Abstract
Rationale
Gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) are prodrugs for gamma-hydroxybutyrate (GHB). Like GHB, GBL and 1,4-BD are drugs of abuse, but their behavioral effects may differ from GHB under some conditions.
Objectives
The first study compared the behavioral effects of GBL (32−240 mg/kg) and 1,4-BD (32−240 mg/kg) with each other and to effects previously reported for GHB (32−420 mg/kg). A second study determined GHB pharmacokinetics following intragastric administration of GHB, GBL, and 1,4-BD.
Methods
Operant responding for food, observed behavioral effects, and a fine-motor task occurred at multiple time intervals after administration of drug or vehicle. In a separate pharmacokinetics study, blood samples were collected across multiple time points after administration of GHB, GBL, and 1,4-BD.
Results
Like GHB, GBL, and 1,4-BD impaired performance on the fine-motor task, but the onset of motor impairment differed across drugs. GBL and 1,4-BD dose dependently decreased the number of food pellets earned, but at lower doses than previously observed for GHB. Similar to GHB, both GBL and 1,4-BD produced sedation, muscle relaxation, gastrointestinal symptoms, and tremors/jerks. Administration of GBL and 1,4-BD produced higher maximum concentrations of GHB with shorter times to maximum concentrations of GHB in plasma when compared to GHB administration.
Conclusions
GBL and 1,4-BD produced behavioral effects similar to those previously reported with GHB and the time course of effects were related to blood levels of GHB. Given their higher potency and faster onset of effects, the abuse liability of GBL and 1,4-BD may be greater than GHB.
Keywords: GABA, Behavior, Drug abuse, Operant
Gamma-hydroxybutyrate (GHB) is a drug of abuse and a prescribed treatment for narcolepsy under the name Xyrem (sodium oxybate). Gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) are GHB precursors that are metabolized into GHB after ingestion, although via different pathways (Arena and Fung 1980; Barker et al. 1985; Lettieri and Fung 1978; Maxwell and Roth 1972; Roth and Giarman 1965, 1968; Snead et al. 1989). GBL is metabolized to GHB via serum lactonase. 1,4-BD is metabolized in a two-step conversion via alcohol dehydrogenase, which first converts it to gamma-hydroxybutyraldehyde and then to GHB. The behavioral effects of GBL and 1,4-BD are likely associated with their conversion to GHB (Shannon and Quang 2000).
Following the scheduling of GHB, illicit use of GHB precursors increased (Palmer 2004). GBL can be found in products including fingernail polish remover, pesticides, ink jet cleaner, and various industrial solvents, while 1,4-BD is found in hair tonics and is used in the synthesis of resins, polyurethanes, and GBL. Using instructions easily found on the Internet, GBL is often converted to GHB prior to ingestion and it has been marketed on the internet as a safe alternative to GHB (Ingels et al. 2000; Shannon and Quang 2000; Winickoff et al. 2000). Recreational users of GHB, GBL, and 1,4-BD generally report feelings of euphoria, relaxation, drowsiness, and disinhibition, although adverse effects, such as nausea, vomiting, anxiety, dizziness, agitation, respiratory depression leading to coma, and sometimes death, are also reported (Galloway et al. 2000; Miotto et al. 2001; Teter and Guthrie 2001).
It is unclear if the behavioral effects of GBL and 1,4-BD are entirely due to their conversion to GHB in vivo, and studies examining possible differences in the behavioral effects of GHB, GBL, and 1,4-BD in rodents have reported inconsistent results. Two studies examining the discriminative stimulus effects of GHB reported GBL failed to produce discriminative stimulus effects that substituted for those of GHB in rats (Carter et al. 2003; Winter 1981). Baker and colleagues (2005), however, reported that both GBL and 1,4-BD fully substituted for GHB. In addition, 1,4-BD and GHB substituted for GBL in a second group of rats trained to discriminate GBL from vehicle (Baker et al. 2005). Rodent studies also suggest that GHB, GBL, and 1,4-BD may differentially effect locomotor behavior (Davies 1978; de Fiebre et al. 2004) and the onset and duration of action of the three compounds in rodents differ (Carter et al. 2003). In addition, GBL significantly increased acetylcholine in rat striatum, hippocampus, and cortex while GHB did not (Ladinsky et al. 1983). When tested in a food-maintained operant procedure, GBL decreased response rate 90 min after administration while 1,4-BD did not suppress responding until 150 min after administration (McMahon et al. 2003). Both GHB and GBL produced physical dependence and had similar withdrawal syndromes after chronic administration in baboons (Goodwin et al. 2006; Weerts et al. 2005). A comparison of the acute behavioral effects and pharmacokinetics of GHB, GBL, and 1,4-BD has not been evaluated in nonhuman primates.
The present study sought to characterize and compare the acute behavioral effects of GBL and 1,4-BD with each other and to previously collected GHB behavioral data in baboons (Goodwin et al. 2005). Following drug administration, effects on food-maintained operant behavior, fine-motor skills, and observed behaviors were determined across multiple time points. In addition, a second study examined pharmacokinetic parameters of GHB, GBL, and 1,4-BD following intragastric administration.
Materials and methods
Subjects
Subjects were five adult male baboons (Papio anubis; primate imports, New York, NY, USA and Southwest Foundation for Biomedical Research, San Antonio, TX, USA) that weighed between 20 and 31 kg at the beginning of the study. Each baboon had a chronic indwelling intragastric (IG) catheter implanted using procedures described previously (Lukas et al. 1982). The catheter was protected by a tether/harness/vest system that permitted the baboon-free movement inside the cage.
Four of the baboons (PF, WL, HA, KH) had previously been subjects in studies examining the acute and chronic effects of GHB (Goodwin et al. 2005; Weerts et al. 2005), but had not received chronic drug for at least 2 months prior to acute administration of GBL. After participating in the present study examining the acute behavioral effects of GBL, all four baboons were subjects in a study examining the dependence potential of chronically administered GBL (Goodwin et al. 2006). Prior to the start of acute administration of 1,4-BD, baboons had not been exposed to chronically administered GBL for at least 3 months. A fifth baboon (GR) was used in the pharmacokinetics studies.
Baboons had continuous access to tap water from a drinking spout located on the front of their home cage and 20 h/day access to food pellets (1 g banana flavored, Bio-SERV, Inc., Frenchtown, NJ, USA or P.J. Noyes, Lancaster, NH, USA) as described below. Baboons also received supplemental feeding with one or two pieces of fresh produce and a multivitamin at the same time each day (noon). Every 2−3 weeks, baboons were anesthetized with ketamine HCl (preceded by atropine sulfate) in order to allow physical examinations, catheter care, weighing, and cage washing. The protocol was approved by the Johns Hopkins University Animal Care and Use Committee and followed the “Guide for the Care and Use of Laboratory Animals” (1996). Facilities were maintained in accordance with United States Department of Agriculture and American Association for Laboratory Animal Care standards.
Apparatus
Subjects were individually housed in standard stainless steel primate cages that also served as the experimental chambers. Cages were equipped with a bench that ran along a side wall and an “intelligence panel” on the rear wall that contained a Lindsley operandum, a colored stimulus light (“jewel light”), a food hopper for pellet delivery, and a speaker for delivery of a stimulus tone as described previously (Weerts et al. 1998). The overhead lights in the room were illuminated for 13 h/day (6:00 am–7:00 pm) and were dimly illuminated for the remaining 11 h/day.
Catheters were connected to an 18-gauge liquid swivel with a strain relief mount (Model SR-750B, Instech-Soloman, Plymouth Meeting, PA, USA). Approximately 500−550 ml of distilled water was slowly infused (0.3 ml/min) via a peristaltic pump (Harvard Model 1201 or 1203, Harvard Apparatus, S. Natick, MA, USA) over 24 h to maintain catheter patency.
Operant behavior sessions were controlled using IBM compatible personal computers with Med-PC software and instrumentation (Med Associates, Inc., East Fairfield, VT, USA). Observational data were collected using laptop computers with The Observer software (Noldus Information Technology, Wageningen, The Netherlands).
Drugs
GBL and 1,4-BD stock solutions were purchased from Sigma-Aldrich (St. Louise, MO, USA) in concentrations of 1.17 g/ml for 1,4-BD,and 1.12 g/ml for GBL. For drug administration, GBL and 1,4-BD grams per milliliter stock solutions were diluted with reoxidized water so that the total dose (grams) for each baboon was administered in 150 ml. Each dose of GBL, 1,4-BD, or vehicle was administered as a single bolus infusion via the IG catheter at 9:00 am (±30 min). Doses of GBL (32−240 mg/kg) and 1,4-BD (32−240 mg/kg) were selected in order to target doses comparable to those we used previously for GHB (32−320 mg/kg) and from the relevant literature based on calculations using molecule weights of the different drug forms (molar conversion) and interspecies dose conversions (Dews 1976; Mordenti and Chappell 1989). Doses (milligrams/kilogram) were progressively increased until disruption of food-maintained behavior and sedation were observed in all subjects, and then, doses were repeated in mixed order so that each dose was tested two to three times in each baboon; the mean of these data was used for analysis. Drug was never given more than once every 3 days. A dose of 240 mg/kg GBL was evaluated once in one baboon (HA) but resulted in convulsions and so was not administered to any other subjects.
GHB sodium salt was used for the pharmacokinetics studies (Sigma-Aldrich, St. Louise, MO, USA). Doses of GHB were calculated based on the salt and dissolved in reoxidized water to a final volume of 150 ml and then administered as described for GBL and 1,4-BD.
Behavioral procedures
Food-maintained operant behavior procedure
Food pellets were available for 20 h/day and contingent upon completion of a fixed number of responses on the Lindsley operandum (i.e., a fixed ratio schedule of reinforcement). Sessions began at 9:00 am immediately after drug or vehicle administration. Drug or vehicle was administered only if the number of pellets earned on the previous day was within the range of the baseline condition. The start of each session was signaled by a tone and the illumination of a jewel light above the lever. The fixed ratio (FR) value was adjusted prior to beginning the experimental conditions so that (1) the number of pellets delivered per day was stable (i.e., no increasing or decreasing trends) for at least 14 days, (2) virtually all pellets delivered per day were consumed (i.e., no more than two to three pellets were found in the pans), and (3) the number of pellets delivered per day was sufficient to maintain body weights in adult baboons of their size and activity level. Using these criteria, the FR value maintained for the study was 10 for PF, WL, and HA and 5 for KH. Food-maintained operant behavior data were collected in 2-h bins across the 20-h session. After 20 h had elapsed, all programmed stimuli were turned off and responses did not result in pellet delivery. Cage pans were inspected and the number of pellets found, if any, was recorded in the daily record.
Behavioral observation procedures
To characterize and compare behavioral effects of GBL and 1,4-BD, trained observers completed a 30-min continuous observation session using laptop computers; observations were initiated 60 min after administration of GBL or 1,4-BD. The procedures were similar to those used previously such that results from the present study could be easily compared to those of GHB (Goodwin and Weerts 2005). Briefly, a trained observer recorded the frequency and duration of all behaviors and postures (as defined in Weerts et al. 1998)in “real time”. The behaviors and postures have been used previously in our laboratory to characterize the behavioral effects of GHB (Goodwin et al. 2005, 2006; Weerts et al. 2005).
In addition, a paper and pencil checklist with an abbreviated list of behaviors was completed at 30-min intervals before and after the 30-min continuous observation sessions until 4 h postdrug administration (i.e., at 0.5, 2, 2.5, 3, 3.5, and 4 h). Checklists were then completed every hour until behaviors not typically observed during vehicle conditions (e.g., vomiting, retching, ataxia, lip droop, tremors, and jerks) were no longer detected. Baboons were observed for 5 min and observers recorded whether or not target behaviors were observed. In this way, the onset and time to recover from overt drug effects (i.e., return to vehicle control) were documented.
A total of seven people were used as observers and observers were not blind to treatment conditions. Before the study began, 30-min continuous observation sessions were conducted in which two observers recorded behavior for the same baboon at the same time on laptop computers. Each observer completed observations with each of the other observers until the concordance between observers for the frequency of all behaviors and postures that occurred, as well as agreement on nonoccurrence of each behavior and posture, for these dual observation sessions was greater than 90%. Interrater reliability was calculated using a frequency- and sequence-based method provided as part of The Observer software (Noldus Information Technology, Wageningen, The Netherlands). Reliability of 90% or greater among observers for drug-induced behavior had also previously been determined in prior drug studies (Goodwin et al. 2005, 2006; Weerts et al. 2005).
Fine-motor task
The effects of acutely administered GBL and 1,4-BD on fine-motor coordination were assessed with a 2-min food item retrieval task as described previously (Weerts et al. 1998). The food item used was based on each baboon's preference (shelled peanuts or raisins). Once preference was determined, the same food item was used throughout the study for each baboon. Briefly, one food item was placed in each of the six equally spaced cups, and the tray was placed against the front of the cage. Observers recorded the duration (seconds) to retrieve all six items, or the maximum time of 120 s, whichever occurred first. Observers also recorded the number of items retrieved/dropped and specific comments about behaviors observed during the task. Behavioral definitions were identical as described above. Prior to drug administration, baboons completed the task daily until performance was stable. On test days, the task was presented 30, 60, and 120 min after infusion of vehicle or drug.
Pharmacokinetics of GHB, GBL and 1,4-BD
Pharmacokinetic parameters of GHB, GBL, and 1,4-BD were determined separately from the above behavioral experiments. Using the same administration procedures as described above, doses of 32, 100, and 180 mg/kg of GBL and 1,4-BD and doses of 32, 100, and 320 mg/kg of GHB were administered as a single bolus infusion via the IG catheter. Doses were administered in mixed order across baboons. To allow handling and blood collection, baboons were anesthetized with ketamine (200−300 mg) and approximately 5 ml of blood was collected from a saffenous vein of each baboon using a vacutainer with a lithium-heparinized tube. Blood samples were collected at multiple time points postdrug administration (30 min and 1, 2, 4, 6, and 24 h). After the induction dose of ketamine, later time points (e.g., 2, 4, and 6 h) required very little ketamine administration (e.g., 50 mg) due to the ongoing sedation from the experimental compounds and the previous ketamine administration. After the initial analysis of the plasma data revealed peak levels of GHB 6 h after 320 mg/kg GHB administration, additional time points were added for this dose of GHB. Doses were repeated and blood was collected at 8, 10, and 12 h post-GHB dose administration. After collection, all blood samples were immediately centrifuged at 3,200 rpm for 12 min. The plasma was then drawn off and transferred to two separate polypropylene tubes and frozen for subsequent analyses. Samples were shipped on dry ice via overnight mail to Dr. Gibson's laboratory at the University of Pittsburgh and Dr. Jakobs Laboratory at VU University Medical Center, Amsterdam, where they were stored at −70 until subsequent analysis. Levels of GHB in plasma after GBL, 1,4-BD, and GHB administration were determined using isotope dilution gas chromatography–mass spectrometry (GCMS): Isotope dilution GCMS is an accepted and common practice for accurate quantification of metabolites in tissue extracts and physiological fluids (Gibson et al. 1990). The procedure includes addition of a known amount of isotopically labeled internal standard (deuterium or carbon-13, nonradioactive) prior to analytical workup. Following administration of 1,4-BD, levels of 1,4-BD in plasma were analyzed by comparable methodology using labeled 1,4-BD as internal standard. Levels of GBL in plasma after GBL administration could not be quantified.
Data analyses
Food-maintained behavioral data were summarized as the number of pellets earned for each of the ten 2-h time bins (e.g., 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 h postadministration of drug or vehicle). Performance of the fine-motor task was summarized as duration of time (seconds) to complete the task for each of the times conducted (30, 60, and 120 min postadministration of drug or vehicle). Separate repeated measures two-way (drug dose vs. time since administration) analyses of variance (ANOVA) were conducted for GBL and 1,4-BD to compare the number of food pellets earned and the duration of time to complete the fine-motor task across doses in each experiment. Significance was accepted at p<0.05. When a significant effect was found, Bonferroni multiple comparison post-hoc t-tests were used to compare each dose to vehicle. In addition, food-maintained behavior data previously collected for GHB (Goodwin et al. 2005) were reanalyzed using the bin-by-bin summary of pellets per 2 h as in the current study.
The 30-min continuous observation session data collected using a laptop computer were first summarized as total frequency scores for behaviors, and total frequency and duration scores for postures were recorded over the 30-min session. For data reduction of observed behaviors, the frequency scores for some related behaviors (e.g., vomit and retch, tremor and jerk) were added to yield a combined score. Frequency and duration scores of all observations were analyzed using separate one-way ANOVAs to compare the frequency of each behavior and the frequency and duration of each posture under drug conditions to vehicle in each experiment. Bonferroni multiple comparison post-hoc t-tests were used when significance was found.
The data collected using the paper and pencil checklists are presented in table form such that the number of subjects displaying the maximal effect for each behavior, the time to maximal effect, the duration of effects, and the time to recovery of baseline are presented for the two highest doses of GBL (100 and 180 mg/kg) and 1,4-BD (180 and 240 mg/kg).
Pharmacokinetic data were calculated using GraphPad Prism (San Diego, CA, USA) software. The elimination half-life (T1/2) and area under the concentration–time curve (AUC) of 1,4-BD in plasma after 1,4-BD administration were calculated, as was the T1/2 and AUC of GHB in plasma after administration of each of the three compounds (GHB, GBL, and 1,4-BD). The maximum concentrations (Cmax) in plasma postdrug administration and time to maximum concentration (Tmax) were also determined.
Results
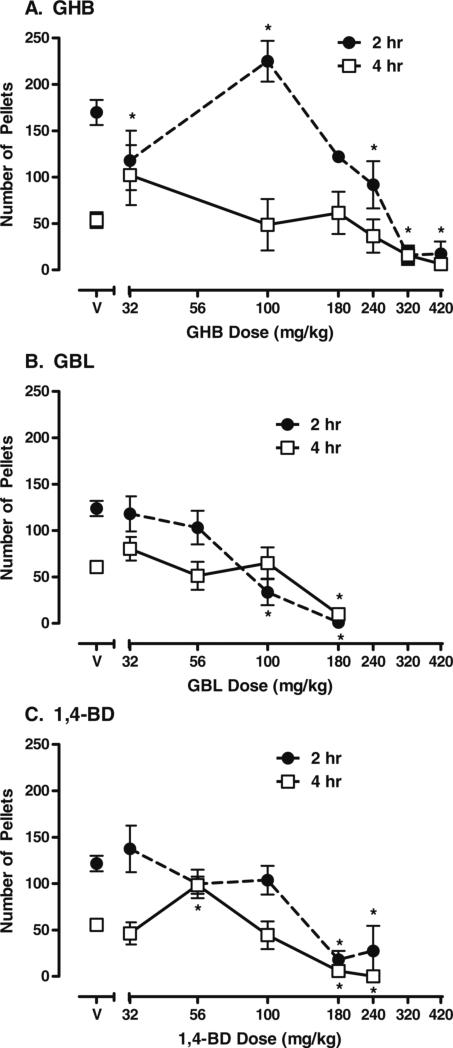
There were significant interactions between dose and time for GHB (F=3.49, p<0.0001), GBL (F=5.50, p<0.0001), and 1,4-BD (F=3.38, p<0.0001) on food-maintained behavior. These effects were dose-related and biphasic when compared to vehicle. Figure 1 shows the significant effects of GHB (a), GBL (b), and 1,4-BD (c) on food-maintained behavior. GHB data shown were reanalyzed from data from a previous study (Goodwin et al. 2005)to allow direct comparison of changes in food-maintained behavior produced by GHB, GBL, and 1,4-BD. Specifically, when compared to vehicle, 100 mg/kg GHB significantly increased food-maintained behavior during the first 2 h after drug administration (p<0.05). Higher doses (320 and 420 mg/kg) of GHB significantly decreased food-maintained behavior during the first 2 h after drug administration (p< 0.05). Food-maintained behavior was not significantly altered 4 h after GHB administration. For GBL, none of the doses increased food-maintained behavior. When compared to vehicle, food-maintained behavior was decreased by 100 and 180 mg/kg 2 h after drug administration (both p<0.001) and was also decreased 4 h after administration of 180 mg/kg GBL (p<0.001). In the one subject (HA) that received a dose of 240 mg/kg of GBL, food-maintained behavior was suppressed over the entire 20 h of food pellet availability (data not shown). For 1,4-BD, increases in food-maintained behavior were also observed, but at a lower dose (56 mg/kg) and not until 4 h after administration (p<0.05). When compared to vehicle, higher doses (180 and 240 mg/kg) of 1,4-BD decreased food-maintained behavior 2 (both p<0.001) and 4 h postadministration (both p<0.05). Thus, there were differences in the time course of effects on food-maintained behavior between the different compounds.
Fig. 1.
Food-maintained behavior after administration of a GHB, b GBL, and c 1,4-BD. Data shown are group means (±1 standard error of the mean) for the number of food pellets earned 2 and 4 h after administration of vehicle (V) or each drug dose (milligrams/kilogram). Data shown are the number of pellets earned in each 2-h period and are not cumulative across time. *p<0.05 represents significant differences between a drug dose and vehicle in pair-wise post hoc tests
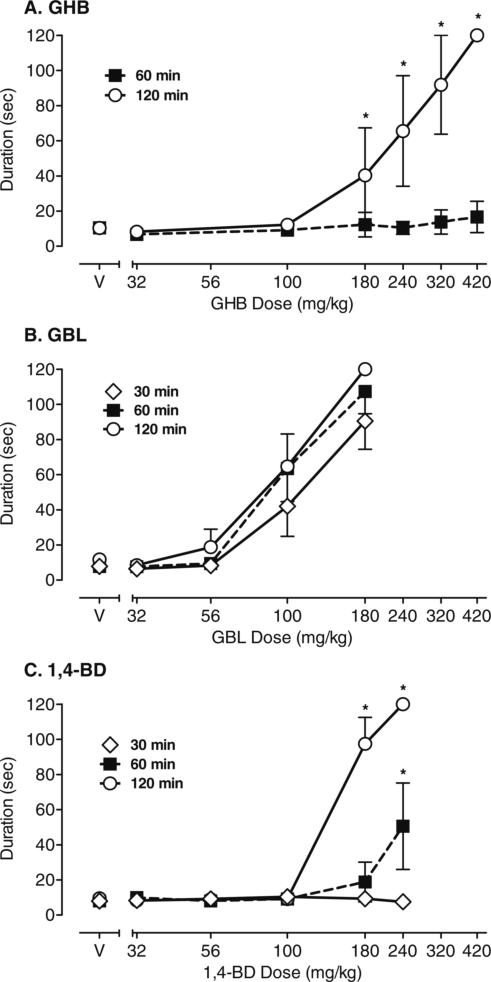
There were also differences between drugs in the onset of disruptive effects during the fine-motor coordination task, as measured by increased duration of time to complete the task. Figure 2 shows the effects of GHB, GBL, and 1,4-BD on duration (seconds) to complete the fine-motor task. Data shown for GHB (a) from a previous study (Goodwin et al. 2005) have been replotted to allow direct comparison of the effects of GHB, GBL, and 1,4-BD on fine-motor behavior. There was a significant dose × time interaction for GHB (F=9.53, p<0.0001). As shown in Fig. 2, GHB did not alter duration to complete the task at any dose tested 60 min postadministration. In contrast, 180−420 mg/kg GHB increased the duration to complete the fine-motor task at 120 min postadministration. There was a significant main effect for GBL dose (F=99.67, p<0.0001) on performance of the fine-motor task. Doses of 100 and 180 mg/kg GBL increased duration to complete the task when compared to vehicle (p<0.001); there was no interaction effect as dose-related increases in duration to complete the task were observed across all times (30, 60, and 120 min postdrug administration). In contrast, there was a significant dose × time interaction for 1,4-BD (F=27.99, p<0.0001). High doses of 1,4-BD significantly increased duration to complete the fine-motor task at 60 (180 mg/kg) and 120 min (180 and 240 mg/kg) postadministration (all p<0.001); at 240 mg/kg 1,4-BD, all subjects failed to complete the task within the maximum time of 120 s. 1,4-BD did not alter the duration to complete the fine-motor task at any dose 30-min postadministration.
Fig. 2.
Time to complete the fine-motor task following administration of a GHB, b GBL, and c 1,4-BD. Data shown are group means (±1 standard error of the mean) for duration (seconds) to complete the fine-motor task at 30, 60, and 120 min postadministration of vehicle (V) or each drug dose (milligrams/kilogram). Data for GHB are replotted from Goodwin et al. (2005), and the task was completed only at 60 and 120 min postadministration of GHB. *p<0.05 represents significant interaction between a drug dose and vehicle at the same time (30, 60, or 120) as determined in pair-wise post hoc tests
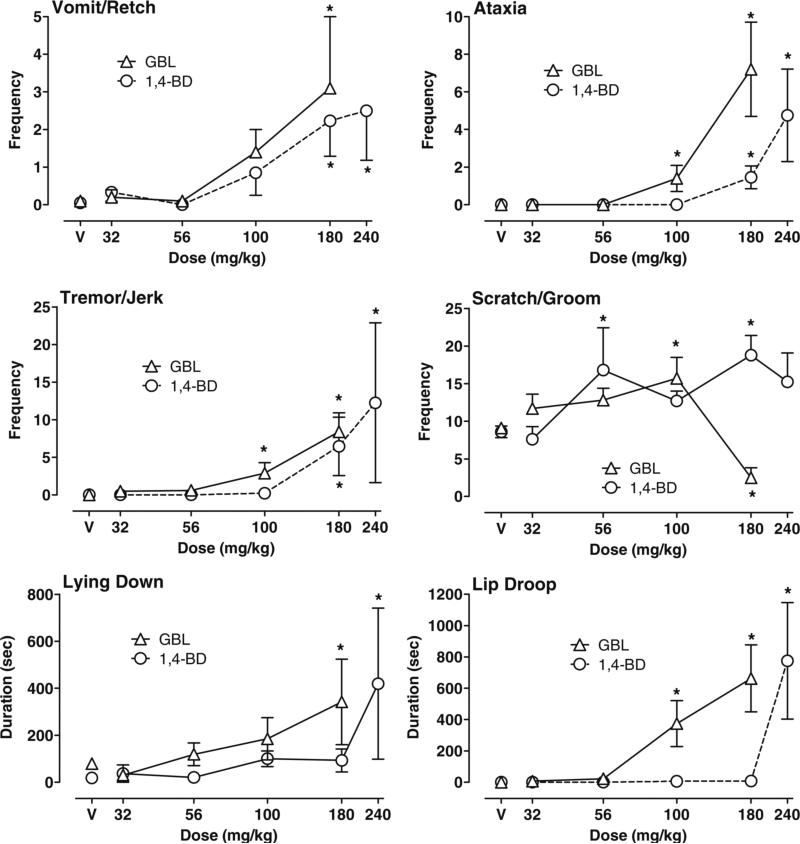
Figure 3 illustrates the significant effects of GBL and 1,4-BD on behaviors recorded via laptop computers during the 30-min continuous observation session. Due to significant differences in how behavioral observation data were collected, data from the previous acute GHB study (Goodwin et al. 2005) were not replotted for visual comparison with the GBL and 1,4-BD data. There were significant main effects of GBL dose on the frequency of scratch/groom (F=10.15, p<0.0001), vomit/retch (F=6.11, p<0.001), ataxia (F=19.76, p<0.0001), and tremor/jerks (F=18.73, p<0.0001 for GBL) and on the duration of lip droop (F=17.95, p< 0.0001 for GBL) and time spent lying down (F=3.31, p< 0.05). When compared to vehicle, 100 mg/kg GBL significantly increased ataxia (p<0.0001), tremor/jerks (p< 0.0001), lip droop (p<0.001), and scratch/groom (p<0.001). Scratch groom was then decreased at the 180 mg/kg dose of GBL (p<0.01). Increases in ataxia (p<0.0001), tremor/jerks (p<0.0001), and lip droop (p<0.001) were also significantly higher at 180 mg/kg GBL. In addition, duration of lying down (p<0.001) and vomit/retch were increased by 180 mg/kg. A high dose of 240 mg/kg GBL administered to one subject (HA) resulted in vomit/retch, ataxia, tremor/jerks, sedation/anesthesia, and seizures at 2 h postadministration (data not shown). Other behaviors recorded but not shown were not significantly changed.
Fig. 3.
Effects of GBL and 1,4-BD on behaviors recorded during the 30-min continuous observation session using a laptop computer. During the session, the frequency and duration of all behaviors that occurred were continuously recorded from 60 to 90 min after drug or vehicle (V) was administered. Data shown are group means (±1 standard error of the mean) for frequency of vomit/retch, ataxia, tremor/jerk, and scratch/groom and the duration (seconds) of lying down and lip droop. *p<0.05 represents significant difference between a drug dose and vehicle as determined by post hoc tests
As shown in Fig. 3, effects of 1,4-BD were very similar to those of GBL. There were significant main effects of 1,4-BD dose on the frequency of scratch/groom (F=5.26, p< 0.001), vomit/retch (F=7.16, p<0.001), ataxia (F=17.96, p<0.0001), tremor/jerks (F=5.79, p<0.0001), duration of lip droop (F=30.06, p<0.0001), and time spent lying down (F=6.95, p<0.0001). When compared to vehicle, 180 and 240 mg/kg 1,4-BD significantly increased frequency of vomit/retch (both p<0.01), ataxia (both p<0.0001), and tremor/jerks (both p<0.0001). The frequency of scratch/groom was also increased at 56 and 180 mg/kg 1,4-BD (p< 0.05). At 240 mg/kg 1,4-BD, duration of lip droop and time lying down were also significantly increased (all p<0.001). Other behaviors recorded but not shown were not significantly changed.
Examination of the paper and pencil behavioral checklists indicated that the onset of these behavioral effects differed for GBL and 1,4-BD. In addition, the duration of behavioral effects of GBL and 1,4-BD was dependent on the dose and behavioral measure. As shown in Table 1, time to maximal effect for ataxia and tremor/jerks was 30 min postadministration of 100 and 180 mg/kg GBL. Maximal effect for vomit/retch was observed at 1 h postadministration of 100 and 180 mg/kg GBL, and this behavior was not observed in any of the subsequent time points. In contrast, maximal effects of 100 and 180 mg/kg GBL on ataxia were observed 30 min through 1 h after administration, and ataxia continued to be observed for 4 h in 50% of the subjects administered with 180 mg/kg GBL. A return to baseline for these behaviors after 100 mg/kg GBL occurred sooner than the 180 mg/kg dose. For example, tremor/jerk was no longer present by 2 h postadministration of 100 mg/kg GBL, but was observed in 50% of the subjects 5 h postadministration of 180 mg/kg GBL. For 1,4-BD, maximal effects for ataxia, tremor/jerk, and lip droop were observed 1−2 h after administration of the 240 mg/kg dose and continued to be observed in 75% of the subjects 3−4 h postadministration. Maximal effects for lip droop occurred at 1 (240 mg/kg) and 2 h (180 mg/kg) postadministration of 1,4-BD and continued in half of the subjects at 2.5−3.5 h. Lip droop was no longer observed by 5 h postadministration of 1,4-BD. Behavioral checklists were not completed when the acute effects of GHB were studied previously (Goodwin et al. 2005).
Table 1.
Time (h) course of effects of the two highest doses (mg/kg) of GBL and 1,4 BD on drug-induced behaviors recorded with paper and pencil checklists
| Dose | Vomit | Lip droop | Ataxia | Tremor/jerk | |
|---|---|---|---|---|---|
| GBL | |||||
| Number of subjects at maximal effect | 100 | 2/4 | 3/4 | 3/4 | 1/4 |
| 180 | 4/4 | 4/4 | 4/4 | 4/4 | |
| Time to maximal effects (h) | 100 | 1.0 | 1.0 | 0.5 | 0.5 |
| 180 | 1.0 | 0.5 | 0.5 | 0.5 | |
| Duration of effects in ≥50% of subjects | 100 | 1.0 | 2.0 | 1.0 | n/a |
| 180 | 1.0 | 2.5 | 4.0 | 5.0 | |
| Recovery of baseline (100% of subjects) | 100 | 2.0 | 3.5 | 2.5 | 2.0 |
| 180 | 2.0 | 6.0 | 6.0 | 6.0 | |
| 1,4-BD | |||||
| Number of subjects at maximal effect | 180 | 3/4 | 2/4 | 4/4 | 4/4 |
| 240 | 3/4 | 3/4 | 4/4 | 4/4 | |
| Time to maximal effects (h) | 180 | 2.5 | 2.0 | 2.0 | 1.0 |
| 240 | 1.0 | 1.0 | 2.0 | 2.0 | |
| Duration of effects in ≥50% of subjects | 180 | 2.5 | 2.5 | 4.0 | 5.0 |
| 240 | 1.0 | 3.5 | 4.0 | 6.0 | |
| Recovery of baseline (100% of subjects) | 180 | 7.5 | 4.0 | 7.0 | 7.5 |
| 240 | 7.5 | 5.0 | 6.0 | 8.0 |
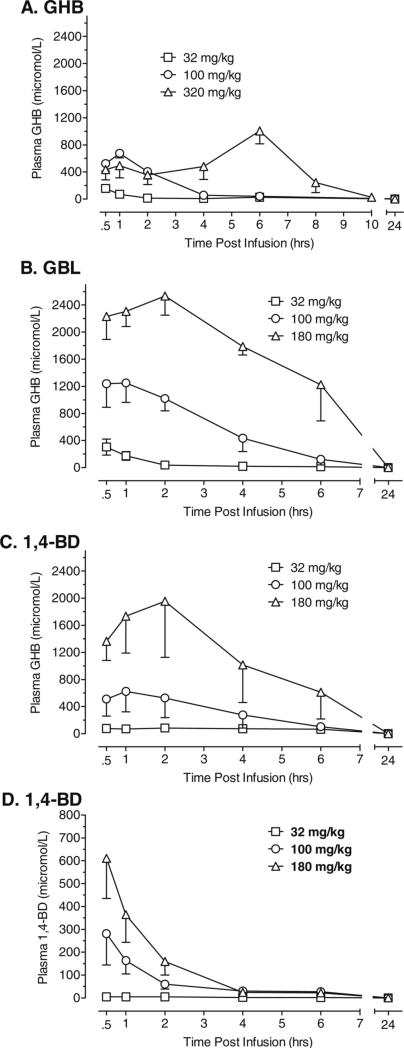
Figure 4 shows plasma levels of GHB across time following administration of GHB (a), GBL (b), and 1,4-BD (c) and plasma levels of 1,4-BD across time after administration of 1,4-BD (d). As shown in Fig. 4, GBL and 1,4-BD were metabolized to GHB in the baboon and plasma levels of GHB were increased in a dose-dependent manner for all three compounds. The time after dosing to reach maximum concentrations (Tmax) of GHB in plasma was 30 min for the lowest dose (32 mg/kg) of GHB, GBL, and 1,4-BD; 1 h for 100 mg/kg of GHB, GBL, and 1,4-BD; and 2 h for 180 mg/kg GBL and 1,4-BD. The Tmax for the highest dose of GHB (320 mg/kg), however, was 6 h. As shown in Fig. 4, the mean maximum concentration (Cmax) of GHB in plasma was higher following administration of GBL across doses when compared to the Cmax of GHB following administration of 1,4-BD or GHB. In contrast, the Tmax for plasma levels of 1,4-BD was 30 min after acute administration of 1,4-BD (see Fig. 4d) and decreased across time. Table 2 shows the pharmacokinetic parameters of GHB in plasma following administration of GHB, GBL, and 1,4-BD. As shown in Table 2, there were some differences in pharmacokinetic parameters of GHB in plasma following administration of GBL and 1,4-BD. The GHB area under the curve following GBL administration was substantially greater when compared to GHB area under the curve following GHB or 1,4-BD. There was also some variability across subjects in metabolism of GBL and 1,4-BD to GHB, as shown by the wide range of maximum concentrations of GHB in plasma that were observed. As shown in Table 3, the T1/2 of 1,4-BD in plasma was less than 1 h at the 100 and 180 mg/kg doses of 1,4-BD. The T1/2 for GHB in plasma was five- and sevenfold longer than the T1/2 of 1,4-BD for 100 and 180 mg/kg 1,4-BD, respectively.
Fig. 4.
Plasma concentrations of GHB across time following administration of GHB (a), GBL (b), and 1,4-BD (c) and plasma levels of 1,4-BD across time after administration of 1,4-BD (d). Data shown are group means (±1 standard error of the mean) for concentrations (micromoles per liter) of GHB (a–c) or 1,4-BD (d)
Table 2.
Pharmacokinetic parameters of GHB in plasma after acute intragastric doses of GHB, GBL, and 1,4-BD
| Dose (mg/kg) | GHB Cmax (μmol/l) | GHB T1/2 (h) | GHB AUC (mmol/l/h) |
|---|---|---|---|
| GHB | |||
| 32 mg/kg GHB | 89.5−269.0 | 0.4218 | 379.5 |
| 100 mg/kg GHB | 485−820 | 1.716 | 1800 |
| 320 mg/kg GHB | 598−1942 | 12.17 | 4578 |
| 32 mg/kg GBL | 15−588 | 0.5394 | 423.8 |
| 100 mg/kg GBL | 757−2160 | 2.43 | 4865 |
| 180 mg/kg GBL | 1837−3183 | 8.673 | 21884 |
| 32 mg/kg 1,4-BD | 58.5−152 | 0 | 997 |
| 100 mg/kg 1,4-BD | 85.4−1370 | 3.266 | 2958 |
| 180 mg/kg 1,4-BD | 821−4330 | 5.658 | 12708 |
Data shown are the dose and drug administered, the range of maximum concentration (Cmax) of drug detected in plasma, the mean half-life (T1/2), and AUC
Table 3.
Pharmacokinetic parameters of 1,4-BD in plasma following 1,4-BD administration
| Dose (mg/kg) | 1,4-BD Cmax (μmol/l) | 1,4-BD T1/2 (h) | 1,4-BD AUC (mmol/l/h) |
|---|---|---|---|
| 1,4-BD | |||
| 32 mg/kg 1,4-BD | 5.3−7.7 | 4.13 | 33.43 |
| 100 mg/kg 1,4-BD | 47.8−590 | 0.589 | 612.2 |
| 180 mg/kg 1,4-BD | 289−1104 | 0.7114 | 942 |
Data shown are the dose and drug administered, the range of maximum concentration (Cmax) of drug detected in plasma, the mean half-life (T1/2), and AUC
Discussion
The current data indicate the acute behavioral effects of GBL, and 1,4-BD are very similar to those of GHB. The behavioral effects of the classic sedative hypnotic compounds (e.g., benzodiazepines and barbiturates) have often been compared to GHB. Indeed, similar to classic sedative hypnotic compounds, GHB and its prodrugs increased behaviors indicative of motor impairment (ataxia), sedation (lying down), and muscle relaxation (lip droop). GBL and 1,4-BD also produced decreases in food-maintained behavior and impaired performance on the fine-motor task, which is similar to effects observed in baboons treated with benzodiazepines (Ator et al. 2000; Weerts et al. 1998). GBL and 1,4-BD, however, also produce changes that are distinctly different from benzodiazepines. For example, rather than the traditional sedation observed with benzodiazepines, higher doses of GHB actually produce a cataleptic state (Itzhak and Ali 2002; Navarro et al. 1998; Sevak et al. 2004). In the current study, both GBL and 1,4-BD produced gastrointestinal symptoms (i.e., vomit/retch) and tremors/jerks. Tremor/jerks were observed across a range of doses and a dose of 240 mg/kg of GBL given to one subject produced both seizures and catalepsy. These effects have also been observed in baboons administered with GHB (Goodwin et al. 2005) and in rhesus monkeys administered with GHB and GBL (Snead 1978a, b, c). The proconvulsant effects of GHB and its prodrugs clearly differentiate these compounds from classic sedative hypnotics.
Although GHB, GBL, and 1,4-BD produced similar behavioral effects, there were differences in the onset and time course of effects. Interestingly, the onset of motor impairment was faster for GBL when compared to 1,4-BD and GHB, and the onset of motor impairment for 1,4-BD was faster when compared to GHB. Onset of sedation, muscle relaxation, and ataxia for GBL was also faster when compared to 1,4-BD. Differences in onset of behavioral effects of GHB, GBL, and 1,4-BD in baboons in the current study are consistent with reported results in other species (Carter et al. 2003; de Fiebre et al. 2004). For example, McMahon et al. (2003) reported that GBL had a more rapid onset of action on response rate when compared to 1,4-BD in rhesus monkeys. In humans, the effects of GHB on psychomotor skills and changes in subject-reported drug strength began as early as 30 min postadministration of high doses of GHB (8 g/70 kg) but, similar to baboons, maximal drug effects of GHB were observed 1−2 h postadministration (Carter et al. 2006). In addition to differences in the onset of action, the duration of action also appears to vary somewhat between compounds. Drug-induced behavioral effects (e.g., ataxia, vomit/retch, tremor/jerk, lip droop) were observed in at least half of the subjects from 0.5 to 5 h for GBL and from 1 to 6 h for 1,4-BD. At high doses, both drugs also suppressed food-maintained behavior for 4 h after administration. The observed behavioral effects of GBL had all returned to baseline by 6 h postadministration, while return to baseline for all behavioral effects of 1,4-BD occurred 7−8 h postadministration.
The onset and duration of behavioral effects of GBL and 1,4-BD tracked concentrations of GHB in plasma. In addition, the behavioral effects of 1,4-BD do not seem to be related to blood levels of 1,4-BD detected in plasma. These findings are consistent with previous studies in rodents that have reported the behavioral effects of GBL and 1,4-BD are not related to levels of the prodrug in blood or brain, but are correlated with GHB levels (Giarman and Roth 1964; Guidotti and Ballotti 1970; Roth and Giarman 1968). Since GBL and 1,4-BD are converted to GHB via different metabolic pathways, differences in onset and time course of effects are likely related to differences in formation, elimination, and distribution in tissues (Arena and Fung 1980; Irwin 1996). In addition, GBL is reportedly less polar and so is more easily absorbed than its hydrolyzed free acid form (i.e., GHB; Irwin 1996) and is converted into GHB so rapidly that there is a greater bioavailability of GHB after administration of GBL when compared to an equivalent dose of GHB (Vree et al. 1978). Another factor that may influence onset and time course of effects is that when administered orally or intragastrically, GHB may inhibit its own absorption and disposition and/or be absorbed more slowly. The differences in Cmax, Tmax, and T1/2 for the highest dose of GHB (320 mg/kg) in the current study is suggestive of both of these possibilities.
In the current study, GBL appears to the most potent of the three compounds, followed by 1,4-BD and then GHB. Consistent behavioral disruption (i.e., changes from baseline observed both within and between subjects) in food-maintained behavior, fine-motor performance, and observed behavior generally occurred at lower doses of GBL than for 1,4-BD. When similar doses (32−180 mg/kg) were administered, GBL produced higher concentrations of GHB in plasma than did 1,4-BD. The high conversion ratio of GBL to GHB is an important factor as ingestion of lower doses of GBL results in high blood levels of GHB and greater likelihood of overdose.
A possible limitation to the generalization of these findings is the use of the N-methyl d-aspartate (NMDA) receptor antagonist ketamine to anesthetize baboons and permit handling for blood draws. It is possible that the plasma levels of GHB in plasma were altered by ketamine. It has been previously reported that the NMDA receptor antagonist dizocilpine significantly enhanced GHB-induced catalepsy in rats (Sevak et al. 2004), indicating NMDA receptor antagonists may have an additive effect on behavior when given in combination with GHB or related compounds. It is not known, however, if such effects are related to changes in GHB pharmacokinetics or some other mechanism.
The previous pharmacokinetic studies in human subjects have evaluated 25−50 mg/kg GHB. The typical therapeutic dose of GHB in humans is 37.5−112.5 mg/kg administered in two divided doses and given 2−4 h apart (Cook 2003). For comparison, the 320 mg/kg dose of GHB evaluated in the baboons would translate to an estimated 222-mg/kg acute dose in humans using interspecies dose calculations (Dews 1976; Mordenti and Chappell 1989). Thus, the doses examined in baboons for the current study included doses in the range of those used therapeutically, as well as doses above the therapeutic range. When the concentrations of drug measured in the different studies are converted to the same units of measure (micrograms/milliliter), the pharmacokinetics of low doses of GHB in baboons and humans appear to be similar. The time to maximum concentration and elimination half-life followed similar time courses for both baboons and humans. Likewise, there is considerable individual variability in the maximum concentrations of GHB in plasma. For example, the maximum concentration ranged from 30 to 103 μg/ml human subjects administered total doses of 3−4.5 g GHB (i.e., 42.8 mg/kg; Scharf et al. 1998). In other studies in which 50 mg/kg GHB was administered, the mean maximum concentrations GHB in plasma ranged from to 20 (+7 standard deviation (SD)) to 83.1 (+10.7 SD) μg/ml (Palatini et al. 1993 and Abanades et al. 2007, respectively). Similarly, the 32 and 100 mg/kg doses of GHB in baboons produced mean maximum concentrations of 18.46 (±8.66 SD) and 72.2 (±15.73 SD) μg/ml.
In humans, administration of 25 mg/kg 1,4-BD resulted in mean maximum concentrations of 3.84 μg/ml 1,4-BD (range 0.33−14.4) and 45.6 μg/ml GHB (range 22.2−85.7; Thai et al. 2006). In our study, a dose of 32 mg/kg 1,4-BD produced maximum concentrations ranging from 0.48 to 0.69 μg/ml 1,4-BD and 8.07 to 24.24 μg/ml GHB. At higher doses, however, there is a marked difference in the time to maximum concentration for GHB, GBL, and 1,4-BD. For example, the time to maximum concentration after the 320 mg/kg dose of GHB was 6 h, compared to an hour or less for the 32 and 100 mg/kg doses. Similar to human data, GHB levels in plasma were dose dependent but nonlinear in the baboon. Indeed, it has been demonstrated that both the absorption and elimination of GHB is dose dependent and nonlinear (i.e., capacity limited; Lettieri and Fung 1979; Palatini et al. 1993; Shumate and Snead 1979). Thus, although concentrations were slightly lower in the baboon, it seems reasonable to conclude that the pharmacokinetics for GHB, GBL, and 1,4-BD were similar in the baboon, as data fall within the range observed in a larger sample of human subjects. Given the higher potency of GBL and 1,4-BD when compared to GHB and that both are metabolized into GHB after ingestion, additional investigations of the pharmacokinetic and behavioral effects of high doses of GHB, GBL, and 1,4-BD are warranted.
In conclusion, the current data suggest that GBL and 1,4-BD produce behavioral effects that are similar to each other as well as to GHB, and these effects appear to be due to the conversion of the prodrugs to GHB. Given that chronic administration of GBL produced physical dependence (Goodwin et al. 2006) and that GBL and 1,4-BD were more potent with a quicker onset and a longer duration of action when compared to GHB, GBL, and 1,4-BD may have greater abuse liability than GHB.
Acknowledgments
This research was supported by the National Institutes on Drug Abuse R01 DA 14919 (E.M.W.) and NS 40270 (K.M.G.). Protocols were approved by the Johns Hopkins University Animal Care and Use Committee and followed the Guide for Care and Use of Laboratory Animals (National Academy of Sciences, 1996). Facilities were maintained in accordance with USDA and AALAC standards.
Supported by: NIH R01 DA 14919 and NS 40270.
Contributor Information
A. K. Goodwin, Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
P. R. Brown, Departments of Molecular and Comparative Pathobiology and Surgery, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
K. M. Gibson, Departments of Pediatrics, Pathology and Human Genetics, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
E. M. Weerts, Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
References
- Abanades S, Farre M, Barral D, Torrens M, Closas N, Langohr K, Pastor A, de la Torre R. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. J Clin Psychopharmacol. 2007;27(6):625–630. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69:356–358. doi: 10.1002/jps.2600690331. [DOI] [PubMed] [Google Scholar]
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Baker LE, Van Tilburg TJ, Brandt AE, Poling A. Discriminative stimulus effects of gamma-hydroxybutyrate (GHB) and its metabolic precursor, gamma-butyrolactone (GBL) in rats. Psychopharmacology (Berl) 2005;181:458–466. doi: 10.1007/s00213-005-0003-x. [DOI] [PubMed] [Google Scholar]
- Barker SA, Snead OC, Poldrugo F, Liu CC, Fish FP, Settine RL. Identification and quantitation of 1,4-butanediol in mammalian tissues: an alternative biosynthetic pathway for gamma-hydroxybutyric acid. Biochem Pharmacol. 1985;34:1849–1852. doi: 10.1016/0006-2952(85)90662-8. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABA-B receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: a comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of traizolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Cook HOM. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol. 2003;41:131–135. doi: 10.1081/clt-120019128. [DOI] [PubMed] [Google Scholar]
- Davies JA. The effect of gamma-butyrolactone on locomotor activity in the rat. Psychopharmacology (Berl) 1978;60:67–72. doi: 10.1007/BF00429181. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, de Fiebre NE, Coleman SL, Forster MJ. Comparison of the actions of gamma-butyrolactone and 1,4-butanediol in Swiss-Webster mice. Pharmacol Biochem Behav. 2004;77:705–710. doi: 10.1016/j.pbb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Dews PB. Interspecies differences in drug effects: behavioral. In: Usdin E, Forrest IS, editors. Psychotherapeutic drugs, part I. Marcel Dekker; New York: 1976. pp. 175–214. [Google Scholar]
- Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE. Abuse and therapeutic potential of gamma-hydroxybutyric acid. Alcohol. 2000;20:263–269. doi: 10.1016/s0741-8329(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Giarman NJ, Roth RH. Differential estimation of gamma-butyrolactone and gamma-hydroxybutyric acid in rat blood and brain. Science. 1964;145:583–584. doi: 10.1126/science.145.3632.583. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Aramaki S, Sweetman L, Nyhan WL, DeVivo DC, Hodson AK, Jakobs C. Stable isotope dilution analysis of 4-hydroxybutyric acid: an accurate method for quantification in physiological fluids and the prenatal diagnosis of 4-hydroxybutyric aciduria. Biomed Environ Mass Spectrom. 1990;19:89–93. doi: 10.1002/bms.1200190207. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Weerts EM. College on Problems of Drug Dependence. Orlando, FL: 2005. Comparison of the behavioral effects of gamma-butyrolactone (GBL) and gamma-hydroxybutyrate (GHB) in baboons. [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacolgy. 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM, Weerts EM. Chronic intragastric administration of gamma-butyrolactone (GBL) produces physical dependence in baboons. Psychopharmacology. 2006;189:71–82. doi: 10.1007/s00213-006-0534-9. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Ballotti PL. Relationship between pharmacological effects and blood and brain levels of gamma-butyrolactone and gamma-hydroxybutyrate. Biochem Pharmacol. 1970;19:883–894. doi: 10.1016/0006-2952(70)90251-0. [DOI] [PubMed] [Google Scholar]
- Ingels M, Rangan C, Bellezzo J, Clark RF. Coma and respiratory depression following the ingestion of GHB and its precursors: three cases. J Emerg Med. 2000;19:47–50. doi: 10.1016/s0736-4679(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Irwin RD. NTP summary report on the metabolism, disposition, and toxicity of 1,4-butanediol (CAS no 110−63−4). National Toxicology Program. Toxic Rep Ser. 1996;54:1–28. [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann N Y Acad Sci. 2002;965:451–460. doi: 10.1111/j.1749-6632.2002.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Ladinsky H, Consolo S, Zatta A, Vezzani A. Mode of action of gamma-butyrolactone on the central cholinergic system. Naunyn Schmiedebergs Arch Pharmacol. 1983;322:42–48. doi: 10.1007/BF00649351. [DOI] [PubMed] [Google Scholar]
- Lettieri JT, Fung HL. Improved pharmacological activity via pro-drug modification: comparative pharmacokinetics of sodium gamma-hydroxybutyrate and gamma-butyrolactone. Res Commun Mol Pathol Pharmacol. 1978;22:107–118. [PubMed] [Google Scholar]
- Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208:7–11. [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR, Bradford LD, Brady JV, Daley L. A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol Biochem Behav. 1982;17:823–829. doi: 10.1016/0091-3057(82)90366-5. [DOI] [PubMed] [Google Scholar]
- Maxwell R, Roth RH. Conversion of 1,4-butanediol to -hydroxybutyric acid in rat brain and in peripheral tissue. Biochem Pharmacol. 1972;21:1521–1533. doi: 10.1016/0006-2952(72)90301-2. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Coop A, France CP, Winger G, Woolverton WL. Evaluation of the reinforcing and discriminative stimulus effects of 1,4-butanediol and gamma-butyrolactone in rhesus monkeys. Eur J Pharmacol. 2003;466:113–120. doi: 10.1016/s0014-2999(03)01486-9. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am J Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokenetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokenetics and new drug development. Pergamon; New York: 1989. pp. 42–96. [Google Scholar]
- Navarro JF, Pedraza C, Martin M, Manzaneque JM, Davila G, Maldonado E. Tiapride-induced catalepsy is potentiated by gamma-hydroxybutyric acid administration. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:835–844. doi: 10.1016/s0278-5846(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, Gallimberti L, Gessa GL, Ferrara SD. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45:353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- Palmer RB. Gamma-butyrolactone and 1,4-butanediol: abused analogues of gamma-hydroxybutyrate. Toxicol Rev. 2004;23:21–31. doi: 10.2165/00139709-200423010-00003. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Preliminary report on the metabolism of g-butyrolactone and g-hydroxybutyric acid. Biochem Pharmacol. 1965;14:177–178. doi: 10.1016/0006-2952(65)90073-0. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Evidence that central nervous system depression by 1,4-butanediol is mediated through a metabolite, gammahydroxybutyrate. Biochem Pharmacol. 1968;17:735–739. doi: 10.1016/0006-2952(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Lai AA, Branigan B, Stover R, Berkowitz DB. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep. 1998;21:507–514. doi: 10.1093/sleep/21.5.507. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, France CP, Koek W. Neuroleptic-like effects of ghydroxybutyrate: interactions with haloperidol and dizocilpine. Eur J Pharmacol. 2004;483:289–293. doi: 10.1016/j.ejphar.2003.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M, Quang LS. Gamma-hydroxybutyrate, gamma-butyrolactone, and 1,4-butanediol: a case report and review of the literature. Pediatr Emerg Care. 2000;16:435–440. doi: 10.1097/00006565-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Shumate JS, Snead OC., 3rd Plasma and central nervous system kinetics of gamma-hydroxybutyrate. Res Commun Chem Pathol Pharmacol. 1979;25:241–256. [PubMed] [Google Scholar]
- Snead OC. Gamma hydroxybutyrate in the monkey. I. Electroencephalographic, behavioral, and pharmacokinetic studies. Neurology. 1978a;28:636–642. doi: 10.1212/wnl.28.7.636. [DOI] [PubMed] [Google Scholar]
- Snead OC. Gamma hydroxybutyrate in the monkey. II. Effect of chronic oral anticonvulsant drugs. Neurology. 1978b;28:643–648. doi: 10.1212/wnl.28.7.643. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd Gamma hydroxybutyrate in the monkey. III. Effect of intravenous anticonvulsant drugs. Neurology. 1978c;28:1173–1178. doi: 10.1212/wnl.28.11.1173. [DOI] [PubMed] [Google Scholar]
- Snead OC, Furner R, Liu CC. In vivo conversion of gamma-aminobutyric acid and 1,4-butanediol to gamma-hydroxybutyric acid in rat brain. Studies using stable isotopes. Biochem Pharmacol. 1989;38:4375–4380. doi: 10.1016/0006-2952(89)90645-x. [DOI] [PubMed] [Google Scholar]
- Teter CJ, Guthrie SK. A comprehensive review of MDMA and GHB: two common club drugs. Pharmacotherapy. 2001;21:1486–1513. doi: 10.1592/phco.21.20.1486.34472. [DOI] [PubMed] [Google Scholar]
- Thai D, Dyer JE, Jacob P, Haller CA. Clinical pharmacology of 1,4-butanediol and gamma-hydroxybutyrate after oral 1,4-butanediol administration to healthy volunteers. Clin Pharmacol Ther. 2006;81:178–184. doi: 10.1038/sj.clpt.6100037. [DOI] [PubMed] [Google Scholar]
- Vree TB, van Dalen R, vander Kleijn E, Gimbrere JSF. Pharmacokinetics of 1,4-butanediol and 4-hydroxybutyric acid in man, rhesus monkey, and dog. Anasthesiol Intensivmed Notfallmed Schmerzther. 1978;110:66–73. [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther. 1998;285:41–53. [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: deadly new internet drugs. Pediatrics. 2000;106:829–830. doi: 10.1542/peds.106.4.829. [DOI] [PubMed] [Google Scholar]
- Winter JC. The stimulus properties of gamma-hydroxybutyrate. Psychopharmacology. 1981;73:372–375. doi: 10.1007/BF00426468. [DOI] [PubMed] [Google Scholar]