CES-D questionnaire, and the diagnosis of depression was not confirmed by a clinical psychiatric diagnosis. However, the CES-D is a commonly used scale to measure depressive symptoms and has been widely used in older population-based studies (25,27). Moreover DSM affective disorders are not highly prevalent among elderly persons in the community, whereas subsyndromal chronic depression is more common (19). Another limitation is that the study design did not allow us to detect depressive episodes that started and remitted between subsequent follow-up visits. Furthermore, the results could have been affected by the use of antidepressant drugs; some studies (45,46) found that antidepressant agents have negative immuno-regulatory effect through stimulation of IL-10 release. However, when we adjusted the analysis for antidepressant medication use, the results did not change substantially. Finally, in our database there was no measure of cognition more selective than MMSE to test the confounding effect of cognitive decline on the association between inflammation and depression.
Despite this limitation, we believe that our findings suggest a potential causal role for inflammatory process in the onset of depressive symptoms in elderly patients. Accumulation of diseases and cardiovascular risk factor with age or dysregulation due to immunosenescence could slowly increase the “low-grade proinflammatory state” (18); this could lead over time to the development of depression that worsens the prognosis of the patient. Modulation of the inflammatory process might in the future become a strategy to reduce depressive mood in elderly patients and prevent its deleterious consequences on morbidity and mortality (47).
Acknowledgments
The InCHIANTI Study is currently supported by a Grant (Contract N 01-AG-5-0002 “InCHIANTI Follow up Study”) from the National Institute on Aging (National Institutes of Health, Bethesda, Maryland).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Maes M, Vandoolaeghe E, Ranjan R, Bosmans E, Bergmans R, Desnyder R. Increased serum interleukin-1-receptor-antagonist concentrations in major depression. J Affect Disord. 1995;36:29–36. doi: 10.1016/0165-0327(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 2.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 3.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 4.Leonard BE. Stress depression and the activation of the immune system. World J Biol Psychiatry. 2000;1:17–25. doi: 10.3109/15622970009150562. [DOI] [PubMed] [Google Scholar]
- 5.Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:767–780. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 6.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Maes M, Bosmans E, Meltzer HY, Scharpé S, Suy E. Interleukin-1 beta: A putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- 8.Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 10.Rief W, Pilger F, Ilhe D, Bosmans E, Egyed B, Maes M. Immunological differences between patients with major depression and somatization syndrome. Psychiatry Res. 2001;105:165–174. doi: 10.1016/s0165-1781(01)00338-9. [DOI] [PubMed] [Google Scholar]
- 11.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interpheron-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 12.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 14.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 15.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: Findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 18.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, et al. The natural history of late-life depression: A 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- 20.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 21.Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.van den Biggelaar AH, Gussekloo J, de Craen AJ, Frölich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM for the InCHIANTI Group. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 25.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 26.Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. Clin Psychol. 1983;39:249–251. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The WHAS: Health and Social Characteristics of Older Women with Disability. NIH Publication No. 95–4009. Bethesda, Maryland: National Institute on Aging; 1995. [Google Scholar]
- 29.Kendal FP, McCreary EK. Muscle Testing and Function. Baltimore: Williams & Wilkins; 1983. [Google Scholar]
- 30.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 31.McNutt LA, Wu C, Xue X, Hafner P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: Implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 35.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–2084. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 36.Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, Dinarello CA. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991;338:1423–1424. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- 37.Banks WA, Farr SA, Morley JE. –2003): Entry of blood-borne cytokines into the central nervous system: Effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 39.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 40.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maes M, Scharpé S, Meltzer HY, Okayli G, Bosmans E, D’Hondt P, et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: Further evidence for an immune response. Psychiatry Res. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 43.Maes M, Meltzer HY, Scharpé S, Bosmans E, Suy E, De Meester I, et al. Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 1993;49:151–165. doi: 10.1016/0165-1781(93)90102-m. [DOI] [PubMed] [Google Scholar]
- 44.Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): Relevance for depression—and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett. 2007;28:826–831. [PubMed] [Google Scholar]
- 45.Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, et al. Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuro-psychopharmacology. 1999;20:370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 46.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psycho-pharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]