Abstract
OBJECTIVE
Glycogen synthase kinase (GSK)-3β plays an important role in cardiomyopathies. Cardiac-specific metallothionein-overexpressing transgenic (MT-TG) mice were highly resistant to diabetes-induced cardiomyopathy. Therefore, we investigated whether metallothionein cardiac protection against diabetes is mediated by inactivation of GSK-3β.
RESEARCH DESIGN AND METHODS
Diabetes was induced with streptozotocin in both MT-TG and wild-type mice. Changes of energy metabolism–related molecules, lipid accumulation, inflammation, nitrosative damage, and fibrotic remodeling were examined in the hearts of diabetic mice 2 weeks, 2 months, and 5 months after the onset of diabetes with Western blotting, RT-PCR, and immunohistochemical assays.
RESULTS
Activation (dephosphorylation) of GSK-3β was evidenced in the hearts of wild-type diabetic mice but not MT-TG diabetic mice. Correspondingly, cardiac glycogen synthase phosphorylation, hexokinase II, PPARα, and PGC-1α expression, which mediate glucose and lipid metabolisms, were significantly changed along with cardiac lipid accumulation, inflammation (TNF-α, plasminogen activator inhibitor 1 [PAI-1], and intracellular adhesion molecule 1 [ICAM-1]), nitrosative damage (3-nitrotyrosin accumulation), and fibrosis in the wild-type diabetic mice. The above pathological changes were completely prevented either by cardiac metallothionein in the MT-TG diabetic mice or by inhibition of GSK-3β activity in the wild-type diabetic mice with a GSK-3β–specific inhibitor.
CONCLUSIONS
These results suggest that activation of GSK-3β plays a critical role in diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Metallothionein inactivation of GSK-3β plays a critical role in preventing diabetic cardiomyopathy.
Metallothionein, a potent antioxidant, protects against diabetic cardiomyopathy (1–5). However, the mechanisms by which metallothionein protects the heart from diabetes are largely unknown (6). The diabetic heart is characterized by diminished glucose utilization and increased fatty acid oxidation, usually resulting in cardiac lipid accumulation (7,8). The lipotoxicity may be a major trigger for cardiac inflammation and oxidative damage and, eventually, cardiac dysfunction (7,8).
For glucose metabolism, insulin via its receptor initiates phosphorylation of insulin receptor intracellular substrates. Insulin receptor substrate family members serve as docking proteins for downstream signaling molecules, one of which is phosphatidylinositol (PI) 3-kinase. Activation of PI 3-kinase and its downstream protein kinase B (Akt) is essential for almost all insulin-induced glucose and fatty acid or lipid metabolism such as glucose uptake, glycogen synthesis, and suppression of triglyceride synthesis (9). GLUT4 and hexokinase II (HK II) both play critical roles in glucose transport and phosphorylation to glucose 6-phosphate for further glycolysis. HK II was found to be negatively regulated by glycogen synthase kinase (GSK)-3 (10).
GSK-3 is a ubiquitously expressed serine/threonine kinase that has versatile biological functions in cells, including regulation of metabolism, cell growth/death, and gene transcription and translation (9). Although both GSK-3α and GSK-3β appear to be equally important in certain aspects, GSK-3α has a more critical role than GSK-3β in regulating hepatic glucose metabolism and insulin sensitivity, and GSK-3β is the predominant regulator of glycogen synthase in skeletal muscle including the heart (9,11,12). Unlike most protein kinases, GSK-3 remains active in its dephosphorylated form and is inactivated upon phosphorylation by other protein kinases, such as Akt. In response to insulin, therefore, activated Akt inhibits the activity of GSK-3α or GSK-3β isoforms by phosphorylating their NH2-terminal serine residues (Ser21 in GSK-3α and Ser9 in GSK-3β), releasing their inhibitory effects on glycogen synthase. Dephosphorylated glycogen synthase and phosphorylated glycogen synthase (Ser641) are active and inactive forms, respectively, in glycogen synthesis (9). In diabetes, GSK-3β was activated by decreasing its phosphorylation (13,14). In contrast, inactivation of GSK-3β, either by increased Akt phosphorylation (13,14) or by cardiac-specific overexpression of dominant negative GSK-3β (11,15), increased cardiac glucose utilization and lipid metabolism. These studies thus suggest that GSK-3β phosphorylation plays a critical role in cardiac glucose metabolism.
In addition, glucose and lipid metabolism in the heart also are transcriptionally regulated by the peroxisome proliferator–activated receptor (PPAR) family (PPARα, -β/δ, and -γ) of ligand-activated transcription factors. Both PPARα and PPARβ play critical roles in the heart in regulating glucose and fatty acid metabolisms, while PPARγ is a major regulator of lipid storage in the adipose tissue where it regulates the differentiation of adipocytes by sensing lipid availability and adapting appropriate gene expression programs (7,8,16). The cardiomyopathic remodeling that occurred in diabetic mice was accompanied by striking lipid and reactive oxygen species accumulation in the heart, suggesting a lipotoxic contribution to cardiomyopathy (7,8,16). Peroxisome proliferator–activated γ coactivator (PGC)-1α is essential for the main tenance of maximal, efficient cardiac mitochondrial fatty acid oxidation, ATP synthesis, and myocardial lipid homeostasis (17).
The present study, therefore, tested our hypothesis that GSK-3β activation plays a critical role in diabetes-induced imbalance in glucose/lipid metabolism, leading to lipid accumulation that, in turn, evokes cardiac inflammation and oxidative stress/damage and, eventually, cardiac remodeling and dysfunction. Furthermore, we investigated whether and how metallothionein prevents diabetic activation of GSK-3β–mediated pathogenesis.
RESEARCH DESIGN AND METHODS
Diabetes models.
Metallothionein-overexpressing transgenic (MT-TG) mice were produced from the FVB mice that have been well characterized (2,18). Both 8- to 10-week-old MT-TG positive mice (heterozygotes) and negative littermates (wild type) were kept in the same cages with free access to rodent chow and tap water. All animal procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care. Streptozotocin (STZ) (Sigma, St. Louis, MO) was dissolved in sodium citrate buffer (pH 4.5). Male mice were given an intraperitoneal injection of STZ at 40 mg/kg body wt daily for 5 days. Whole-blood glucose obtained from the mouse tail vein was detected using a SureStep complete blood glucose monitor (LifeScan, Milpitas, CA) 5 days after the last STZ injection. STZ-injected mice with glucose levels >12 mmol/l were considered diabetic, and mice serving as controls were given the same volume of sodium citrate (2,4).
For the GSK-3β inhibitor experiment, diabetic mice, diagnosed on day 5 after the last STZ injection, were randomly divided into two groups (n = 6 for each), diabetic control and diabetes/inhibitor, to receive SB216763 (Tocris, Ellisville, MO), a selective inhibitor of GSK-3β. SB216763 was given by tail intravenous injection at 600 μg/kg body wt every 2 days for 2 months. Chronic injection with SB216763 to inhibit GSK-3β has not previously been reported; however, acute doses of SB216763 with 600 μg/kg for mice have been documented (19). Therefore, we selected this dose of SB216763 given every other day to avoid excess side effects and keep concentration at a high level. SB216763 injection for 2 months was selected because we found that diabetic mice 2 months after the onset of diabetes started to show cardiac dysfunction (20).
Cell cultures for in vitro experiments.
Embryonic rat heart–derived cells (H9c2) were cultured as previously described (21). H9c2 cells (American Type Culture Collection CLR-1446; Rockville, MD) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS from Atlanta Biologicals (Norcross, GA). When cell populations reached 60–70% confluence, the cultures were exposed to flavone acetic acid or tumor necrosis factor-α (TNF-α) in a final concentration of 50 μmol/l or 1.0 ng/ml in cultures for 24 h. The monolayer cultures were then washed with PBS, and the cells were harvested by trypsinization for measurements of mRNA expression of TNF-α and plasminogen activator inhibitor 1 (PAI-1), examined by RT-PCR assay or triglyceride contents by biochemical assay.
Oil Red O staining for lipid accumulation.
Cryosections of heart (10-μm thick) were fixed in 10% buffered formalin for 5 min at room temperature, stained with Oil Red O for 10 min, washed, and then counterstained with hematoxylin (DAKO, Carpinteria, CA) for 45 s. A Nikon microscope (Nikon, Melville, NY) was used to capture the Oil Red O–stained tissue sections at 400× magnification.
RNA isolation and real-time RT-PCR.
Total RNA was extracted from heart tissues or cardiac H9C2 cells using Trizol reagent (RNA STAT 60 Tel-Test; Ambion, Austin, TX). RNA concentrations were determined spectrophotometrically, and 1 μg total RNA was reversely transcribed using an avian myeloblastosis virus reverse-transcriptase kit (Promega, Madison, WI). PCR primers were designed using PAI-1, TNF-α, and intracellular adhesion molecule 1 (ICAM-1) (Applied Biosystems, Foster City, CA). Primers were designed to cross introns to ensure that only cDNA, not DNA, was amplified. TaqMan Universal PCR Master Mix (Applied Biosystems) was used to prepare the PCR mix. The amplification reactions were carried out in the ABI Prism 7700 sequence detection system (Applied Biosystems) with initial hold steps (50°C for 2 min, followed by 95°C for 10 min) and 50 cycles of a two-step PCR (92°C for 15 s and 60°C for 1 min). The fluorescence intensity of each sample was measured at each temperature change to monitor amplification of the target gene. The comparative cycle time (CT) method was used to determine fold differences between samples. The comparative CT method determines the amount of target, normalized to an endogenous reference (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) and relative to a calibrator (2−ΔΔCt).
Triglyceride determinations.
Mouse hearts or H9C2 cells were homogenized in 2 × PBS. Tissue lipids were extracted with methanol:chloroform (1:2), dried in an evaporating centrifuge and resuspended in 5% fat-free BSA. Colorimetric assessment of cardiac and H9C2 cell triglyceride levels was carried out using Sigma Diagnostics Triglyceride Reagent (Sigma Chemical, St. Louis, MO). Values were normalized to protein in homogenate before extraction determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Western blotting assays.
Western blotting assays were used to detect total GSK-3β, phosphorylated GSK-3β at Ser9, total glycogen synthase, phosphorylated glycogen synthase at Ser641, PGC-1α, and 3-nitrotyrocin (3-NT) (Cell Signaling, Boston, MA); connective tissue growth factor (CTGF) (Chemicon, Temecula, CA); and HK II, PPARα, and PAI-1 (BD Bioscience, Franklin Lakes, NJ). Heart tissues were homogenized in lysis buffer using homogenizer. Tissue or cell proteins were collected by centrifuging at 12,000g at 4°C in a Beckman GS-6R centrifuge for 10 min. The protein concentration was measured by Bradford assay. The sample, diluted in loading buffer and heated at 95°C for 5 min, was then subjected to electrophoresis on 10% SDS-PAGE gel at 120 V. After electrophoresis of the gel and transfer of the proteins to nitrocellulose membrane, the membranes were rinsed briefly in tris-buffered saline, blocked in blocking buffer (5% milk and 0.5% BSA) for 1 h, and washed three times with tris-buffered saline containing 0.05% Tween 20. The membranes were incubated with different primary antibodies at a dilution of 1:1,000 for 2 h and then washed and reacted with secondary horseradish peroxidase–conjugated antibody for 1 h. Antigen-antibody complexes were then visualized using ECL kit (Amersham, Piscataway, NJ).
Sirius Red staining of collagen.
Tissue sections of 5 μm were used for Sirius Red staining of collagen using 0.1% Sirius Red F3BA and 0.25% Fast Green FCF. The sections stained for Sirius Red then were assessed for the proportion of fibrosis (collagen) using a computer-assisted image analysis system as described in our previous study (4).
Statistical analysis.
Data from repeated experiments were presented as means ± SD (n ≥6). One-way ANOVA, followed by post hoc multiple comparisons tests with the Bonferroni test, was performed for the statistical analysis. Differences were considered significant at P < 0.05.
RESULTS
Inactivation of GSK-3β in MT-TG mice is accompanied by a significant prevention of diabetes-induced alterations in energy utilization–related molecules.
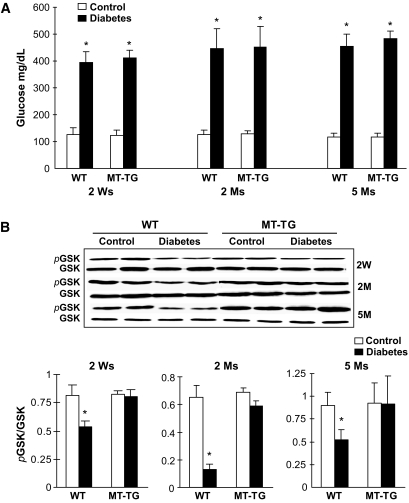
Wild-type and MT-TG diabetic mice showed similar, persistent increases in whole-blood glucose levels until experimental termination 5 months after the onset of diabetes induced by STZ (Fig. 1A). To test whether metallothionein protects the heart from diabetes mediated by its inactivation of GSK-3β, the total expression and phosphorylation of GSK-3β were examined from the hearts of the wild-type and MT-TG diabetic mice at indicated times after the onset of diabetes (Fig. 1B). We found that diabetes induced a significant decrease of GSK-3β phosphorylation in the wild-type diabetic hearts—an effect that was not observed in the MT-TG diabetic hearts.
FIG. 1.
Diabetic hyperglycemia and activation of GSK-3β. Before mice were killed at the indicated times, blood glucose was monitored at indicated times, using tail-vein blood (A), and cardiac tissues were used for Western blotting analysis of total and phosphorylated GSK-3β (B). *P < 0.05 vs. corresponding controls. Ms, months; Ws, weeks; WT, wild type.
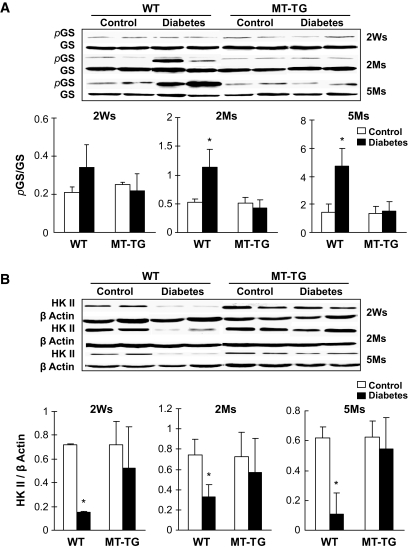
Two major functions of GSK-3β are its negative regulation of glucose metabolism and positive involvement in apoptotic cell death pathway (9). We have demonstrated the protection of metallothionein against diabetes-induced cardiac cell death (22). In the following experiments, therefore, we focus on effects of GSK-3β on energy metabolism–related molecules and associated pathogenic damages. Significantly increased glycogen synthase phosphorylation was observed in the hearts of wild-type diabetic mice 2 and 5 months, but not 2 weeks, after diabetes onset (Fig. 2A). Diabetes also significantly decreased HK II protein levels in the hearts of wild-type mice from 2 weeks until 5 months (Fig. 2B). However, both altered glycogen synthase phosphorylation and HK II abundance were normalized in the MT-TG diabetic hearts (Fig. 2), suggesting that glycogen synthesis and glucose metabolism decreased in the hearts of wild-type diabetic mice but not MT-TG diabetic mice.
FIG. 2.
Diabetes-induced glycogen synthase (GS) phosphorylation and decrease of HK II expression. Cardiac tissues were collected, as indicated in Fig. 1, for detecting total and phosphorylated glycogen synthase (Ser641) (A) and HK II expression (B) by Western blotting. *P < 0.05 vs. control. Ms, months; Ws, weeks; WT, wild type.
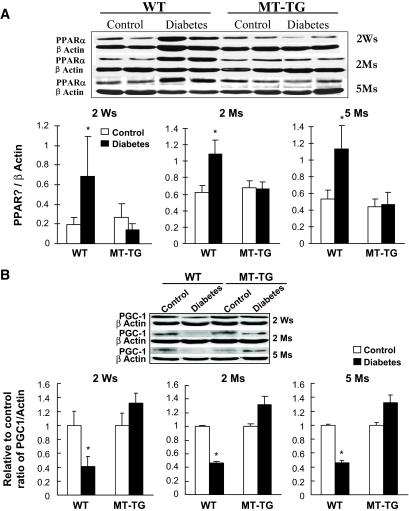
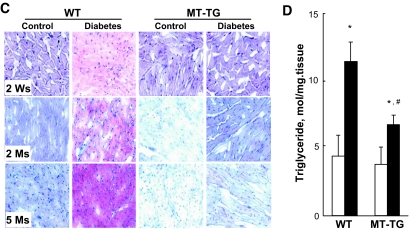
The decreased glucose metabolism in the wild-type diabetic hearts was accompanied by increased cardiac PPARα expression (Fig. 3A), a positive mediator for fatty acid uptake and utilization. Furthermore, PGC-1α that acts as an essential regulator for the maintenance of maximal and efficient cardiac mitochondrial fatty acid oxidation was significantly depressed in the wild-type diabetic hearts but not in the MT-TG diabetic hearts (Fig. 3B). The increased PPARα and decreased PGC-1α indicate the imbalance of fatty acid metabolism. Consistent with this notion, diabetes significantly increased lipid accumulation in the hearts of wild-type mice but not MT-TG mice from 2 weeks to 5 months after the onset of diabetes, which was evidenced by Oil Red O staining (Fig. 3C) and biochemical measurement of triglyceride levels (Fig. 3D).
FIG. 3.
Diabetes-increased PPARα expression and lipid accumulation. Cardiac tissues were collected, as indicated in Fig. 1, for detecting PPARα (A) and PGC-1α (B) expressions by Western blotting and cardiac lipid accumulation by Oil Red O staining (400×) (C) and triglyceride measurement (D). Panel D presents the data only from diabetic mice 2 weeks (Ws) after diabetes. *P < 0.05 vs. control; #P < 0.05 vs. wild-type (WT) diabetic group. Ms, months. (A high-quality digital representation of this figure is available in the online issue.).
Inactivation of GSK-3β in MT-TG mice prevents diabetes-induced pathogenic changes.
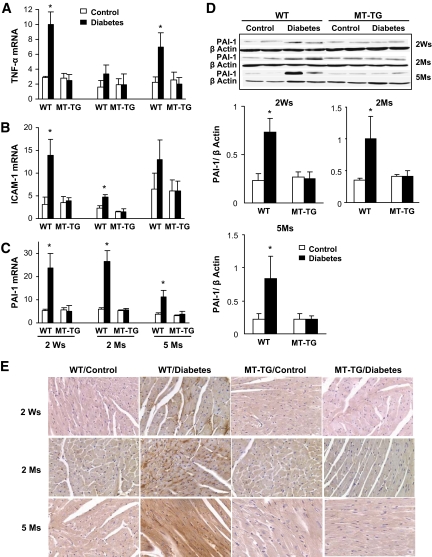
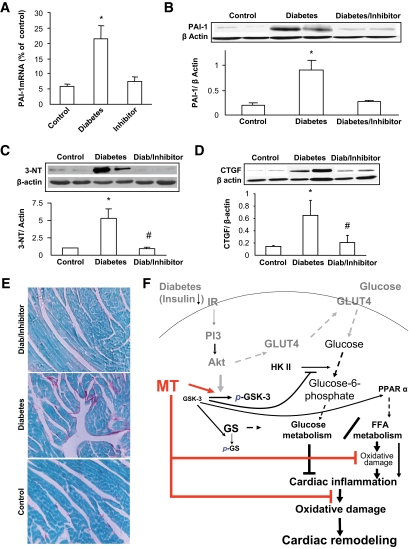
We next determined whether altered glucose and lipid metabolisms are accompanied by cardiac inflammation by detecting a few inflammatory cytokines that were found to play critical roles in the pathogenesis of diabetic cardiomyopathy (23,24). Real-time RT-PCR analysis showed that TNF-α and ICAM-1 mRNA expression in the hearts of wild-type diabetic mice was significantly increased 2 weeks and 5 months (Fig. 4A) and 2 weeks and 2 months (Fig. 4B) after diabetes, respectively. PAI-1 mRNA expression was significantly increased in the hearts of wild-type diabetic mice from 2 weeks to 5 months after diabetes (Fig. 4C). In addition, the increased PAI-1 expression in the hearts of wild-type diabetic mice was further confirmed by Western blot analysis (Fig. 4D) and immunohistochemical localization (Fig. 4E). However, the induction of inflammatory cytokine expression was not observed in the hearts of MT-TG diabetic mice.
FIG. 4.
Diabetes-induced cardiac inflammation. Real-time RT-PCR was used to examine the expression of TNF-α (A), ICAM-1 (B), and PAI-1 mRNA (C) in the hearts of diabetic mice at indicated postdiabetic times. TNF-α, ICAM-1, and PAI-1 expressions were normalized to GAPDH, by which the fold changes in expression were calculated using 2−ΔΔCt method. Expression of PAI-1 was further confirmed for its protein level by Western blotting (D) and localization by immunohistochemical staining (E). *P < 0.05 vs. corresponding controls. Ms, months; Ws, weeks; WT, wild type. (A high-quality digital representation of this figure is available in the online issue.)
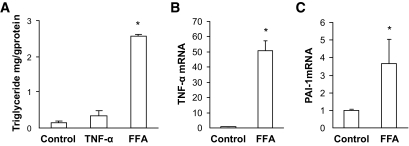
The causative effect of cardiac lipid accumulation (steatosis) on inflammation and oxidative damage has been previously established (14,23–25). In line with this notion, we also provided in vitro evidence that direct exposure of cultured cardiac H9c2 cells to TNF-α at a final concentration of 1.0 ng/ml for 24 h did not increase triglyceride levels (Fig. 5A). In contrast, direct exposure of cardiac cells to free fatty acid (FFA) (palmitate at 50 μmol) for 24 h not only significantly increased intracellular triglyceride levels (Fig. 5A) but also significantly increased mRNA expression of inflammatory cytokines TNF-α and PAI-1 (Fig. 5B and C).
FIG. 5.
FFA-induced upregulation of inflammatory cytokines. Cultured cardiac (H9c2) cells were directly exposed to TNF-α at a concentration of 1.0 ng/ml for 24 h, which did not increase intracellular triglyceride levels (A). Direct exposure of cardiac cells to FFA (palmitate) at 50 μmol/l for 24 h significantly increased intracellular triglyceride levels (A) and also significantly increased mRNA expression of inflammatory cytokines TNF-α (B) and PAI-1 (C). *P < 0.05 vs. control.
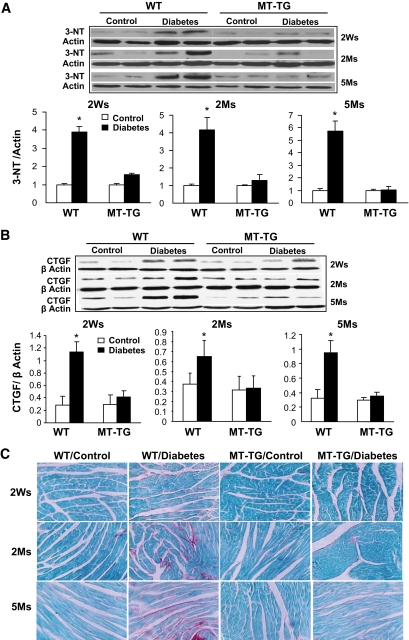
Cardiac lipid accumulation and associated inflammation were accompanied by significant cardiac nitrosative damage, examined by Western blotting assay for 3-NT as an index of nitrosative damage in the wild-type diabetic mice—an effect not observed in the MT-TG diabetic mice (Fig. 6A). In addition, because the proinflammatory cytokine PAI-1 is also profibrotic given its inhibition of the conversion of plasminogen to plasmin, we next examined the diabetic effect on cardiac fibrosis. As shown in Fig. 6, diabetes increased cardiac CTGF expression (Fig. 6B) and collagen deposition (evidenced by Sirius Red staining) (Fig. 6C) in the wild-type, but not MT-TG, mice.
FIG. 6.
Diabetes-increased cardiac nitrosative damage and fibrosis. Cardiac nitrosative damage was examined by 3-NT accumulation with Western blotting analysis (A). Cardiac fibrosis was examined by Western blotting analysis of CTGF expression (B) and Sirius Red staining of collagen (C). *P < 0.05 vs. corresponding controls. Ms, months; Ws, weeks; WT, wild type. (A high-quality digital representation of this figure is available in the online issue.)
Pharmacological inactivation of GSK-3β completely prevents diabetes-induced cardiac energy metabolism changes, inflammation, and fibrosis.
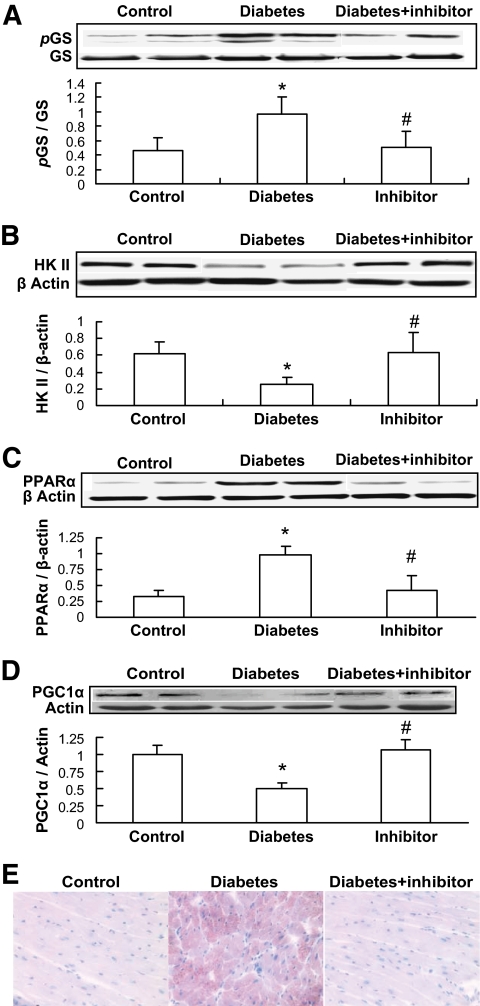
The above results indicate that inactivation of GSK-3β by metallothionein in the MT-TG diabetic mice seems to lead to the prevention of diabetes-induced cardiac energy metabolism derangement, lipid accumulation, inflammation, oxidative damage, and remodeling. To define whether the activation of GSK-3β by its dephosphorylation is directly related to diabetes-induced pathogenic effects, GSK-3β–specific inhibitor SB216763 was given to the wild-type diabetic mice at 600 μg/kg body wt every other day for 2 months immediately after the onset of diabetes. Inhibition of GSK-3β activity completely prevented diabetes-induced glycogen synthase phosphorylation (Fig. 7A), HK II downregulation (Fig. 7B), PPARα upregulation (Fig. 7C), PGC-1 downregulation (Fig. 7D), and lipid accumulation (Fig. 7E) in the heart of the wild-type diabetic mice.
FIG. 7.
Inhibition of GSK-3β by its inhibitor attenuated diabetes-induced changes related to glucose and lipid metabolism. Once diabetes was diagnosed on day 3 after mice were injected with STZ, half of these diabetic mice were immediately administered GSK-3β–specific inhibitor SB216763 at 600 μg/kg every other day for 2 months, and then cardiac glycogen synthase (GS) phosphorylation (A), HK II expression (B), PPARα expression (C), and PGC-1α expression (D) were examined by Western blotting. Cardiac lipid accumulation was examined by Oil Red O staining (400×) (E). *P < 0.05 vs. corresponding controls; #P < 0.05 vs. corresponding diabetes. (A high-quality digital representation of this figure is available in the online issue.)
Inhibition of GSK-3β activity also significantly prevented diabetes-increased cardiac PAI-1 mRNA (Fig. 8A) and protein expression (Fig. 8B) and 3-NT accumulation (Fig. 8C) and fibrosis (Fig. 8D and E). This study ensures that GSK-3β activation plays a critical role in diabetes-induced cardiac glucose and lipid metabolism changes, inflammation, and fibrosis.
FIG. 8.
Inhibition of GSK by its inhibitor attenuated diabetes-induced cardiac inflammation, nitrosative damage, and fibrosis. Experimental approaches and cardiac tissue sampling are the same as those in Fig. 6. Samples were examined for cardiac expression of PAI-1 by real-time RT-PCR (A) and Western blotting (B), cardiac 3-NT accumulation (C), and cardiac fibrosis by Western blotting for CTGF expression (D) and Sirius Red staining of collagen (200×) (E). *P < 0.05 vs. corresponding controls; #P < 0.05 vs. corresponding diabetes. F: schematic illustration of the mechanisms by which metallothionein (MT) preserves GSK-3β phosphorylation to maintain glucose and lipid metabolism balance under diabetic conditions and, consequently, inhibit cardiac lipid accumulation, inflammation, oxidative/nitrosative damage, and remodeling. Solid lines present the experimental finding from the present study, and dashed lines indicate well-known pathways from the literature. GS, glycogen synthase; IR, insulin receptor. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
The present study investigated the critical role of diabetic activation of GSK-3β in the alteration of cardiac energy metabolism, lipotoxicity, inflammation, nitrosative damage, and remodeling and reveals the following innovative findings. First, metallothionein preservation of GSK-3β phosphorylation in the heart resulted in a significant prevention of diabetic metabolic derangements, i.e., decreased glucose metabolism–related mediators (glycogen synthase inactivation and HK II downregulation) and increased lipid metabolism–related mediators (PPARα upregulation, PGC-1α downregulation, and lipid accumulation). Second, metallothionein prevention of the metabolic derangements was accompanied by a significant prevention of cardiac inflammation, nitrosative damage, and remodeling (fibrosis). Third, the association of metallothionein inactivation of GSK-3β with its prevention of diabetic energy derangement, lipid accumulation, inflammation, and remodeling was ensured by the findings of the GSK-3β inhibitor study. These findings indicate that activation of GSK-3β plays a critical role in the pathogenesis of diabetic cardiomyopathy.
Impaired regulation of glucose utilization is a main feature in the heart of both type 1 and type 2 diabetes (26). GSK-3 was originally identified as a regulator of glycogen synthase, a rate-limiting enzyme that promotes glycogen deposition. In the absence of insulin, active GSK-3 phosphorylates the serine residues in the COOH-terminal domain of glycogen synthase and negatively regulates its activity, reducing the capacity of cells to synthesize and store glycogen. Expression and activity of GSK-3 are significantly increased in the brain of mice with STZ-induced diabetes (27,28) and in skeletal muscle samples from humans with type 2 diabetes (29). Consistent with previous studies (13,14), our study showed the significant activation of GSK-3β in the hearts of diabetic mice from 2 weeks to 5 months. Inhibition of GSK-3 by various approaches resulted in an activation of glycogen synthase that increases rates of glycogen synthesis and offers cardiac protection (15,27,28,30). However, it remains unclear whether and how inactivation of GSK-3 results in cardiac protection against diabetes.
PPARs play important roles in metabolic homeostasis and protection from a variety of stress-induced damages; however, each member of the PPAR family (PPARα, -β/δ, and -γ) has a distinct role in different tissues under various conditions (31). For instance, activation of both PPARα and PPARγ attenuated diabetic kidney disease in the apolipoprotein E knockout mouse (32). Interestingly, PPARγ may potentially damage the heart (33) but is essential in protecting cardiomyocytes from oxidative damage (34). Cardiac PPARα transgenic mice showed excessive FFA oxidation, lipid accumulation, reduced glucose utilization, and cardiomyopathy—a phenotype that is remarkably similar to the diabetic heart (7,35). In contrast to PPARα, cardiac PPARβ/δ transgenic mice had increased myocardial glucose utilization, did not accumulate myocardial lipid, and had normal cardiac function (16). Furthermore, different roles of PPARα in the liver and brain also have been noted recently (36). Therefore, the roles of PPARs in metabolic homeostasis, anti-inflammation, and cytotoxic protection are distinct in different tissues under different conditions. In general, however, PPARα expressions and activity are elevated in diabetes and reduced in both cardiac ischemia and hypertrophy (7,31,35).
The different roles of these PPARs may be related to the expression of their comediators, such as the expressions of PGC-1α under certain conditions (37). PGC-1α is essential for the maintenance of maximal, efficient cardiac mitochondrial fatty acid oxidation, ATP synthesis, and lipid homeostasis (17). Under normal condition, PGC-1α expression in the liver is relatively low compared with that in other tissues such as heart and brain that rely mainly on aerobic metabolism for ATP production (17,37). Expression of PGC-1α in the liver increased in the animals with fasting, insulin resistance, and diabetes (37); in contrast, PGC-1α expression decreased in the heart of insulin-resistant mice (5), in skeletal muscles of type 2 diabetic patients (38), and in blood vessel media of STZ-injected diabetic rats (39).
In the present study, the innovative finding is the restoration of diabetic inhibition of HK II by inactivation of GSK-3β in MT-TG diabetic mice (Fig. 1) or its specific inhibitor (Fig. 7). To our knowledge, this is the first report demonstrating that HK II is negatively regulated by GSK-3β in the heart. This was an in vitro study in which chronic inhibition of GSK-3 protected against rotenone-induced apoptosis in cultured neuronal cells as a result of an enhancement of HK II–dependent glycolysis (40).
We also provide evidence that inhibition of diabetic activation of GSK-3β either by metallothionein in MT-TG diabetic mice (Fig. 2) or by GSK-3β inhibitor (Fig. 8) significantly attenuated diabetic upregulation of PPARα expression and downregulation of PGC-1α expression, respectively (Fig. 6). To date, there has been no previous study showing the direct link between GSK-3β and PPARα, but there were two studies showing that upregulation of Akt can significantly downregulate PPARα expression (41,42), suggesting that PPARα expression is regulated positively by GSK-3β and negatively by Akt. Whether the Akt's negatively regulated expression of PPARα is also mediated by its suppression of GSK-3β remains to be further investigated. The finding of the inhibition of PGC-1α expression by inactivating GSK-3β is in line with results from other previous studies demonstrating that GSK-3β reduces PGC-1α expression by stimulating its proteasomal degradation (43). The present study provides evidence that diabetic activation of GSK-3β plays a critical role in cardiac pathological changes.
Therefore, we propose a mechanism, as illustrated in Fig. 8E. Impaired insulin signaling in the heart of both type 1 and type 2 diabetic subjects (13,14,26) depresses Akt-dependent GSK-3β phosphorylation (Fig. 1), which alters energy metabolism, leading to lipid accumulation (Figs. 2 and 3). The lipotoxic effects in the heart evoke the cardiac inflammation, oxidative/nitrosative damage, remodeling (Figs. 4–6), and eventual cardiac dysfunction (2,22). The proposed mechanism is based on the following evidence. First, cardiac lipid accumulation in the wild-type diabetic mice was accompanied by increased cardiac inflammation (Fig. 4) and nitrosative damage (Fig. 6A), and inhibition of cardiac lipid accumulation by inactivation of GSK-3β (Fig. 7) is accompanied by prevention of cardiac inflammation and nitrosative damage (Fig. 8). Second, in an in vitro study, we found that exposure of cardiac cells to inflammatory factor TNF-α did not increase triglyceride levels (Fig. 5A) but that exposure to fatty acid significantly increased the inflammatory factors TNF-α and PAI-1 mRNA expression (Fig. 5B and C). Third, mice with cardiac-restricted overexpression of PPARα that enhances cardiac fatty acid uptake and lipid accumulation and also represses glucose metabolic genes showed a typical diabetic cardiomyopathy (7). The diabetic cardiomyopathy in this model can be rescued by deletion of the long-chain fatty acid transporter CD36 (35), suggesting the critical role of increased lipid metabolisms and lipotoxicity in the diabetic cardiomyopathy. Lastly, recent studies demonstrated the prevention of diabetic cardiomyopathy by inhibition of inflammation (23,24,44), suggesting that inflammation may be a consequence of cardiac lipid accumulation and toxicity. In support of this notion, administration of mycophenolate mofetil that specifically inhibited renal inflammation, but did not affect renal lipid accumulation, significantly prevented diabetic nephropathy (25). Atorvastatin reduced diabetes-induced cardiac inflammation and fibrosis, resulting in improved left ventricle function in the absence of LDL cholesterol lowering, also suggesting that the cardiac inflammation under diabetic condition is a downstream, important mediator in diabetic lipotoxicity and remodeling (44).
The last significant finding from our study is that metallothionein attenuated diabetes-induced GSK-3β activation, leading to the restoration of a diabetic switch from glucose to FFA metabolism in the heart that, in turn, causes the subsequent pathological changes, illustrated in Fig. 8F. Although it remains unclear how exactly metallothionein inhibits GSK-3β activation, a possible preservation of Akt expression and activation as upstream mediator of GSK-3 (Fig. 8F) may be an explanation. Diabetes suppresses Akt expression and activation, and a few studies showed that metallothionein protective effects are mediated by stimulating Akt signaling (3,5). Nonetheless, an investigation into the exact mechanism responsible is warranted in the future study.
In summary, by using the MT-TG mouse model and pharmacological inhibitor, the present study provides evidence for the first time that activation of GSK-3β plays a pivotal role in diabetes-induced energy metabolic derangement and, consequently, pathological remodeling in the heart. There is a clinical potential of the present study because zinc deficiency often occurs in diabetic patients and also increases the prevalence of cardiac dysfunction in diabetic patients (45). Upregulation of cardiac metallothionein in diabetic mice by zinc supplementation significantly prevented the development of diabetic cardiomyopathy (21). Diabetic patients treated with zinc also had reduced risk of vascular diseases such as nephropathy, retinopathy, and neuropathy (46,47). Therefore, the studies underlying the mechanisms by which metallothionein protects the diabetic heart will encourage development of a metallothionein inducer of potential clinical use for diabetic patients.
Acknowledgments
This work was supported in part by grants from the American Diabetes Association (02-07-JFA-10 and 05-07-CD-02 [to L.C.] and 07-07-JF-23 [to W.F. and L.C.]) and the National Natural Scientific Foundation of China (30672495 [to X.-K.L.]) and also by a start-up fund for the Chinese-American Research Institute for Diabetic Complications from Wenzhou Medical College (to X.-K.L. and L.C.).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008, and at the Basic Cardiovascular Sciences Annual Conference—2008 Heart Failure: Molecular Mechanisms and Therapeutic Targets, Keystone, Colorado, 28–31 July 2008.
We are grateful to Dr. Y. James Kang (the Department of Medicine and Pharmacology, University of Louisville, Louisville, Kentucky), who kindly provided MT-TG founder mice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ye G, Metreveli NS, Ren J, Epstein PN: Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes 2003; 52: 777– 783 [DOI] [PubMed] [Google Scholar]
- 2.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ: Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 2005; 54: 1829– 1837 [DOI] [PubMed] [Google Scholar]
- 3.Fang CX, Dong F, Ren BH, Epstein PN, Ren J: Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia 2005; 48: 2412– 2421 [DOI] [PubMed] [Google Scholar]
- 4.Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ: Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 2006; 48: 1688– 1697 [DOI] [PubMed] [Google Scholar]
- 5.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J: Metallothionein prevents high-fat diet–induced cardiac contractile dysfunction: role of peroxisome proliferator–activated receptor γ coactivator 1α and mitochondrial biogenesis. Diabetes 2007; 56: 2201– 2212 [DOI] [PubMed] [Google Scholar]
- 6.Cai L: Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications. Curr Med Chem 2007; 14: 2193– 2203 [DOI] [PubMed] [Google Scholar]
- 7.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP: The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002; 109: 121– 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poornima IG, Parikh P, Shannon RP: Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006; 98: 596– 605 [DOI] [PubMed] [Google Scholar]
- 9.Muniyappa R, Montagnani M, Koh KK, Quon MJ: Cardiovascular actions of insulin. Endocr Rev 2007; 28: 463– 491 [DOI] [PubMed] [Google Scholar]
- 10.Pastorino JG, Hoek JB, Shulga N: Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res 2005; 65: 10545– 10554 [DOI] [PubMed] [Google Scholar]
- 11.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH: Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal 2008; 20: 206– 218 [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR: Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol 2008; 28: 6314– 6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajoie C, Calderone A, Trudeau F, Lavoie N, Massicotte G, Gagnon S, Beliveau L: Exercise training attenuated the PKB and GSK-3 dephosphorylation in the myocardium of ZDF rats. J Appl Physiol 2004; 96: 1606– 1612 [DOI] [PubMed] [Google Scholar]
- 14.Montanari D, Yin H, Dobrzynski E, Agata J, Yoshida H, Chao J, Chao L: Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes 2005; 54: 1573– 1580 [DOI] [PubMed] [Google Scholar]
- 15.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J: Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res 2007; 101: 1164– 1174 [DOI] [PubMed] [Google Scholar]
- 16.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP: Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 2007; 117: 3930– 3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, Kelly DP: The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 2008; 295: H185– H196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN: Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 1997; 100: 1501– 1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross ER, Hsu AK, Gross GJ: The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol 2006; 291: H827– H834 [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Wang J, Li Y, Du Y, Arteel GE, Saari JT, Kang YJ, Cai L: Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol 2005; 167: 17– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L: Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 2006; 113: 544– 554 [DOI] [PubMed] [Google Scholar]
- 22.Cai L: Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med 2006; 41: 851– 861 [DOI] [PubMed] [Google Scholar]
- 23.Westermann D, Rutschow S, Jäger S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschöpe C: Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 2007; 56: 641– 646 [DOI] [PubMed] [Google Scholar]
- 24.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Escher F, Bucker-Gartner C, Spillmann F, Noutsias M, Riad A, Schultheiss HP, Tschope C: Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 2007; 56: 1834– 1841 [DOI] [PubMed] [Google Scholar]
- 25.Dominguez J, Wu P, Packer CS, Temm C, Kelly KJ: Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol 2007; 293: F670– F679 [DOI] [PubMed] [Google Scholar]
- 26.Bugger H, Abel ED: Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008; 114: 195– 210 [DOI] [PubMed] [Google Scholar]
- 27.Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E: Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer's disease and correction by insulin. J Neurosci Res 2008; 86: 3265– 3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collino M, Aragno M, Castiglia S, Tomasinelli C, Thiemermann C, Boccuzzi G, Fantozzi R: Insulin reduces cerebral ischemia/reperfusion injury in the hippocampus of diabetic rats: a role for glycogen synthase kinase-3β. Diabetes 2009; 58: 235– 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR: Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 2000; 49: 263– 271 [DOI] [PubMed] [Google Scholar]
- 30.Das A, Xi L, Kukreja RC: Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 2008; 283: 29572– 29585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W: From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 2006; 45: 120– 159 [DOI] [PubMed] [Google Scholar]
- 32.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC: PPAR-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol Dial Transplant 2006; 21: 2399– 2405 [DOI] [PubMed] [Google Scholar]
- 33.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ: Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 2007; 117: 2791– 2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q: Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res 2007; 76: 269– 279 [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP: CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 2007; 100: 1208– 1217 [DOI] [PubMed] [Google Scholar]
- 36.Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, Martinez E, Bringart M, Waget A, Kersten S, Desvergne B, Gremlich S, Wahli W, Seydoux J, Delzenne NM, Thorens B, Burcelin R: Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: role in liver and brain. Endocrinology 2006; 147: 4067– 4078 [DOI] [PubMed] [Google Scholar]
- 37.Ward WF, Liang H, Balas B, Tantiwong P, Dube JJ, Goodpaster BH, O'Doherty RM, Defronzo RA, Richardson A, Musi N: Whole-body overexpression of PGC-1{alpha} has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab 2009; 296: E945– E954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P: Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 2007; 31: 1302– 1310 [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Sun G, Zhang H, Zhang Y, Chen X, Jiang X, Jiang X, Krauss S, Zhang J, Xiang Y, Zhang CY: PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS ONE 2009; 4: e4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J: Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem 2009; 284: 3001– 3011 [DOI] [PubMed] [Google Scholar]
- 41.Cook SA, Matsui T, Li L, Rosenzweig A: Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 2002; 277: 22528– 22533 [DOI] [PubMed] [Google Scholar]
- 42.Li R, Zheng W, Pi R, Gao J, Zhang H, Wang P, Le K, Liu P: Activation of peroxisome proliferator-activated receptor-alpha prevents glycogen synthase 3beta phosphorylation and inhibits cardiac hypertrophy. FEBS Lett 2007; 581: 3311– 3316 [DOI] [PubMed] [Google Scholar]
- 43.Anderson RM, Barger JL, Edwards MG, Braun KH, O'Connor CE, Prolla TA, Weindruch R: Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 2008; 7: 101– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Linthout S, Riad A, Dhayat N, Spillmann F, Du J, Dhayat S, Westermann D, Hilfiker-Kleiner D, Noutsias M, Laufs U, Schultheiss HP, Tschope C: Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 2007; 50: 1977– 1986 [DOI] [PubMed] [Google Scholar]
- 45.Soinio M, Marniemi J, Laakso M, Pyörälä K, Lehto S, Rönnemaa T: Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007; 30: 523– 528 [DOI] [PubMed] [Google Scholar]
- 46.Al-Maroof RA, Al-Sharbatti SS: Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J 2006; 27: 344– 350 [PubMed] [Google Scholar]
- 47.Gupta R, Garg VK, Mathur DK, Goyal RK: Oral zinc therapy in diabetic neuropathy. J Assoc Physicians India 1998; 46: 939– 942 [PubMed] [Google Scholar]