Abstract
Although the introduction of highly active antiretroviral therapy (HAART) has led to a strong reduction of HIV-associated dementia (HAD) incidence, the prevalence of minor HIV-1 associated neurocognitive disorder (HAND) is rising among AIDS patients. HAART medication has shifted neuropathology from a subacute encephalitic condition to a subtle neurodegenerative process involving synaptic and dendritic degeneration, particularly of hippocampal neurons that are spared prior to HAART medication. Considerable neuroinflammation coupled with mononuclear phagocyte activation is present in HAART-medicated brains, particularly in the hippocampus. Accumulating evidence suggests that the resultant elevated secretion of pro-inflammatory cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) can increase amyloid-β peptide (Aβ) generation and reduce Aβ clearance. Recent advancements in Alzheimer’s disease (AD) research identified Aβ biogenesis and clearance venues that are potentially influenced by HIV viral infection, providing new insights into beta-amyloidosis segregation in HIV patients. Our study suggests enhanced beta-amyloidosis in ART-treated HAD and HIVE brains, and suppression of Aβ clearance by viral infection of human primary macrophages. A growing awareness of potential convergent mechanisms leading to neurodegeneration shared by HIV and Aβ points to a significant chance of comorbidity of AD and HAND in senile HIV patients, which calls for a need of basic studies.
Keywords: Alzheimer’s disease, amyloid-β peptide, cytokines, Human immunodeficiency virus (HIV), HIV-associated neurocognitive disorders (HAND), macrophage, microglia, microtubule-associated protein tau, neurodegeneration, neurofibrillary tangle, neurotoxicity
Introduction
Alzheimer’s disease (AD), characterized by progressive cognitive decline and disability, is the most common form of senile dementia (Selkoe 2001b). To date there is still no curative treatment for AD. A similar troubling situation applies to HIV-1-associated neurocognitive disorders (HAND), the neurological complication of viral infection. With the widespread use of highly active antiretroviral therapy (HAART), HIV infected patients’ life spans have been prolonged (Besson et al. 2001). This longer lifespan coupled with the adverse effects of HAART and HIV-1 viral neurovirulence will lead to an expanding population being ravaged by both HAND and AD. This review addresses how the reciprocal influence of HIV neuroinvasion and beta-amyloidosis might accelerate neurodegeneration.
HAART medication -associated complications
Since the implementation of HAART, the incidence of acquired immunodeficiency syndrome (AIDS)-defining illnesses like opportunistic infections and central nervous system (CNS) neoplasms has decreased, leading to a significant improvement in the survival of HIV-infected patients (Hogg et al. 1997; Besson et al. 2001). This means more HIV patients will live to an age where AD and cardiovascular system complications are common. Furthermore, patients are inflicted with several adverse effects associated with HAART medication, which predispose them to AD development. For instance, immune reconstitution syndrome, an autoimmune condition that occurs when reconstituted T cell populations attack opportunistic pathogens that have proliferated while the T cells were under siege from HIV, produces connective tissue disease symptoms or vasculitis (Stoll and Schmidt 2003; 2004; Gray et al. 2005; Kumarasamy et al. 2008), lypodystrophic and metabolic effects causing hyperlipidemia, alterations in body fat distribution to metabolically inactive areas, diabetes and coronary artery disease, which are all known AD risk factors (Heath et al. 2001; Heath et al. 2002; Newman et al. 2005; Guallar et al. 2008). Other complications reported in HAART-medicated patients include chemotherapy disability, osteopenia/osteoporosis (Lima et al. 2007), severe demyelination (Langford et al. 2002; Gray et al. 2005; Vendrely et al. 2005) non-AIDS – defining malignancies such as leukemia (Pantanowitz et al. 2006) and depression (Berger-Greenstein et al. 2007).
HAND
HAND is a collective term used to denote the neurological complications of AIDS, which are typically subcortical, consisting of the triad of cognitive, behavior, and motor dysfunction (Ances and Ellis 2007). HAND manifests as HIV-associated minor cognitive/motor disorder (MCMD), a milder form, and HIV-associated dementia (HAD), the most devastating form (Letendre et al. 2008 380). HIV penetrates into the CNS early after peripheral infection of circulating T cells and monocytes (Koenig et al. 1986; Davis et al. 1992; An et al. 1999). The process for HIV entry into the CNS revolves around products secreted from immune-activated and virus-infected perivascular macrophage and microglia that affect blood-brain barrier (BBB) function, expression of cell adhesion molecules and chemokines, and lead to a disruption of brain microvessel integrity. To date, the four possible mechanisms that are supportive of viral entry into the CNS and are currently under investigation include: the surreptitious transmission of virus in infected macrophages (the Trojan horse model), direct infection of the BBB by HIV; transcytosis of HIV; and BBB disruption (Buescher et al. 2007). This neuroinvasion in turn elicits a series of neuroinflammatory responses, resulting in neurologic dysfunctions in a significant number of individuals with AIDS. The neuropathological correlates are collectively termed HIV associated encephalitis (HIVE), which are characterized by BBB disruption, leukocyte infiltration into the CNS, formation of microglia nodules and multinucleated giant cells, astrocyte activation and eventual damage and/or loss of neurons (Gendelman et al. 1997; Kramer-Hammerle et al. 2005; Buckner et al. 2006). To date the mechanisms leading to dementia in AIDS patients are not fully understood; however, it is thought that activated macrophage, microglia, and astrocytes produce chemokines and cytokines that in conjunction with secreted viral proteins damage the neuronal synaptodendritic arbor, resulting in loss of synaptic integrity and function, and eventual neuronal demise (Giulian et al. 1990; Gonzalez-Scarano and Martin-Garcia 2005; Bellizzi et al. 2006; Rumbaugh and Nath 2006).
In the era of HAART medication, HIVE may have shifted from a subcortical pathology towards a cortical pattern (Brew 2004), from a subacute rapidly progressive condition to a more subtle neurodegenerative process involving synapses, dendrites, and neuronal populations usually not affected by acute HIVE. Variants of HIV neuropathology in HAART-medicated patients that have been reported include severe white matter injury, extensive perivascular lymphocytic infiltration and Aβ accumulation of AD-like lesions (Gray et al. 2003; Green et al. 2005). Recent studies suggest that HIVE prevalence is actually rising as the number of treated subjects with chronic HIV infection increases, despite HAART medication (McArthur 2004; Everall et al. 2005). Consequently, considering the link between chronic neuroinflammation, neuronal/synaptodenritic degeneration and AD, the HAART-associated side effects and HAART-induced neuropathology are significant risks factors for AD development.
Enhanced amyloidosis in HAND brains
Amyloidosis composed of serum amyloid-A protein or lambda light chains has been reported in clinical HIV- seropositive patients in multiple sites of the body, including the kidney, and gastrointestinal tract (Chinnakotla et al. 2001; Chan-Tack et al. 2006; Miranda et al. 2007; Newey et al. 2007). HIV induces deposition of the same amyloid-β peptide (Aβ) found in AD (Finch and Morgan 2007), and Aβ immunoreactivity has been found predominantly in the neuronal soma, dystrophic axonal processes, and extracellular space (often as perivascular plaques) (Esiri et al. 1998; Izycka-Swieszewska et al. 2000; Green et al. 2005). In contrast, Gelman et al (Gelman and Schuenke 2004) reported that there was no statistically significant difference in Aβ plaques counts between AIDS patients and control subjects; instead, there were increased accumulations of ubiquitinated protein and decreased synaptic proteins in HIV-infected patients compared with HIV-seronegative subjects, suggesting HIV infection-mediated neuroinflammation might perturb synaptic protein turnover through the proteasome. The failure for them to see difference in Aβ plaques counts might be attributable to differences in experimental design. Firstly, all examined brain samples were restricted to patients who did not have a successful history of viral suppression by HAART, while Green et al have shown that HAART-medicated brains have significantly higher percentage of Aβ plaques as compared to pre-HAART patients (Green et al. 2005); secondarily, the majority (15 out of 25) of the patients was below 60 years of age from patients and sample size for each stratified group was small. Therefore, a more comprehensive study is needed to resolve this inconsistency.
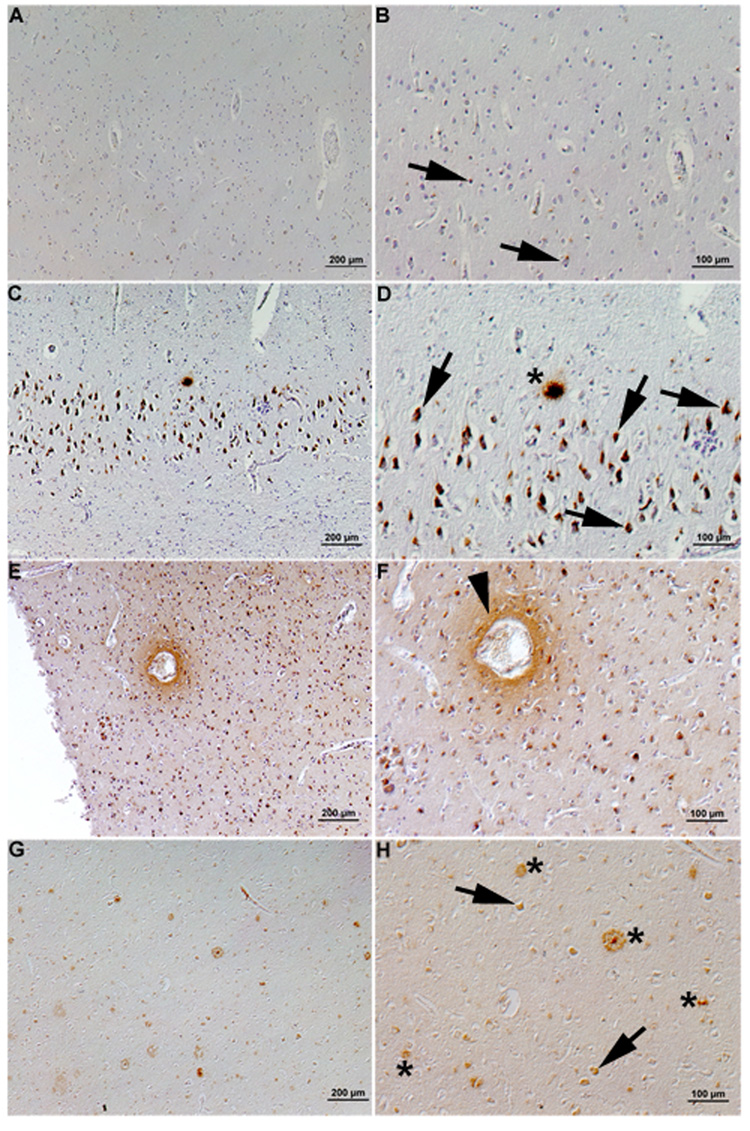
Our neuropathological examination of autopsy brains of HIVE and HIV-seronegative cases revealed similar findings. Although intraneuronal Aβ immunoreactivity is also seen in aged control brains (Figure 1, A and B), it is significantly increased in ART-treated HIVE brains (Figure 1, C and D). Of note is that Aβ plaques are also observed in HIVE brains, in which perivascular diffuse Aβ immunoreactivity is also observed (Figure 1, E and F). Extracellular Aβ deposition was also found in ART-treated HAD brains but ART-untreated HAD show only intraneuronal Aβ accumulation (data not shown). The prevalence of this intraneuronal Aβ staining is about 30–40%, and extracellular Aβ is present in 4–13% of HIV-infected brains, with a significantly higher percentage of extracelluar Aβ present in HAART-treated patients (Green et al. 2005). Accumulation of amyloid precursor protein (APP) with globular and/or bundled structures has been reported in HIVE brains, suggesting widespread axonal injury (Giometto et al. 1997). The observation of enhanced beta-amyloidosis in HAART-treated HAND brain is intriguing, but the underpinning molecular mechanisms are unclear. One potential mechanism is the effect of HIV protease inhibitor, which is a standard component of ART protocol, on Aβ degradation. Indeed, nelfinavior, a classic HIV protease, can inhibit activity of insulin degrading enzyme, a known Aβ degrading enzyme (Hamel et al. 2006). Thus, choric treatment of HIV patients with HIV protease inhibitor in HAART or ART protocol may accelerate the onset of extracellular Aβ deposition, and other ART drug should also be tested for their effect on Aβ clearance and production. According to the prevalent Aβ theory this might be multifactorial, involving a number of pathways whose consequences ultimately are centered on Aβ biogenesis and its clearance. Several significant potential contributors will be discussed below in light of recent findings from our group and others.
Figure 1. Enhanced β-amyloid depositions in HIVE brains.
Anti-Aβ polyclonal antibody immunostaining of HIV-seronegative (A–B), HIVE (C–F) and AD (G–H) brains. Aβ–immunoreactive plaques were found in HIVE brains (asterisk, D), resembling those found in AD brain (asterisks, H). Intracellular Aβ immunoreactivity was observed in HIV-seronegative, HIVE and AD brains (arrows, B, D and H). Perivascular amyloid deposition was evident in HIVE brains (arrowhead, F). Original magnification, panels A, C, E, and G ×200, B, D, F and H ×400)
Tauopathy-related pathology in HAND brains
Microtubule-associated protein tau is one of major cytoskeletal molecules in neuronal axons, and hyperphosphorylated tau (pTau) is a major component of neurofibrillary tangles (NFT), the other pathological hallmark of AD. The amyloid cascade hypothesis proposes that Aβ pathology precedes and induces tau pathology (Selkoe 2001b). The accelerated Aβ amyloidosis in HIV-infected brains may explain theaberrations of tau or pTau levels in HIV-infected patients, since Aβ deposition precede and accelerate NFT formation in AD brains and animal models of AD, respectively. Cerebrospinal fluid (CSF) tau protein concentration was significantly higher in patients with HAD compared with neuro-asymptomatic HIV-1 cases and HIV-negative controls (Andersson et al. 1999). Compared with age-matched controls, pTau concentrations were also increased in HAD brains (Brew et al. 2005; Anthony et al. 2006). The highest levels of pTau are noted in HAART-treated subjects. However, there is also a conflicting report that CSF tau is not elevated in HAD subjects (Ellis et al. 1998; Green et al. 2005). This discrepancy might arise from differences in sampling size or severity of disease progression.
In accord, tau aggregation and NFT is neuropathologically more correlated with the progression of HAD, with the greatest levels of pTau being noticed in HAART-medicated patients (Anthony et al. 2006). However, within the age groups studied, these significant neuropathological changes remained subclinical and were not yet associated with cognitive impairment (Anthony et al. 2006). The molecular mechanism of tau phosphorylation and NFT formation in HIV-infected patients is poorly investigated. It might be secondary to altered amyloidosis process such as neuronal stimulation by viral proteins or pro-inflammatory cytokines both of which are produced by microglia. Indeed, microglial activation is correlated with pTau levels (Tan et al. 1999), and activated microglia induce pTau in neurons in IL-1 receptor antagonist (IL-1ra) and anti-IL-1β antibody-sensitive manner (Sheng et al. 2000; Sheng et al. 2001; Li et al. 2003). Thus, activated microglia may contribute to neurofibrillary pathology through IL-1 production in HIV brain.
Aβ biogenesis and clearance balance
Aβ production pathway
The prevalent Aβ theory hypothesizes that the primary influence driving AD pathogenesis is the accumulation of Aβ in the brain, which is proposed to result from an imbalance between Aβ production and Aβ clearance (Hardy and Higgins 1992; hardy and Selkoe 2002). APP, which is a ubiquitously expressed type I integral membrane, can be successively cleaved by proteases known as β- and γ-secretases in the late endosomes to liberate heterogeneous Aβ species (39–43 amino acid, predominantly Aβ40 and in a lesser amount Aβ42) into the extracellular matrix in a function referred to as amyloidogenic processing. Alternatively, APP may be cleaved within the Aβ domain by β-secretases precluding the formation of Aβ (non-amyloidogenic processing) which leads to the shedding of a large soluble N-terminal fragment of APP (sAPPβ) into the extracellular or intra-luminal space (Checler 1995; Carey et al. 2005; Newman et al. 2007). In addition, Aβ can be generated in the macroautophagy pathway, which is activated in early AD mouse models due to the impaired maturation of autophagic vacuoles (AVs) to lysosomes (Yu et al. 2005).
Aβ clearance pathway
Accumulating evidence underscores the notion that faulty clearance or enzyme-mediated Aβ degradation may account for Aβ accumulation as well, in particular for late-onset AD cases (Zlokovic et al. 2000; Selkoe 2001a; Tanzi 2005; Holtzman and Zlokovic 2007). It is known that intact soluble Aβ may be cleared from the brain via several routes: transport across vessel walls into the circulation mediated by blood vessel wall-expressed low-density lipoprotein receptor-related protein-1 (LRP-1) (Shibata et al. 2000; Lam et al. 2001), clearance from brain via binding of Aβ to soluble LRP-1 circulating in blood (acting as an endogenous peripheral 'sink') (Sagare et al. 2007), transport of Aβ across the blood-brain barrier from the abluminal to the luminal side via the P-glycoprotein (PgP/MDR1/ABCB1) efflux pump (Lam et al. 2001), and drainage along perivascular basement membranes, possibly into CSF (Weller 1998). It has been shown that the receptor for advanced glycation end products (RAGE) is a primary transporter of Aβ across the BBB into the brain from systemic circulation (Donahue et al. 2006). Aβ also undergoes enzymatic degradation. Multiple enzymes within the CNS are capable of degrading Aβ, most of which are produced by neurons or glia. Some of these enzymes are produced in the cerebral vasculature, where reduced Aβ-degrading activity may contribute to the development of cerebral amyloid angiopathy (CAA) (Miners et al. 2008). Two major peptidases that have been studied intensively and hold great therapeutic promise are neprilysin and insulin-degrading enzyme (IDE, also called insulysin) (Iwata et al. 2001; Farris et al. 2003). The levels of both of these enzymes are reduced in AD, although the correlation with enzyme activity is still not entirely clear, and over-expression of NEP and IDE has been shown to reduce Aβ levels in different systems ranging from cultured neurons, drosophila, and AD mouse models (Iwata et al. 2001; Farris et al. 2003; Leissring et al. 2003; Hemming et al. 2007; El-Amouri et al. 2008).
Overproduction of amyloidogenesis-promoting cytokines
Within the CNS, the major cell types infected by invading HIV are of macrophage/microglia lineage, and astrocytic infection, though to a much lesser extent. These cells can also be activated by released cytokines and shed viral proteins such as gp120, resulting in up-regulation of cytokines, chemokines, and endothelial adhesion molecules (Gartner 2000; Kaul et al. 2001; Kaul et al. 2005), among which the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) play an important role in the induction of neuronal injury and death or neuroprotection (Brabers and Nottet 2006). In the brain, TNF-α is synthesized predominantly in microglia as a 26-KDa membrane-bound polypeptide precursor that is cleaved to be a bioactive 17-KDa subunit by TNF-α converting enzyme (TACE). The biological actions of released TNF-α are mediated through two distinct cell surface receptors, TNFR1 (p55) and TNFR2 (p75) which are constitutively expressed in neurons, glial cells and blood vessels, to which TNF-α exhibits fairly equal affinity, though each mediates distinct cellular responses (Hsu et al. 1996; Quintana et al. 2005). Through the action of these two receptors, TNF-α has a broad range of actions on neurons and glial cells, which may be either neuroprotective or neurotoxic, as reviewed elsewhere (Vitkovic et al. 2000; Pickering et al. 2005). Potently elevated levels of TNF-α have been associated with the pathological effects in a variety of conditions including AD and HIVE, in addition, expression levels of both TNF-α receptors are increased in the brains of patients with AIDS compared with normal control brains (Sippy et al. 1995).
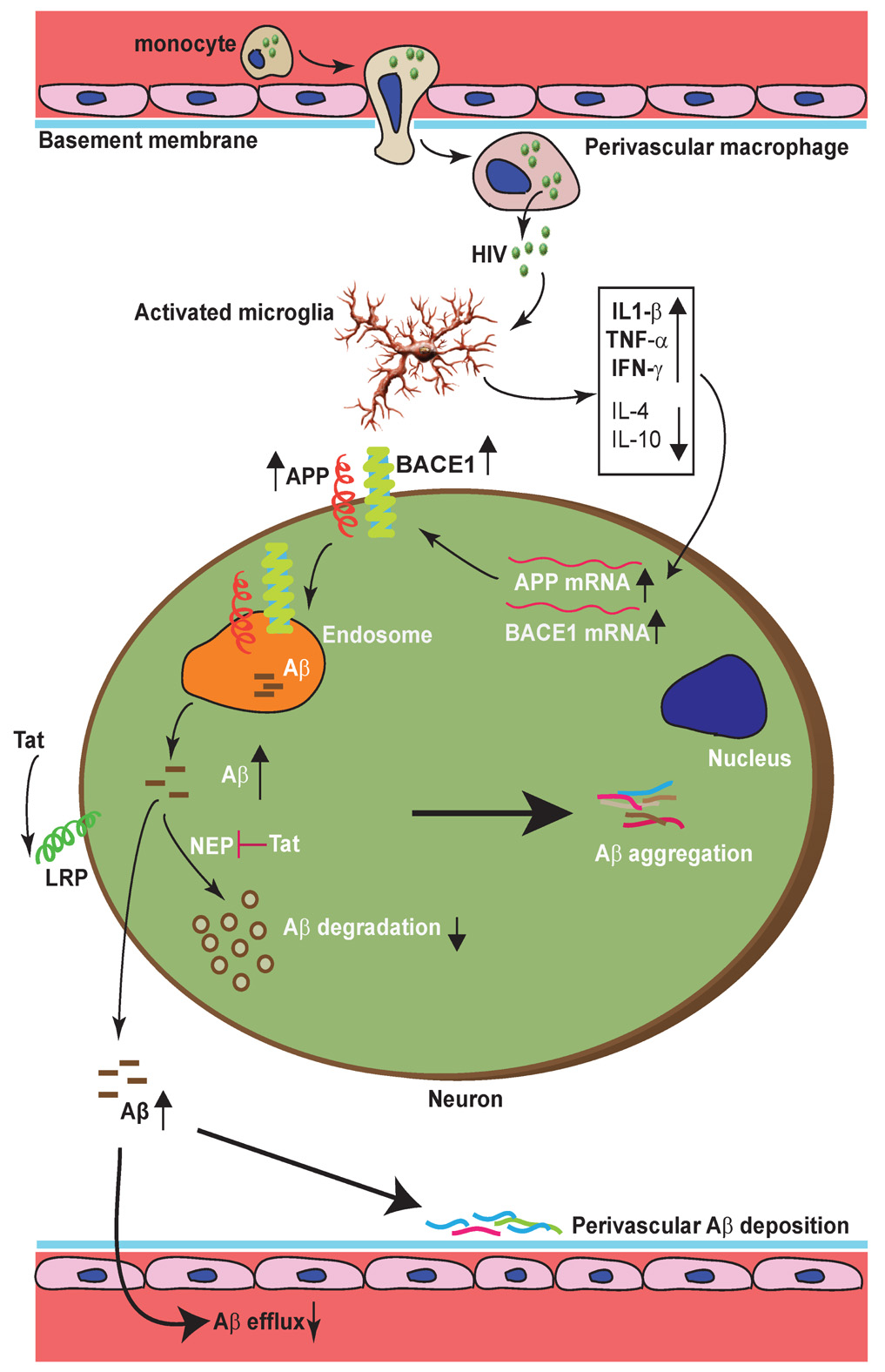
High levels of TNF-α expressed in microglia infiltrating with amyloid plaques is featured in AD brains and mouse models (Meda et al. 1995; Patel et al. 2005; Yamamoto et al. 2007a; Frank et al. 2008), and have pleiotrophic contributions to neuroinflammation-mediated cell death, synaptic transmission and synaptic plasticity (scaling) abnormalities implicated in AD (Albensi and Mattson 2000; Beattie et al. 2002; Volterra and Steinhauser 2004; Pickering et al. 2005; Stellwagen and Malenka 2006; Pickering and O'Connor 2007). TNF-α is not only contributing to neurodegeneration, but it is also directly involved in Aβ generation and degradation. Work from our lab and other groups has demonstrated that TNF-α directly stimulates astrocytic BACE1 expression to enhance β-site processing of APP, and suppresses Aβ degradation in microglia (Blasko et al. 2000; Rossner et al. 2001; Hartlage-Rubsamen et al. 2003; Yamamoto et al. 2007a). This result is bolstered by a genetic manipulation study in which genetic deletion of TNFR1 gene in APP23 transgenic mice inhibits Aβ generation and plaque formation in the brain via reduction of BACE1 levels and activity (He et al. 2007). Apart from this, TNF-α can stimulate β-secretase activity to increase Aβ production (Liao et al. 2004). Interferon-γ and IL-1β, whose expression levels are elevated in HIVE, act synergistically to increase astrocytic BACE1 levels and activity (Blasko et al. 2000; Hong et al. 2003; Liao et al. 2004; Cho et al. 2007; Yamamoto et al. 2007a). Glutamate neurotoxicity is implicated as a mediator of neuronal degeneration in HIVE, which is triggered primarily by massive Ca2+ influx arising from over-stimulation of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors (Erdmann et al. 2007; Tian et al. 2008). Interestingly, it is reported that sub-lethal NMDA receptor activation can inhibit the α-secretase candidate TACE to increase neuronal expression of Kunitz protease inhibitor domain - containing APP (KPI-APP) to promote neuronal Aβ production (Lesne et al. 2005). Consequently, the dramatic surge of above cytokines in HIVE, together with other factors like free radicals that also can up-regulate BACE1 expression (Tamagno et al. 2002; Tamagno et al. 2005; Sastre et al. 2006), have a significant chance to promote Aβ generation and subsequent accumulation in HIVE brains, as schematized in Figure 2.
Figure 2. Proposed mechanisms leading to enhanced intraneuronal and perivascular β-amyloid depositions in HIVE brains.
Activated microglia secreted elevated levels of proinflammatory cytokines (IL1-β, TNF-α, IFN-γ) that can up-regulate BACE1 and APP expression in neurons, resulting in increased Aβ generation. HIV viral protein Tat can be up-taken via LRP into neurons where it can inhibit Neprilysin activity, blocking Aβ degradation. The reduced secretion of anti-inflammatory cytokines (IL-4 and IL-10) also contributes to impaired Aβ degradation. Accelerated perivascular Aβ deposition is a consequence of impaired efflux of Aβ into plasma, and/or inhibition of perivascular Aβ degrading enzymes by viral proteins.
The cytokine IL-1β is a powerful driving force for leukocyte recruitment to the CNS (Gibson et al. 2004). It can override the intrinsic resistance of the CNS to leukocyte infiltration, resulting in acute cellular recruitment to the brain parenchyma (Depino et al. 2005; Shaftel et al. 2007a). It is a key mediator of inflammation and neuronal death in acute CNS injuries, such as stroke and brain trauma (Allan et al. 2005), and has been implicated in chronic neurodegenerative diseases such as AD and HAD (Griffin et al. 1989; Royston et al. 1992; Corasaniti et al. 2001; Wyss-Coray 2006). An increase of IL-1β concentration in the brain leads to deficits in cognitive and synaptic function typified by an attenuation in hippocampal-dependent learning and memory (Gibertini et al. 1995; Pugh et al. 2000; Shaw et al. 2001; Song et al. 2004), and in long-term potentiation (LTP) (Katsuki et al. 1990; Murray and Lynch 1998; Vereker et al. 2000; Kelly et al. 2003; Minogue et al. 2003; Maher et al. 2005; Griffin et al. 2006). Interestingly, mice deficient for IL-1R (the only known receptor for IL-1) exhibited deficits in a number of learning paradigms, as well as in LTP, suggesting a physiological role for IL-1β (Avital et al. 2003).
In addition to its detrimental role in neuronal injury (Allan et al. 2005), IL-1β has been found to be involved in Aβ metabolism process, e.g., transcriptional upregulation of expression of APP gene by promoting binding of NF-kappaB/Rel to 5′-regulatory region of the APP gene (Grilli et al. 1996; Yang et al. 1998), or supporting beta-secretase cleavage of the immature APP molecule (Blasko et al. 2000), and augmenting gamma-secretase activity (Liao et al. 2004). Nonetheless, a recent report demonstrated that IL-1β up-regulated TACE to enhance alpha-cleavage of APP and reduced beta-cleavage in mouse neurons (Tachida et al. 2008). IL-1β may also impair the microglia-mediated Aβ clearance (Hickman et al. 2008). More intriguingly, sustained hippocampal overexpression of IL-1β in APPsw/PS1 mice mediated chronic inflammation to ameliorate plaque pathology through enhancement of microglia-mediated plaque degradation (Shaftel et al. 2007b), presumably through enhancing recruitment of bone-marrow-derived microglia into the CNS via increased leakage of BBB and robust upregulation of MCP-1 (Shaftel et al. 2007a).
These provocative findings underscore the plastic roles that a particular cytokine can play. They act in highly context-dependent ways, not necessarily always being proinflammatory or detrimental (Wyss-Coray 2006). Though the status of neuroinflammation as a “good guy” or a “bad guy” with respect to AD pathogenesis is still controversial (Rogers et al. 1996; Morgan et al. 2005; Wyss-Coray 2006; Lemere 2007), the observation of an ongoing robust activation of microglia and astrocytes, particularly in the hippocampus of HAART-treated patients despite HIVE has become less common (Anthony et al. 2005; Bell et al. 2006) which means it poses a threat as a factor that is more conducive to development of AD.
Impaired enzymatic Aβ degradation
HIV encodes at least nine proteins that can be divided into three classes: structural - Gag, Pol, and Env; regulatory – Tat (transactivator of transcription) and Rev (Regulatory for expression of viral proteins); and accessory –Vpu, Vpr, Vif and Nef. These viral factors can damage neurons and interfere with the function of CNS through respective mechanisms (Ances and Ellis 2007); furthermore, some of these rogue molecules are implicated in Aβ pathology. For instance, Tat inhibited NEP activity by 80% in an in vitro assay, and recombinant Tat added directly to brain cultures resulted in a 125% increase in soluble Aβ (Rempel and Pulliam 2005; Daily et al. 2006). This indicates patients living with HIV may have faulty Aβ degradation. In addition, binding of Tat to LRP mediates neuronal uptake of Tat, and substantial inhibition of binding, uptake and subsequent degradation of several physiological ligands of LRP including Aβ (Liu et al. 2000). Gp41 peptides can impair protein kinase C response to down-regulate IL-1β-induced elevation of secreted sAPPβ (Chong and Lee 1999), which is a potent glial neurotrophic factor (Luo et al. 2001; Wang et al. 2004; Bell et al. 2008).
Our in vitro study showed a significant impairment of Aβ degradation by HIV-infected macrophage when compared to non-infected control cells (Figure 3).
Figure 3. Impaired fibrillar Aβ degradation in HIV-infected human macrophages.
Human monocyte-derived macrophages (MDM) were infected with HIV-1 YU-2 (0.1pg p24/cell) or uninfected, and 3 day post infection subjected to pulse-labeling with 1.0 µM 125I-labeled fibrillar Aβ42 for 1h at 37° C. The cells were washed out and chased for different time points as indicated in graph, when media were collected and fractionated with trichloroacetic acid (TCA) into TCA-soluble fraction containing degraded Aβ42 and TCA-insoluble fraction containing aggregated Aβ42 as described (Yuyama et al. 2008). HIV infection results in intracellular Aβ retention (A), significant reduction in Aβ degradation (B) and a concomitant increase of Aβ aggregation in extracellular space (C). *** denotes p<0.001 against control group at respective time points as determined by two-way repeated measurement ANOVA and Bonferroni post tests.
The exosome connection
Exosome formation and function
Exosomes, small membrane vesicles secreted from cells into the extracellular space, were first observed in association with sheep reticulocytes (Johnstone et al. 1987), and have been observed in cells found in the CNS including macrophage, microglia, and neurons (Verani et al. 2005; Faure et al. 2006). Proteins sequestrated into the limiting membrane of multi-vesicular bodies (MVBs) can be selectively incorporated into intraluminal vesicles (ILV) that are contained within the MVBs, and the internal vesicles can be targeted for lysosomal degradation. Alternatively, they can fuse with the plasma membrane releasing ILV into the extracellular space (Vella et al. 2008). Exosome secretion was previously considered a cellular mechanism to release unnecessary proteins, but recent studies have revealed additional roles such as proteolytic processing of certain target proteins like soluble TNFR (Hawari et al. 2004), intercellular trafficking of HIV (Loomis et al. 2006; Wiley and Gummuluru 2006; Vella et al. 2008), and as vehicles for stimulating anti-tumoral immune response, as reviewed elsewhere (Denzer et al. 2000; Fevrier and Raposo 2004).
Implications of exosome in neurodegenerative disorders
It is intriguing that proteins associated with prion disease and AD can be selectively incorporated into ILV of MVBs and subsequently secreted into the extracellular space. It has been shown that the accumulation of the longer, more amyloidogenic Aβ42 occurs predominantly in MVBs of neurons in normal mouse, rat and human brain, and that this accumulation increases with age in transgenic mice and human AD brains (Takahashi et al. 2002). Also of significance is the enrichment in the exosomes of GM1 ganliosides, which is an important component of cellular plasma membranes and especially enriched in lipid raft, and can bind to Aβ42, Aβ40 (Ariga et al. 2001) and APP (Zhang et al. 2007). There is mounting evidence in support of GM1-bound Aβ (GAβ) found in human brain as a seed for Aβ fibrillogenesis (Yanagisawa et al. 1995; McLaurin and Chakrabartty 1996; Yamamoto et al. 2005; Yamamoto et al. 2007b). Although it is still not clear what causes endocytic pathway abnormalities in AD brains, a recent in vitro study showed that it can result in accumulation of GM1 ganglioside in early endosome and accelerated release of exosome-associated GM1 ganglioside into the extracelluar environment inducing Aβ fibril formation (Yuyama et al. 2008). It is of note that GM1-ganglioside level is elevated in the CSF of subjects seropositive for HIV. This elevation may be due to the fact that HIV budding occurs via exosomes that are enriched for GM1-ganglioside, or might be a result of endosomal pathway alterations owing to budding and accumulation of HIV virions within macrophage (Jouve et al. 2007). The enrichment of GM1 ganglioside in the extracellular space might promote the Aβ fibril formation process; this speculation is supported by results of our in vitro study in which HIV infection resulted in enhancement of Aβ aggregation by 3–4 folds. Therefore the commonality of utilizing exosomes as the same releasing mechanism for both HIV and Aβ is of immense interest and significance. Although a co-lease of both agents from neurons is an unlikely scenario considering no direct viral infection of neurons, it might be likely in infected macrophage. This might result in Aβ deposition within the macrophage, since our recent study demonstrated that fibrillar Aβ degradation in macrophage is dependent on lysosomes (Yamamoto et al. 2008), and may account for the Aβ immunoreactivity localized in perivascular macrophages in HIV-infected brain. However, the accumulation and enrichment of GM1 ganglioside–enriched exosomes from infected macrophages may act as a nexus promoting fibrillogenesis of Aβ secreted by neurons and activated astrocytes. This is summarized in a proposed mechanism shown in Figure 4.
Figure 4. Proposed mechanisms leading to impaired intra-macrophage Aβ degradation and facilitated extracelluar Aβ fibrillogenesis.
Phagocytosed Aβ fibrils can be retrograde transported into aggresome and then refolding occurs with the help of chaperone molecules such as Hsp70; later they can be degraded by IDE or lysosomal enzymes, or excytosed. Endocytosed Aβ fibrils can also be degraded via endosome-lysosome pathway. HIV might impair Aβ fibrils degradation in macrophage through elevating TNF-α and IFN-γ (inhibitory for Aβ fibrils degradation), decreasing IL-4 and IL-10 (stimulatory for Aβ fibrils degradation), and blocking endosome-lysosomal pathway, raising a possibility that Aβ may be co-leased with virions into extracelluar space where it can serve as a seed for fibrillogenesis.
Many amyloidosis facilitates HIV infection
It is very interesting that not only HIV viral infection can influence the amyloidosis process, but also that amyloidosis in turn can facilitate HIV viral infection. Aβ fibrils can stimulate viral infection of target cells expressing CD4 and an appropriate coreceptor by 5–20 folds by multiple HIV-1 isolates (Wojtowicz et al. 2002). In addition, another amyloid fibrils formed from naturally occurring fragments of the abundant semen marker prostatic acidic phosphatase (PAP) can drastically enhance viral infection by several orders of magnitude (Munch et al. 2007). These studies indicate that viral infection and Aβ production can mutually affect each other constituting a vicious cycle, and the complexity of their interaction is underappreciated. Intriguingly , apolipoprotein (apo) E isoform 4, the infamous risk factor for AD, is associated with an accelerated disease course and progression to death in HIV-infected patients, and apoE4 enhanced HIV fusion/cell entry of both R5 and X4 HIV strains in vitro (Burt et al. 2008). The sharing of the same disease risk factor not only suggests that current efforts to convert apoE4 to an "apoE3-like" molecule to treat AD might also have clinical applicability in HIV disease, but also underscores the speculation of a convergent pathogenic pathway for both AD and AIDS.
Summary
HIV neuroinvasion results in chronic inflammation in the brain, which is pathogenically implicated in AD. Recent findings presented above provide new insights as to how HIV infection can cause imbalance of Aβ biogenesis and clearance, accounting for accelerated beta-amyloidosis observed in HAND patients. The shifted neuropathology and adverse effects associated with HAART medication might cause these patients to be more susceptible to AD comorbidity. There is increasing awareness that HIV infection and Aβ may have shared pathogenic mechanisms responsible for neurodegeneration and dementia, hence a closer look into their interaction can provide not only more insights into their pathogenesis, but also helpful information to cope with this comorbidity for each of which there is no cure today.
Acknowledgement
We thank National NeuroAIDS Tissue Banks (University of California, Los Angeles, University of California, San Diego, and University of Texas Galveston) for brain specimens, Departmental Tissue and Cell Core Facility for elutriated human monocytes, Drs. Howard Gendelman, Ben Gelman, Yuri Persidsky, Tomomi Kiyota and Huanyu Dou for consultation in AD and HAND pathogenesis; Ms Robin Taylor for developing schemes, and Russell Swan and Meg Marquardt for editing manuscripts. This work was funded by NIH grant R01 MH072539, P01 NS043985, NCRR P20RR15635 and Vada Kinman Oldfield Research Award (T.I).
Reference
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslen M. Increased cerebrospinal fluid protein tau concentration in neuro-AIDS. J Neurol Sci. 1999;171:92–96. doi: 10.1016/s0022-510x(99)00253-1. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Arch Biochem Biophys. 2001;388:225–230. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29:554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu SM, Gelbard HA. Protecting the synapse: evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmune Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- Berger-Greenstein JA, Cuevas CA, Brady SM, Trezza G, Richardson MA, Keane TM. Major depression in patients with HIV/AIDS and substance abuse. AIDS Patient Care STDS. 2007;21:942–955. doi: 10.1089/apc.2006.0153. [DOI] [PubMed] [Google Scholar]
- Besson C, Goubar A, Gabarre J, Rozenbaum W, Pialoux G, Chatelet FP, Katlama C, Charlotte F, Dupont B, Brousse N, Huerre M, Mikol J, Camparo P, Mokhtari K, Tulliez M, Salmon-Ceron D, Boue F, Costagliola D, Raphael M. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98:2339–2344. doi: 10.1182/blood.v98.8.2339. [DOI] [PubMed] [Google Scholar]
- Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, Grubeck-Loebenstein B. Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor alpha on the synthesis of Abeta1-40 and Abeta1-42 by human astrocytes. Neurobiol Dis. 2000;7:682–689. doi: 10.1006/nbdi.2000.0321. [DOI] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. Aids. 2004;18 Suppl 1:S75–S78. [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol. 2006;1:160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher J, Gross S, Gendelman H, Ikezu T. The neuropathogenesis of HIV-1 infection. In: Portegies P, Berger J, editors. Handbook of Clinical Neurology. 3rd Edition. Elsevier B.V.; 2007. [DOI] [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, Cavrois M, Huang Y, Mahley RW, Dolan MJ, McCune JM, Ahuja SK. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol. 2005;6:30. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Tack KM, Ahuja N, Weinman EJ, Wali RK, Uche A, Greisman LA, Drachenberg C, Hawkins PN, Redfield RR. Acute renal failure and nephrotic range proteinuria due to amyloidosis in an HIV-infected patient. Am J Med Sci. 2006;332:364–367. doi: 10.1097/00000441-200612000-00012. [DOI] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Chinnakotla AK, De Luna AM, Thew ST, Anderson BR, Cantave IM. Symptomatic gastrointestinal amyloidosis in an HIV-infected patient. Am J Gastroenterol. 2001;96:2248–2250. [Google Scholar]
- Cho HJ, Kim SK, Jin SM, Hwang EM, Kim YS, Huh K, Mook-Jung I. IFN-gamma-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia. 2007;55:253–262. doi: 10.1002/glia.20451. [DOI] [PubMed] [Google Scholar]
- Chong YH, Lee MJ. Effect of HIV-1 gp41 peptides on secretion of beta-amyloid precursor protein in human astroglial cell line, T98G. J Mol Neurosci. 1999;12:147–156. doi: 10.1007/BF02736928. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Bilotta A, Strongoli MC, Navarra M, Bagetta G, Di Renzo G. HIV-1 coat protein gp120 stimulates interleukin-1beta secretion from human neuroblastoma cells: evidence for a role in the mechanism of cell death. Br J Pharmacol. 2001;134:1344–1350. doi: 10.1038/sj.bjp.0704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Depino A, Ferrari C, Pott Godoy MC, Tarelli R, Pitossi FJ. Differential effects of interleukin-1beta on neurotoxicity, cytokine induction and glial reaction in specific brain regions. J Neuroimmunol. 2005;168:96–110. doi: 10.1016/j.jneuroim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol. 2008;172:1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Seubert P, Motter R, Galasko D, Deutsch R, Heaton RK, Heyes MP, McCutchan JA, Atkinson JH, Grant I. Cerebrospinal fluid tau protein is not elevated in HIV-associated neurologic disease in humans. HIV Neurobehavioral Research Center Group (HNRC) Neurosci Lett. 1998;254:1–4. doi: 10.1016/s0304-3940(98)00549-7. [DOI] [PubMed] [Google Scholar]
- Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008 doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168:230–237. doi: 10.1016/j.tvjl.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Giometto B, An SF, Groves M, Scaravilli T, Geddes JF, Miller R, Tavolato B, Beckett AA, Scaravilli F. Accumulation of beta-amyloid precursor protein in HIV encephalitis: relationship with neuropsychological abnormalities. Ann Neurol. 1997;42:34–40. doi: 10.1002/ana.410420108. [DOI] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62:429–440. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- Gray F, Bazille C, Adle-Biassette H, Mikol J, Moulignier A, Scaravilli F. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005;11 Suppl 3:16–22. doi: 10.1080/13550280500511741. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. Aids. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Goffi F, Memo M, Spano P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J Biol Chem. 1996;271:15002–15007. doi: 10.1074/jbc.271.25.15002. [DOI] [PubMed] [Google Scholar]
- Guallar JP, Gallego-Escuredo JM, Domingo JC, Alegre M, Fontdevila J, Martinez E, Hammond EL, Domingo P, Giralt M, Villarroya F. Differential gene expression indicates that 'buffalo hump' is a distinct adipose tissue disturbance in HIV-1-associated lipodystrophy. Aids. 2008;22:575–584. doi: 10.1097/QAD.0b013e3282f56b40. [DOI] [PubMed] [Google Scholar]
- Hamel FG, Fawcett J, Tsui BT, Bennett RG, Duckworth WC. Effect of nelfinavir on insulin metabolism, proteasome activity and protein degradation in HepG2 cells. Diabetes Obes Metab. 2006;8:661–668. doi: 10.1111/j.1463-1326.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. science. 2002;5580:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hartlage-Rubsamen M, Zeitschel U, Apelt J, Gartner U, Franke H, Stahl T, Gunther A, Schliebs R, Penkowa M, Bigl V, Rossner S. Astrocytic expression of the Alzheimer's disease beta-secretase (BACE1) is stimulus-dependent. Glia. 2003;41:169–179. doi: 10.1002/glia.10178. [DOI] [PubMed] [Google Scholar]
- Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath KV, Hogg RS, Singer J, Chan KJ, O'Shaughnessy MV, Montaner JS. Antiretroviral treatment patterns and incident HIV-associated morphologic and lipid abnormalities in a population-based chort. J Acquir Immune Defic Syndr. 2002;30:440–447. doi: 10.1097/00042560-200208010-00010. [DOI] [PubMed] [Google Scholar]
- Heath KV, Hogg RS, Chan KJ, Harris M, Montessori V, O'Shaughnessy MV, Montanera JS. Lipodystrophy-associated morphological, cholesterol and triglyceride abnormalities in a population-based HIV/AIDS treatment database. Aids. 2001;15:231–239. doi: 10.1097/00002030-200101260-00013. [DOI] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. 2007;4:e262. doi: 10.1371/journal.pmed.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RS, O'Shaughnessy MV, Gataric N, Yip B, Craib K, Schechter MT, Montaner JS. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Zlokovic BV. Role of Ab transport and clearance in the pathogenesis and treatment of Alzheimer's disease. New York: Springer; 2007. [Google Scholar]
- Hong HS, Hwang EM, Sim HJ, Cho HJ, Boo JH, Oh SS, Kim SU, Mook-Jung I. Interferon gamma stimulates beta-secretase expression and sAPPbeta production in astrocytes. Biochem Biophys Res Commun. 2003;307:922–927. doi: 10.1016/s0006-291x(03)01270-1. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Izycka-Swieszewska E, Zoltowska A, Rzepko R, Gross M, Borowska-Lehman J. Vasculopathy and amyloid beta reactivity in brains of patients with acquired immune deficiency (AIDS) Folia Neuropathol. 2000;38:175–182. [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- Jouve M, Sol-Foulon N, Watson S, Schwartz O, Benaroch P. HIV-1 buds and accumulates in "nonacidic" endosomes of macrophages. Cell Host Microbe. 2007;2:85–95. doi: 10.1016/j.chom.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 Suppl 1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenal B, Lai AR, Saghayam S, Balakrishnan P, Yepthomi T, Poongulali S, Flanigan TP, Solomon S, Mayer KH. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDS. 2008;22:337–344. doi: 10.1089/apc.2007.0093. [DOI] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids. 2002;16:1019–1029. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Lemere CA. A beneficial role for IL-1 beta in Alzheimer disease? J Clin Invest. 2007;117:1483–1485. doi: 10.1172/JCI32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, McCutchan JA, Ellis RJ. Neurologic Complications of HIV Disease and Their Treatment. Top HIV Med. 2008;16:15–22. [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- Lima AL, Zumiotti AV, Camanho GL, Benegas E, dos Santos AL, D'Elia CO, Oliveira PR. Osteoarticular complications related to HIV infection and highly active antiretroviral therapy. Braz J Infect Dis. 2007;11:426–429. doi: 10.1590/s1413-86702007000400012. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Loomis RJ, Holmes DA, Elms A, Solski PA, Der CJ, Su L. Citron kinase, a RhoA effector, enhances HIV-1 virion production by modulating exocytosis. Traffic. 2006;7:1643–1653. doi: 10.1111/j.1600-0854.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JJ, Wallace MS, Hawver DB, Kusiak JW, Wallace WC. Characterization of the neurotrophic interaction between nerve growth factor and secreted alpha-amyloid precursor protein. J Neurosci Res. 2001;63:410–420. doi: 10.1002/1097-4547(20010301)63:5<410::AID-JNR1036>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Miranda BH, Connolly JO, Burns AP. Secondary amyloidosis in a needle phobic intra-venous drug user. Amyloid. 2007;14:255–258. doi: 10.1080/13506120701456293. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey C, Odedra BJ, Standish RA, Furmali R, Edwards SG, Miller RF. Renal and gastrointestinal amyloidosis in an HIV-infected injection drug user. Int J STD AIDS. 2007;18:357–358. doi: 10.1258/095646207780749691. [DOI] [PubMed] [Google Scholar]
- Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- Newman M, Musgrave IF, Lardelli M. Alzheimer disease: amyloidogenesis, the presenilins and animal models. Biochim Biophys Acta. 2007;1772:285–297. doi: 10.1016/j.bbadis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol. 2006;18:469–478. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J Neuroinflammation. 2005;2:9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Johnson JD, Martin D, Rudy JW, Maier SF, Watkins LR. Human immunodeficiency virus-1 coat protein gp120 impairs contextual fear conditioning: a potential role in AIDS related learning and memory impairments. Brain Res. 2000;861:8–15. doi: 10.1016/s0006-8993(99)02445-2. [DOI] [PubMed] [Google Scholar]
- Quintana A, Giralt M, Rojas S, Penkowa M, Campbell IL, Hidalgo J, Molinero A. Differential role of tumor necrosis factor receptors in mouse brain inflammatory responses in cryolesion brain injury. J Neurosci Res. 2005;82:701–716. doi: 10.1002/jnr.20680. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. Aids. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Rossner S, Apelt J, Schliebs R, Perez-Polo JR, Bigl V. Neuronal and glial beta-secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology. J Neurosci Res. 2001;64:437–446. doi: 10.1002/jnr.1095. [DOI] [PubMed] [Google Scholar]
- Royston MC, Rothwell NJ, Roberts GW. Alzheimer's disease: pathology to potential treatments? Trends Pharmacol Sci. 1992;13:131–133. doi: 10.1016/0165-6147(92)90047-a. [DOI] [PubMed] [Google Scholar]
- Rumbaugh JA, Nath A. Developments in HIV neuropathogenesis. Curr Pharm Des. 2006;12:1023–1044. doi: 10.2174/138161206776055877. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain's amyloid cobwebs. Neuron. 2001a;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001b;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007a;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007b;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Zhu SG, Jones RA, Griffin WS, Mrak RE. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol. 2000;163:388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Jones RA, Zhou XQ, McGinness JM, Van Eldik LJ, Mrak RE, Griffin WS. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer's disease: potential significance for tau protein phosphorylation. Neurochem Int. 2001;39:341–348. doi: 10.1016/s0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:511–521. [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard BE, Horrobin DF. Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol Psychiatry. 2004;9:630–638. doi: 10.1038/sj.mp.4001462. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stoll M, Schmidt RE. Immune Restoration Inflammatory Syndromes: The Dark Side of Successful Antiretroviral Treatment. Curr Infect Dis Rep. 2003;5:266–276. doi: 10.1007/s11908-003-0083-x. [DOI] [PubMed] [Google Scholar]
- Stoll M, Schmidt RE. Immune restoration inflammatory syndromes: apparently paradoxical clinical events after the initiation of HAART. Curr HIV/AIDS Rep. 2004;1:122–127. doi: 10.1007/s11904-004-0018-7. [DOI] [PubMed] [Google Scholar]
- Tachida Y, Nakagawa K, Saito T, Saido TC, Honda T, Saito Y, Murayama S, Endo T, Sakaguchi G, Kato A, Kitazume S, Hashimoto Y. Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem. 2008;104:1387–1393. doi: 10.1111/j.1471-4159.2007.05127.x. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Tanzi RE. The synaptic Abeta hypothesis of Alzheimer disease. Nat Neurosci. 2005;8:977–979. doi: 10.1038/nn0805-977. [DOI] [PubMed] [Google Scholar]
- Tian C, Erdmann N, Zhao J, Cao Z, Peng H, Zheng J. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. J Neurochem. 2008;105:994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 2005;109:449–455. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. "Inflammatory" cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Volterra A, Steinhauser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–257. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang JY, Yang F, Ji ZJ, Chakraborty G, Sheng SL. A novel neurotrophic peptide: APP63–73. Neuroreport. 2004;15:2677–2680. doi: 10.1097/00001756-200412030-00025. [DOI] [PubMed] [Google Scholar]
- Weller RO. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropathol Exp Neurol. 1998;57:885–894. doi: 10.1097/00005072-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Farzan M, Joyal JL, Carter K, Babcock GJ, Israel DI, Sodroski J, Mirzabekov T. Stimulation of enveloped virus infection by beta-amyloid fibrils. J Biol Chem. 2002;277:35019–35024. doi: 10.1074/jbc.M203518200. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J Immunol. 2008;181:3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007a;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Fukata Y, Fukata M, Yanagisawa K. GM1-ganglioside-induced Abeta assembly on synaptic membranes of cultured neurons. Biochim Biophys Acta. 2007b;1768:1128–1137. doi: 10.1016/j.bbamem.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yokoseki T, Shibata M, Yamaguchi H, Yanagisawa K. Suppression of Abeta deposition in brain by peripheral administration of Fab fragments of anti-seed antibody. Biochem Biophys Res Commun. 2005;335:45–47. doi: 10.1016/j.bbrc.2005.06.208. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat Med. 1995;1:1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Yang Y, Quitschke WW, Brewer GJ. Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species. Brain Res Mol Brain Res. 1998;60:40–49. doi: 10.1016/s0169-328x(98)00164-8. [DOI] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Yamamoto N, Yanagisawa K. Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality: another putative pathway for GM1-induced amyloid fibril formation. J Neurochem. 2008;105:217–224. doi: 10.1111/j.1471-4159.2007.05128.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ding J, Tian W, Wang L, Huang L, Ruan Y, Lu T, Sha Y, Zhang D. Ganglioside GM1 binding the N-terminus of amyloid precursor protein. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6:718. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]