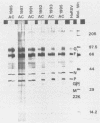
Abstract
Monoclonal antibodies (MAbs) to the fusion protein (F), attachment protein (G), and nucleoprotein (N) of respiratory syncytial (RS) virus were evaluated for use as detector antibodies in immunoglobulin G (IgG), IgA, and IgM capture enzyme immunoassays. MAb assays were tested against assays using polyclonal antibodies (PAbs) with serum specimens from patients with and without evidence of recent RS virus infection. Assays developed with N MAbs were comparable to or better than PAb assays for detecting specific IgG and IgM antibodies but were somewhat less sensitive for IgA. F MAb assays were less sensitive for IgG and IgM antibodies but identified specific IgA in some specimens negative by N MAb assay. G MAb assays were insensitive for IgG and IgM antibodies but did detect about 50% of the IgA antibodies identified by the PAb assay. The basis for the low sensitivity of the G MAb assays is unclear, since many of these specimens were positive for IgG antibodies to G by Western immunoblot. The sensitivity of MAb assays varied with patient age: N MAb assays detected specific antibody responses to RS virus in all immunoglobulin classes in both adults and infants less than 1 year of age, F MAb assays detected specific IgG responses in adults and IgA responses in both adults and infants, and G MAb assays only detected IgA responses in adults. A mixture of N and F MAbs was complementary overall, identifying 54 of 55 (IgG), 51 of 52 (IgA), and 16 of 17 (IgM) serum specimens positive by PAb assay. These MAb assays were also specific with specimens tested from persons without a history of recent RS virus infection. The availability of these MAb-based assays offers other laboratories the opportunity to have long-term, standardized reagents and tests for serological diagnosis of RS virus infection.
Full text
PDF

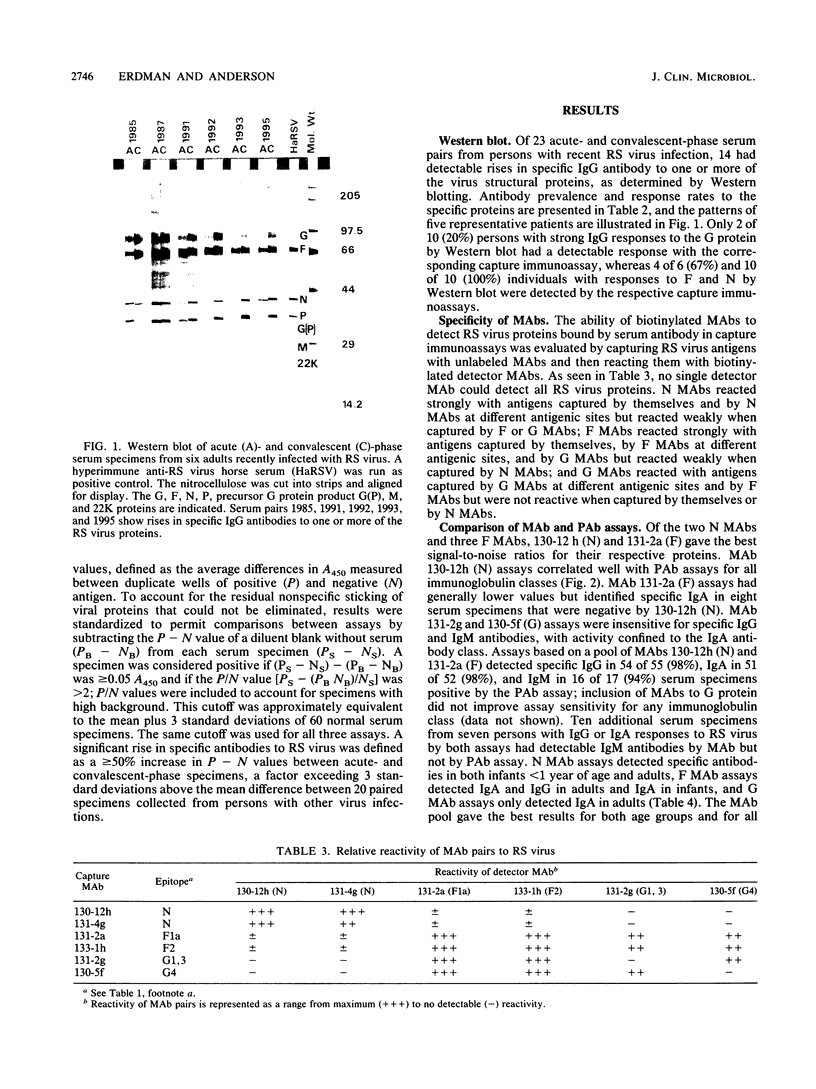
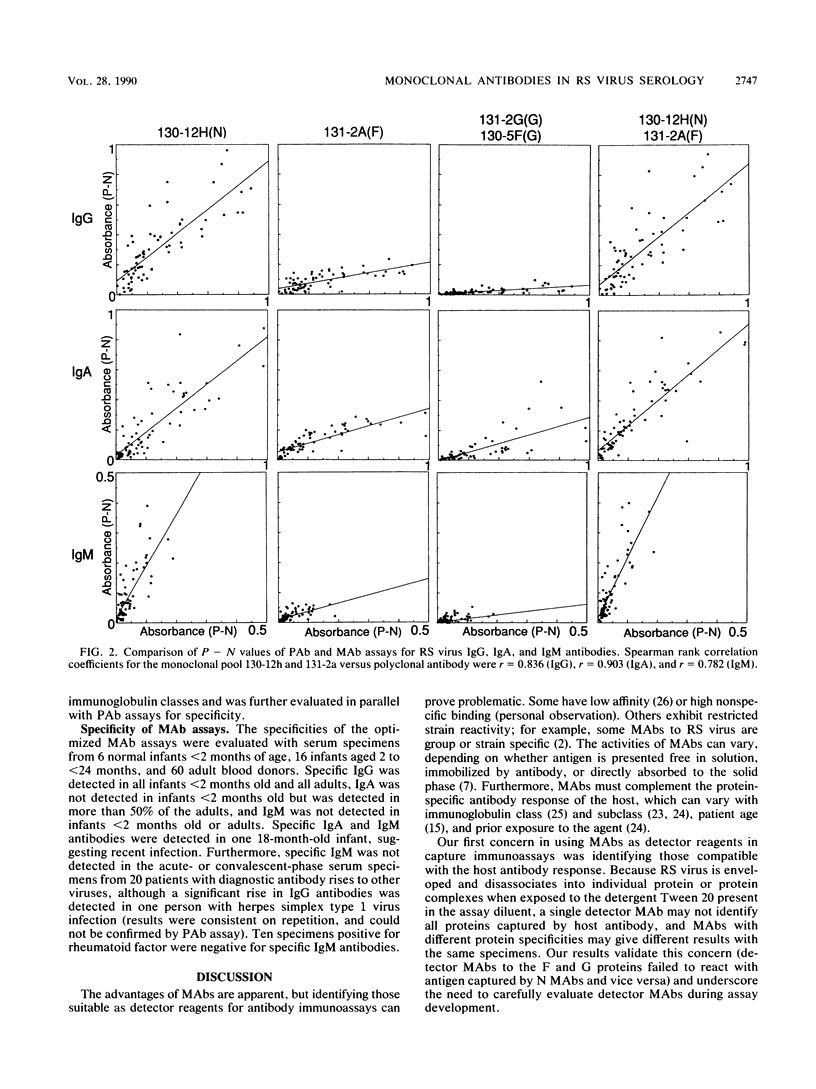
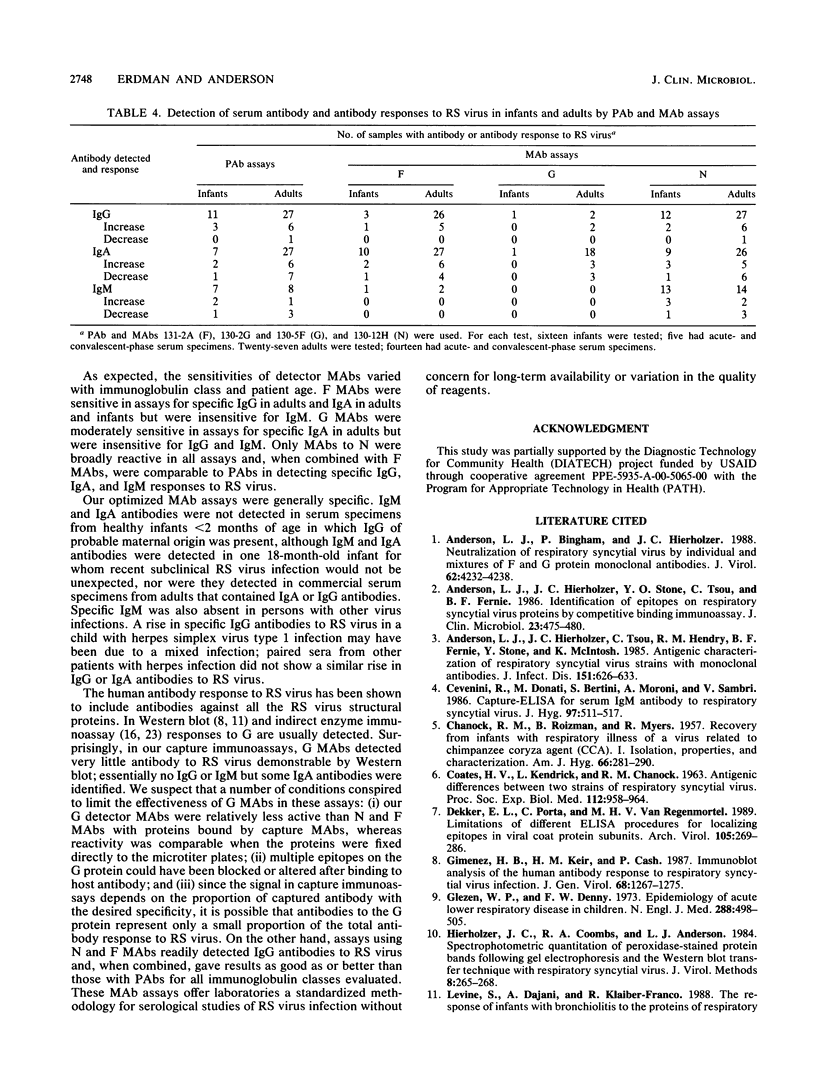

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Bingham P., Hierholzer J. C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988 Nov;62(11):4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Stone Y. O., Tsou C., Fernie B. F. Identification of epitopes on respiratory syncytial virus proteins by competitive binding immunoassay. J Clin Microbiol. 1986 Mar;23(3):475–480. doi: 10.1128/jcm.23.3.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- CHANOCK R., ROIZMAN B., MYERS R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957 Nov;66(3):281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- COATES H. V., KENDRICK L., CHANOCK R. M. Antigenic differences between two strains of respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Apr;112:958–964. doi: 10.3181/00379727-112-28221. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Donati M., Bertini S., Moroni A., Sambri V. Capture-ELISA for serum IgM antibody to respiratory syncytial virus. J Hyg (Lond) 1986 Dec;97(3):511–517. doi: 10.1017/s0022172400063713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker E. L., Porta C., Van Regenmortel M. H. Limitations of different ELISA procedures for localizing epitopes in viral coat protein subunits. Arch Virol. 1989;105(3-4):269–286. doi: 10.1007/BF01311363. [DOI] [PubMed] [Google Scholar]
- Gimenez H. B., Keir H. M., Cash P. Immunoblot analysis of the human antibody response to respiratory syncytial virus infection. J Gen Virol. 1987 May;68(Pt 5):1267–1275. doi: 10.1099/0022-1317-68-5-1267. [DOI] [PubMed] [Google Scholar]
- Glezen P., Denny F. W. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973 Mar 8;288(10):498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Coombs R. A., Anderson L. J. Spectrophotometric quantitation of peroxidase-stained protein bands following gel electrophoresis and the western blot transfer technique with respiratory syncytial virus. J Virol Methods. 1984 May;8(3):265–268. doi: 10.1016/0166-0934(84)90021-1. [DOI] [PubMed] [Google Scholar]
- Levine S., Dajani A., Klaiber-Franco R. The response of infants with bronchiolitis to the proteins of respiratory syncytial virus. J Gen Virol. 1988 Jun;69(Pt 6):1229–1239. doi: 10.1099/0022-1317-69-6-1229. [DOI] [PubMed] [Google Scholar]
- Meddens M. J., Herbrink P., Lindeman J., van Dijk W. C. Serodiagnosis of respiratory syncytial virus (RSV) infection in children as measured by detection of RSV-specific immunoglobulins G, M, and A with enzyme-linked immunosorbent assay. J Clin Microbiol. 1990 Jan;28(1):152–155. doi: 10.1128/jcm.28.1.152-155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman O., Ruuskanen O., Sarkkinen H., Hänninen P., Halonen P. Immunoglobulin class-specific antibody response in respiratory syncytial virus infection measured by enzyme immunoassay. J Med Virol. 1984;14(1):67–72. doi: 10.1002/jmv.1890140110. [DOI] [PubMed] [Google Scholar]
- Meurman O., Sarkkinen H., Ruuskanen O., Hänninen P., Halonen P. Diagnosis of respiratory syncytial virus infection in children: comparison of viral antigen detection and serology. J Med Virol. 1984;14(1):61–65. doi: 10.1002/jmv.1890140109. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Alling D. W., Snyder M. H., Walsh E. E., Prince G. A., Chanock R. M., Hemming V. G., Rodriguez W. J., Kim H. W., Graham B. S. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986 Nov;24(5):894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Graham B. S., Prince G. A., Walsh E. E., Chanock R. M., Karzon D. T., Wright P. F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986 Jun;23(6):1009–1014. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Koenig D. W., Chanock R. M. Antigenic analysis of a putative new strain of respiratory syncytial virus. J Infect Dis. 1985 Apr;151(4):634–637. doi: 10.1093/infdis/151.4.634. [DOI] [PubMed] [Google Scholar]
- Reimer C. B., Phillips D. J., Aloisio C. H., Black C. M., Wells T. W. Specificity and association constants of 33 monoclonal antibodies to human IgA epitopes. Immunol Lett. 1989 Jun 1;21(3):209–215. doi: 10.1016/0165-2478(89)90106-5. [DOI] [PubMed] [Google Scholar]
- Richardson L. S., Yolken R. H., Belshe R. B., Camargo E., Kim H. W., Chanock R. M. Enzyme-linked immunosorbent assay for measurement of serological response to respiratory syncytial virus infection. Infect Immun. 1978 Jun;20(3):660–664. doi: 10.1128/iai.20.3.660-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Vaur L., Agut H., Garbarg-Chenon A., Prud'Homme de Saint-Maur G., Nicolas J. C., Bricout F. Simplified enzyme-linked immunosorbent assay for specific antibodies to respiratory syncytial virus. J Clin Microbiol. 1986 Oct;24(4):596–599. doi: 10.1128/jcm.24.4.596-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. K., Graham B. S., Wright P. F., Walsh E. E., Kim H. W., Reimer C. B., Nelson D. L., Chanock R. M., Murphy B. R. Serum immunoglobulin G antibody subclass responses to respiratory syncytial virus F and G glycoproteins after primary infection. J Clin Microbiol. 1986 Aug;24(2):304–306. doi: 10.1128/jcm.24.2.304-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. K., Muelenaer P., Henderson F. W., Snyder M. H., Reimer C. B., Walsh E. E., Anderson L. J., Nelson D. L., Murphy B. R. Serum immunoglobulin G antibody subclass response to respiratory syncytial virus F and G glycoproteins after first, second, and third infections. J Clin Microbiol. 1989 Mar;27(3):589–592. doi: 10.1128/jcm.27.3.589-592.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R. C., Sun M., Hildreth S. W., Arumugham R., Ogra P. L. Respiratory syncytial virus-specific antibody responses in immunoglobulin A and E isotypes to the F and G proteins and to intact virus after natural infection. J Clin Microbiol. 1989 Feb;27(2):295–299. doi: 10.1128/jcm.27.2.295-299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of microbial antigens and prospects for improved assays. Yale J Biol Med. 1986 Jan-Feb;59(1):25–31. [PMC free article] [PubMed] [Google Scholar]