Abstract
OBJECTIVE
We tested the hypothesis that short-term exercise training improves hereditary insulin resistance by stimulating ATP synthesis and investigated associations with gene polymorphisms.
RESEARCH DESIGN AND METHODS
We studied 24 nonobese first-degree relatives of type 2 diabetic patients and 12 control subjects at rest and 48 h after three bouts of exercise. In addition to measurements of oxygen uptake and insulin sensitivity (oral glucose tolerance test), ectopic lipids and mitochondrial ATP synthesis were assessed using1H and31P magnetic resonance spectroscopy, respectively. They were genotyped for polymorphisms in genes regulating mitochondrial function, PPARGC1A (rs8192678) and NDUFB6 (rs540467).
RESULTS
Relatives had slightly lower (P = 0.012) insulin sensitivity than control subjects. In control subjects, ATP synthase flux rose by 18% (P = 0.0001), being 23% higher (P = 0.002) than that in relatives after exercise training. Relatives responding to exercise training with increased ATP synthesis (+19%, P = 0.009) showed improved insulin sensitivity (P = 0.009) compared with those whose insulin sensitivity did not improve. A polymorphism in the NDUFB6 gene from respiratory chain complex I related to ATP synthesis (P = 0.02) and insulin sensitivity response to exercise training (P = 0.05). ATP synthase flux correlated with O2uptake and insulin sensitivity.
CONCLUSIONS
The ability of short-term exercise to stimulate ATP production distinguished individuals with improved insulin sensitivity from those whose insulin sensitivity did not improve. In addition, the NDUFB6 gene polymorphism appeared to modulate this adaptation. This finding suggests that genes involved in mitochondrial function contribute to the response of ATP synthesis to exercise training.
Lifestyle intervention is the recommended strategy for prevention of type 2 diabetes. First-degree relatives of patients with type 2 diabetes (relatives) have an increased risk of insulin resistance and type 2 diabetes (1,2). Inherited and environmental factors cause insulin resistance via intracellular lipid and inflammatory mediators that interfere with insulin signaling, leading to an impaired rise of glucose 6-phosphate (G6P) due to reduced glucose transport/phosphorylation (2,3). These alterations can coexist with excessive storage of intramyocellular lipids (IMCLs) or hepatocellular lipids (HCLs) and impaired mitochondrial function and/or number in insulin-resistant states such as aging (4), with free fatty acid (FFA) elevation (5), and in some (6–8) but not all (9,10) individuals at risk of or with type 2 diabetes. Nondiabetic relatives with severe insulin resistance present with elevated FFAs, IMCLs, and HCLs along with impaired ATP synthesis possibly due to reduced mitochondrial contents (11). Inherited and acquired factors associate with gene expression of the respiratory chain components, NDUFB6 and COX7A1, and their transcriptional coactivators, peroxisome proliferator–activated receptor-γ coactivator-1α/β (PGC-1α/β), which determine maximum oxygen uptake (Vo2max) and insulin action (12,13). It remains unclear whether altered ATP synthesis results from increased availability of lipids and/or adipokines such as adiponectin, the nicotinamide phosphoribosyltransferase visfatin, and retinol-binding protein-4 (RBP-4) (14).
It is also uncertain whether such abnormalities are reversible by exercising and/or occur independently of effects on insulin action. Long-term endurance exercise training increases insulin sensitivity in sedentary young and elderly individuals (15), relatives (16), and glucose-intolerant, obese, or type 2 diabetic individuals (17). Exercise training for at least 4 weeks enhances fat oxidation along with increased mitochondrial mass and enzyme activities (18,19). However, little is known regarding the time course and onset of changes in glucose and energy metabolism independently of acute exercise effects.
We used magnetic resonance spectroscopy (MRS) to measure in vivo flux of inorganic phosphate (Pi) to ATP through ATP synthase (fATPase) as well as IMCLs and HCLs before and after three bouts of cycling training to test the following hypotheses:1) increased fATPase is an early event in the response to short-term exercise training, 2) responses of fATPase and insulin sensitivity are different in relatives compared with healthy control subjects, and 3) the responses are modulated by polymorphisms in genes that are mutually linked to exercise capacity, energy metabolism, and insulin sensitivity in epidemiological studies.
RESEARCH DESIGN AND METHODS
Volunteers.
We recruited healthy relatives of one (n = 19) or two (n = 5) parents with type 2 diabetes, which was confirmed by hyperglycemia, oral antidiabetic medication, or insulin use. Twelve individuals matched for sex, age, BMI, and physical activity were recruited as control subjects (Table 1). A medical history was obtained, and the participants underwent a physical examination. All participants were weight-stable over the 6 months before the study. None of them was smoking or regularly performing intense exercise.
TABLE 1.
Baseline clinical and laboratory characteristics of control subjects and first-degree relatives of type 2 diabetic patients and relative subgroups: RESP and NRES of ATP-synthesis to exercise training
| Control | Relatives | RESP | NRES | |
|---|---|---|---|---|
| n(women/men) | 12 (6/6) | 24 (13/11) | 10 (4/6) | 14 (9/5) |
| Age (years) | 37 ± 11 (30–44) | 40 ± 12 (35–45) | 41 ± 15 (31–52) | 39 ± 11 (33–46) |
| Body weight (kg) | 71 ± 13 (63–79) | 73 ± 14 (68–79) | 76 ± 14 (65–86) | 72 ± 13 (64–79) |
| BMI (kg/m2) | 23 ± 2 (22–25) | 25 ± 4 (24–27) | 26 ± 4 (23–29) | 25 ± 4 (22–27) |
| Waist-to-hip ratio | 0.83 ± 0.08 (0.78–0.88) | 0.85 ± 0.05 (0.83–0.87) | 0.87 ± 0.04 (0.84–0.90) | 0.84 ± 0.05 (0.81–0.87) |
| Systolic blood pressure (mmHg) | 124 ± 19 (112–135) | 126 ± 11 (121–130) | 127 ± 11 (119–135) | 125 ± 12 (118–132) |
| Diastolic blood pressure (mmHg) | 82 ± 10 (76–88) | 83 ± 8 (79–86) | 82 ± 8 (76–88) | 84 ± 8 (79–88) |
| A1C (%) | 5.3 ± 0.3 (5.1–5.5) | 5.4 ± 0.3 (5.3–5.6) | 5.5 ± 0.4 (5.2–5.7) | 5.4 ± 0.3 (5.3–5.6) |
| Triglycerides (mmol/l) | 0.87 ± 0.50 (0.53–1.20) | 1.23 ± 0.70 (0.94–1.53) | 1.32 ± 0.57 (0.91–1.73) | 1.18 ± 0.80 (0.72–1.64) |
| HDL cholesterol (mmol/l) | 1.7 ± 0.45 (1.4–2.0) | 1.5 ± 0.4 (1.3–1.6) | 1.4 ± 0.4 (1.1–1.7) | 1.5 ± 0.4 (1.3–1.7) |
| LDL cholesterol (mmol/l) | 3.0 ± 0.7 (2.5–3.4) | 3.5 ± 0.9 (3.1–3.9) | 3.8 ± 0.9 (3.2–4.4) | 3.3 ± 1.0 (2.8–3.9) |
| Physical activity (scale 1–5) | 2.7 ± 0.3 (2.5–2.9) | 2.9 ± 0.3 (2.7–3.0) | 2.9 ± 0.3 (2.6–3.1) | 2.9 ± 0.3 (2.7–3.1) |
| VO2max(ml · kg−1· min−1) | 33.6 ± 6.0 (29.8–37.5) | 30.6 ± 6.4 (27.8–33.3) | 29.6 ± 5.5 (25.7–33.6) | 31.2 ± 7.1 (27.1–35.3) |
| VO2RCP(ml · kg−1· min−1) | 26.2 ± 4.8 (23.1–29.2) | 22.8 ± 5.7 (20.4–25.2) | 22.1 ± 4.7 (18.7–25.4) | 23.3 ± 6.4 (19.6–27.0) |
Data are means ± SD (95% CI). NRES, not responding to exercise with stimulation of fATPase; RESP, responding to stimulation of fATPase.
Study protocols.
Volunteers gave written informed consent to participate in the study, which was approved by the institutional ethics board and performed according to the Declaration of Helsinki. All participants consumed an isocaloric diet, refrained from any physical exercise for 3 days, and fasted for 12 h before the start of the studies. On day 1, participants underwent a frequent-sampling 75-g oral glucose tolerance test (OGTT) and MRS. On day 2, they performed exercise testing. On days 3 and 5, they exercised on a cycling ergometer. On day 7, measurements of day 1 were repeated in identical fashion.
Dietary assessment.
Throughout the study, all volunteers maintained a dietary plan reflecting an isocaloric diet in line with the American Diabetes Association recommendations. Dietary intake over the last year and dietary compliance were assessed with a modified interviewer-administered 107-item food frequency questionnaire adjusted for local dietary habits (http://www.uni-hohenheim.de/wwwin140/info/interaktives/foodfreq.htm). During the1-week intervention, volunteers counted all food and beverages consumed, using common household measures, to obtain 6-day dietary records. Nutrient/fluid intake on the days before the studies were analyzed using 24-h recalls. In the evenings before the studies, participants consumed identically composed carbohydrate-enriched dinners at identical times. On study days, participants did not receive any calories except for the OGTT until completion of the MRS measurements.
Genotyping.
Genomic DNA was extracted from blood of all participants with a QIAamp DNA Blood Mini kit (catalog no. 51106; Qiagen). Single nucleotide polymorphisms (SNPs), rs540467 of NDUFB6 and rs8192678 (Gly482Ser) of PPARGC1A, were genotyped using allelic discrimination assays performed with an ABI 7900 system (Applied Biosystems). The following assays were used: for rs540467, Assay-on-Demand, C_2334430 (Applied Biosystems); and for rs8192678, forward primer TGGAGAATTGTTCATTACTGAAATCACTGT and reverse primer GGTCATCCCAGTCAAGCTGTTTT together with two different probes, Vic-CAAGACCGGTGAACTG and Fam-ACAAGACCAGTGAACTG, respectively.
OGTT.
Participants drank a solution containing 75 g glucose, and venous blood samples were collected before and in 30-min intervals for 150 min for measurements of glucose, insulin, and C-peptide. Dynamic insulin sensitivity was assessed by oral glucose insulin sensitivity (OGIS) using the 120-min formula, which yields a measure of glucose clearance that has been widely exploited and validated against whole-body insulin sensitivity obtained from the euglycemic-hyperinsulinemic clamp (20). β-Cell function was assessed from the insulinogenic index (21).
MRS.
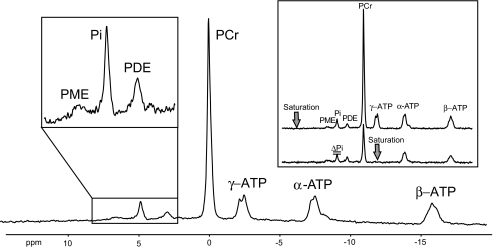
Participants were studied in a 3-T magnetic resonance spectrometer (Bruker, Bremen, Germany). A 10-cm circular double resonant1H/31P surface coil was used for quantifying HCLs and phosphorus metabolites. A 28-cm birdcage coil was positioned over the right lower leg for measuring IMCLs in soleus and tibialis anterior muscles. For nonlocalized31P MRS, the right calf was positioned on the surface coil with the medial head of the right gastrocnemius muscle in the coil center. The integrals of the region of phosphomonoesters covering G6P (7.1–7.4 ppm), phosphodiesters (PDEs), Pi, and phosphocreatine were measured from the ratio of integrated peak intensities and β-ATP resonance intensity in spectra without inversion and saturation, assuming an ATP concentration of 5.5 mmol/l (Fig. 1). The assumption of constant ATP before and after exercise training was supported by unchanged ATP/PDE ratios (data not shown), because PDE levels remain constant under similar conditions (22). Absolute quantification would be required to detect subtle changes of myocellular ATP concentrations, which do not necessarily reflect actual fATPase. The saturation transfer experiment (selective irradiation of γ-ATP) was used to measure the exchange rate (k1) between Pi and ATP and to calculate fATPase from k1 × [Pi ] (5,8) (Fig. 1). IMCLs and HCLs were determined within volumes of interest of 1.73 cm3(23) and 27 cm3(5), respectively.
FIG. 1.
31P magnetic resonance spectrum acquired at 3-T using a surface coil (repetition time = 15 s, number of scans = 16) positioned under the calf muscle of one participant. The spectrum shows intramyocellular phosphomonoesters (PME) including G6P, Pi, PDEs, phosphocreatine (PCr), and ATP. Inset: 31P spectra with saturation of γ-ATP (bottom) and with saturation mirrored around Pi (top), which was always used to account and correct for direct saturation of the resonance frequency pulse.
Habitual physical activity, exercise capacity, and training.
Physical activity was assessed by an interviewer-administered questionnaire on a scale from 1 to 5 (low to high degree of activity) (24). Each participant performed an incremental exhaustive exercise test on an electronically braked cycle ergometer (Lode Excalibur Sport, Groningen, the Netherlands) at 70 revolutions/min. Respiratory gas exchange measures were determined by open-air spirometry (MasterScreen CPX; Jäger/Viasys, Würzburg, Germany). The breath-by-breath measures were recorded and averaged over an interval of eight breath cycles. Heart rate was measured every 5 s (Polar Vantage NV telemetry; Polar Electro, Kempele, Finland). To regulate load intensities of training sessions according to the individual aerobic capacity, a ventilatory threshold (respiratory compensation point [RCP]) was determined (25), which marks the onset of hyperventilation during incremental exercise training driven mainly by the onset of lactic acidosis. RCP was determined independently by two investigators (intraclass correlation, r = 0.9721) from 1) the second upward inflection in the VE (minute ventilation) curve after the first break point at the anaerobic threshold, 2) an upward inflection in the curve of VE/Vco2(ventilatory equivalent for CO2), and 3) a downward inflection in the curve of PE Tco 2 (end-tidal volume for CO2) during the time course of respiratory gas exchange measure variables. The two training sessions (days 2 and 5) consisted of 30 min in three 10-min bouts separated by two 5-min breaks with the training intensity set at 90% work load determined at RCP. Thus, the three bouts of exercise training comprised the exercise testing and the two subsequent training sessions. All participants completed exercise testing, and all training sessions, which were performed under continuous supervision by a sports physiologist. Data analysis revealed variable fATPase responses to exercise training among relatives compared with control subjects. Relatives were therefore divided into two subgroups based on the difference of fATPase between baseline and after exercise training: responder (n = 10) with (fATPaseafter – fATPasebefore) >0 and nonresponder (n = 14) with (fATPaseafter – fATPasebefore) ≤0.
Analytical procedures.
Plasma glucose was measured on a Glucose Analyzer II (Beckman Coulter;http://www.beckmancoulter.com). For FFA measurement (Wako Chemicals USA;http://www.wakousa.com/), blood was collected into vials containing orlistat to prevent in vitro lipolysis. Lactate was determined enzymatically (Roche;http://www.roche.com/home.html), insulin and C-peptide by double-antibody radioimmunoassay and RBP-4 and visfatin in seven control subjects and eight relatives by ELISA (Phoenix Pharmaceuticals, Karlsruhe, Germany;www.phoenixpeptide.com/), with inter- and intra-assay coefficients of variation of <6% (5).
Statistics.
OGIS and fATPase were defined as the primary end points. The sample size calculation was based on previous studies on fATPase using false-positive (Zα = 1.96, two-tailed) and false-negative error rates (Zβ = 0.84, one-tailed), respectively. Data are presented as means ± SD and 95% CIs (text and tables) or means ± SE (figures). Unpaired/paired two-tailed t tests were used for between- and within-group comparisons as appropriate. Bivariate correlations were assessed with a Pearson correlation coefficients. Multiple stepwise linear regression analysis was performed for the dependent variables, fATPase and OGIS, including BMI, waist-to-hip ratio, age, triglycerides, FFAs, physical activity, Vo2max, HCLs, IMCLs, and caloric intake and also for changes in the above parameters during the study. All calculations were done using SPSS for Windows (version 8; SPSS, Chicago, IL;http://www.spss.com). Associations between genotypes and exercise responses for fATPase and OGIS were analyzed with the χ2test (NCSS Statistical Software, Kaysville, UT).
RESULTS
Clinical characteristics, exercise testing, and dietary intake.
The groups showed similar sex distribution, age, and fat mass and distribution (Table 1). Laboratory tests, exercise performance, and physical activity including its individual components (data not shown) were not different between groups and subgroups. Energy and nutrient intake were not different between groups before and during the 24 h before studies (Table 2). The 6-day dietary records confirmed similar nutrient composition (supplementaryTable 1, available at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1240/DC1). Body weights did not change in control subjects (before 71.50 ± 12.82 vs. after 71.35 ± 12.75 kg) or in relatives (73.46 ± 13.54 vs. 73.06 ± 13.22 kg).
TABLE 2.
Nutrient intake obtained from 24-h dietary recalls in control subjects and relatives
| Control subjects |
Relatives |
|||
|---|---|---|---|---|
| Before | After training | Before | After training | |
| Caloric intake (kcal/day) | 2,274 ± 843 (1,738–2,809) | 2,045 ± 904 (1,470–2,619) | 1,937 ± 614 (1,671–2,203) | 1,820 ± 557 (1,579–2,061) |
| % of daily energy intake | ||||
| Carbohydrate (%) | 39 ± 11 (32–46) | 43 ± 10 (37–50) | 44 ± 12 (39–49) | 44 ± 9 (40–48) |
| Fat (%) | 40 ± 10 (34–47) | 37 ± 8 (32–42) | 37 ± 10 (33–41) | 35 ± 8 (31–38) |
| Protein (%) | 17 ± 6 (14–21) | 17 ± 6 (13–20) | 17 ± 6 (15–20) | 19 ± 5 (16–21) |
| Saturated fat (%) | 18 ± 6 (14–21) | 17 ± 4 (15–19) | 16 ± 5 (14–18) | 15 ± 5 (13–17) |
| n-3 fatty acids (%) | 1 ± 0 (1–2) | 1 ± 1 (1–2) | 1 ± 1 (–1) | 1 ± 0 (–1) |
| n-6 fatty acids (%) | 5 ± 3 (3–7) | 4 ± 2 (3–5) | 5 ± 2 (4–6) | 6 ± 3 (5–7) |
| Cholesterol (g/day) | 0.38 ± 0.17 (0.27–0.50) | 0.38 ± 0.20 (0.26–0.51) | 0.34 ± 0.22 (0.25–0.44) | 0.30 ± 0.17 (0.22–0.37) |
Data are means ± SD (95% CI). Dietary data after training were not available from one relative who lost the dietary record. There were no statistical differences within and between groups.
Metabolites, hormones, insulin sensitivity, and secretion.
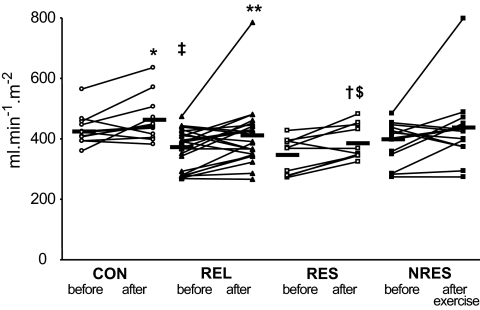
Fasting plasma glucose did not differ between groups (Table 3). The 2-h plasma glucose after oral glucose loading was within the normal range, slightly higher in relatives than in control subjects before (6.3 ± 1.4 vs. 5.0 ± 1.1 mmol/l, P = 0.009) but not after exercise (4.8 ± 1.2 vs. 5.5 ± 1.2 mmol/l). Plasma FFAs were comparable at baseline but were higher in relatives after training (P = 0.019). C-peptide was higher in relatives before (P = 0.006) and after exercise training (P = 0.031). Fasting plasma insulin tended to be increased in relatives. In subgroups, plasma RBP-4 (before training: control subjects 0.36 ± 0.12 and relatives 0.41 ± 0.07 ng/ml; after training: 0.36 ± 0.08 and 0.43 ± 0.09 ng/ml) and visfatin (before training: control subjects 39 ± 26 and relatives 29 ± 9 ng/ml; after training: 35 ± 13 and 26 ± 6 ng/ml) did not differ. At baseline, OGIS was 13% lower (P = 0.012) in relatives than in control subjects and rose slightly in both control subjects by 7% (P = 0.05) and in relatives by 12% (P = 0.012) after training (Fig. 2). The quantitative insulin-sensitivity check and insulinogenic indexes were comparable before and after training in both groups (Table 3).
TABLE 3.
Responses of relatives and of RESP and NRES of ATP synthesis to exercise training
| Before exercise | After exercise | |
|---|---|---|
| Glucose (mmol/l) | ||
| Control subjects | 4.9 ± 0.3 (4.7–5.1) | 4.9 ± 0.3 (4.7–5.1) |
| Relatives | 5.0 ± 0.4 (4.8–5.1) | 5.0 ± 0.4 (4.8–5.1) |
| RESP | 5.0 ± 0.4 (4.8–5.3) | 5.0 ± 0.4 (4.7–5.3) |
| NRES | 4.9 ± 0.4 (4.7–5.2) | 5.0 ± 0.5 (4.7–5.2) |
| Lactate (mmol/l) | ||
| Control subjects | 1.1 ± 0.3 (0.9–1.3) | 1.0 ± 0.2 (0.8–1.1)* |
| Relatives | 1.5 ± 0.5 (1.3–1.7)** | 1.3 ± 0.5 (1.0–1.5)* |
| RESP | 1.3 ± 0.5 (1.0–1.7) | 1.2 ± 0.3 (0.9–1.4) |
| NRES | 1.6 ± 0.4 (1.3–1.8) | 1.3 ± 0.6 (1.0–1.6) |
| FFAs (μmol/l) | ||
| Control subjects | 411 ± 190 (290–531) | 502 ± 154 (404–600) |
| Relatives | 443 ± 160 (376–511) | 540 ± 204 (450–631)* |
| RESP | 488 ± 195 (348–628) | 510 ± 204 (364–655) |
| NRES | 408 ± 128 (338–486) | 566 ± 209 (434–699)* |
| Insulin (pmol/l) | ||
| Control subjects | 27 ± 8 (22–32) | 23 ± 7 (19–27) |
| Relatives | 41 ± 34 (27–55) | 39 ± 31 (26–52) |
| RESP | 36 ± 22 (21–52) | 36 ± 20 (22–50) |
| NRES | 45 ± 41 (22–69) | 40 ± 37 (19–62) |
| C-peptide (nmol/l) | ||
| Control subjects | 1.6 ± 0.4 (1.3–1.8) | 1.5 ± 0.4 (1.3–1.8) |
| Relatives | 2.3 ± 0.8 (1.9–2.6)** | 2.2 ± 1.0 (1.8–2.6)§ |
| RESP | 2.4 ± 0.8 (1.9–3.0) | 2.3 ± 1.0 (1.6–3.0) |
| NRES | 2.2 ± 0.8 (1.7–2.7) | 2.1 ± 1.0 (1.6–2.7) |
| QUICKI | ||
| Control subjects | 0.51 ± 0.05 (0.48–0.54) | 0.52 ± 0.04 (0.50–0.54) |
| Relatives | 0.47 ± 0.07 (0.45–0.50) | 0.48 ± 0.07 (0.46–0.51) |
| RESP | 0.48 ± 0.06 (0.44–0.52) | 0.48 ± 0.05 (0.44–0.51) |
| NRES | 0.47 ± 0.07 (0.43–0.51) | 0.49 ± 0.08 (0.45–0.53) |
| Insulinogenic index | ||
| Control subjects | 4.3 ± 1.5 (3.3–5.2) | 6.1 ± 4.2 (3.4–8.8) |
| Relatives | 4.8 ± 2.4 (3.8–5.9) | 6.0 ± 4.7 (4.0–8.0) |
| RESP | 4.3 ± 2.7 (2.4–6.2) | 5.0 ± 3.6 (2.4–7.5) |
| NRES | 5.2 ± 2.2 (4.0–6.5) | 6.8 ± 5.4 (3.7–9.9) |
Data are means ± SD (95% CI).
*P< 0.05 before vs. after exercise;
**P< 0.05 control subjects vs. relatives at baseline;
§P< 0.05 control subjects vs. relatives after exercise training. NRES, not responding to exercise with stimulation of fATPase; QUICKI, quantitative insulin-sensitivity check index; RESP, responding to exercise with stimulation of fATPase.
FIG. 2.
Dynamic insulin sensitivity as assessed from the OGTT (OGIS) in individuals without (CON, n = 12) or with (REL, n = 24) first-degree relatives with type 2 diabetes and in relative subgroups responding (RESP, n = 10) or not responding (NRES, n = 14) with increased ATP synthesis after exercise training sessions. Black horizontal bars indicate mean values of the respective groups. *P = 0.049 CON before versus after; **P = 0.012 REL before versus after; † P = 0.009 RESP before versus after; ‡ P = 0.012 CON versus REL before; § P = 0.003, CON versus RESP before; $P = 0.031 CON versus RESP after exercise.
In subgroups, responding or not responding to exercise with stimulation of fATPase, fasting glucose, lactate, insulin, insulinogenic index C-peptide (Table 3), and 2-h postload plasma glucose (data not shown) were comparable before and after training. Plasma FFAs were similar at baseline and increased after exercise training by 39% in nonresponders (P = 0.008). OGIS was comparable at baseline but increased only in responders upon exercise training (P = 0.009) (Fig. 2).
Intramyocellular phosphorus metabolism and ectopic lipids.
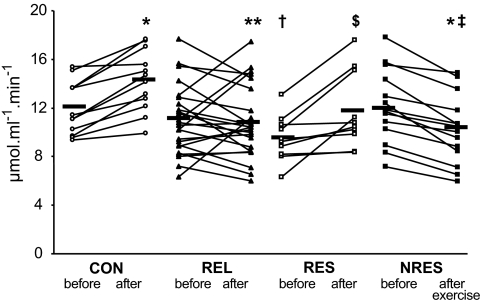
Baseline fATPase did not differ between groups (control subjects 12.0 ± 2.2 vs. relatives 11.1 ± 2.9 μmol · ml−1· muscle−1 · min−1) (Fig. 3). After training, fATPase increased by ∼18% (P = 0.0001) only in control subjects (14.2 ± 2.5 μmol · ml−1· muscle−1 · min−1) and was ∼23% (P = 0.002) higher than in relatives (10.9 ± 3.0 μmol · ml−1 · muscle−1 · min−1), in whom it did not change upon exercise training (Fig. 3). Similarly, k1rose in control subjects (P = 0.001) but not in relatives (P = 0.7). HCLs tended to be higher in relatives before training (control subjects 1.5 ± 1.0 vs. relatives 5.8 ± 7.5%, P = 0.08) (Fig. 4) but were not different after training (control subjects 1.8 ± 1.0 vs. relatives 4.4 ± 5.4%). IMCLs (Fig. 4) and G6P (data not shown) were not different between groups or affected by training.
FIG. 3.
Skeletal muscle fATPase in individuals without (CON, n = 12) or with (REL, n = 24) first-degree relatives with type 2 diabetes and in REL subgroups responding (RESP, n = 10) or not responding (NRES, n = 14) with increased ATP synthesis after exercise training sessions. Black horizontal bars indicate mean values of the respective groups. *P < 0.001 CON and NRES before versus after; **P = 0.002, CON versus REL after; † P = 0.010 CON versus RESP before; $P = 0.009 RESP before versus after; § P = 0.024 RESP versus NRES before; ‡ P < 0.001 CON versus NRES after exercise.
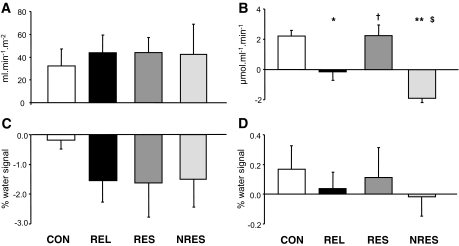
FIG. 4.
Absolute changes (Δ; means ± SE) in dynamic insulin sensitivity (OGIS) (A), fATPase (B), and lipid concentrations in liver (HCL) (C) and soleus muscle (IMCL) (D) in individuals without (CON, n = 12) or with (REL, n = 24) first-degree relatives with type 2 diabetes and in REL subgroups responding (RESP, n = 10) or not responding (NRES, n = 14) with increased ATP synthesis after exercise training sessions. *P = 0.005 CON versus REL; **P < 0.001 RESP versus NRES; § P < 0.001 CON versus NRES; † P = 0.014 REL versus RESP; $P = 0.024 REL versus NRES.
Interestingly, baseline fATPase was higher (P = 0.025) in nonresponders than in responders before exercise training, but by definition only increased in responders by ∼24% (P = 0.009) and decreased in nonresponders by ∼16% (P < 0.001). Similarly, k1 rose in responders (P = 0.01) and decreased in nonresponders (P = 0.02). HCLs and IMCLs in soleus muscle were comparable before and after the two training sessions. Baseline IMCLs in tibialis anterior muscle was similar but decreased by 35% (P = 0.006) in nonresponders after training (data not shown).
Baseline fATPase related positively to Vo2(r = 0.300, P = 0.046) and power output (r = 0.361, P = 0.030) each at maximum and RCP. This finding also held true for fATPase after exercise training (data not shown). Baseline fATPase correlated positively with OGIS (r = 0.360, P = 0.031). HCLs related negatively to OGIS (r = −0.675, P < 0.001) and positively to plasma insulin (r = 0.761, P < 0.001). Multiple regression analysis revealed that HCLs and baseline caloric intake explained ∼49% of the variation in baseline OGIS (P = 10−5). Changes in fATPase (before versus after exercise sessions) were neither related to changes in OGIS (r = 0.001, P = 0.993) nor to changes in caloric intake (before versus mean caloric intake during the intervention week; r = 0.078, P = 0.665), which did not relate to each other, respectively (r = −0.201, P = 0.261). Multiple regression analysis did not identify any anthropometric, dietary, or laboratory parameters as independent predictors of the postexercise changes in fATPase.
Associations between SNPs in the NDUFB6 and PPARGC1A genes and exercise responses of fATPase and insulin sensitivity.
The NDUFB6 SNP, rs540467 G/A, was associated with resistance to stimulation of fATPase and OGIS upon exercise training (Table 4). Exercise training increased fATPase in 74% of the G/G carriers but only in 33% of those carrying the A allele (P = 0.02 for a dominant model). More G/G genotype carriers (84%) increased OGIS after exercise training than A allele carriers (53%, P = 0.05 for a dominant model). The PPARGC1A SNP (rs8192678, Gly482Ser) related to neither fATPase nor OGIS responses to exercise training (Table 4).
TABLE 4.
Association between SNPs in the NDUFB6(rs540467) and PPARGC1A(rs8192678,Gly482Ser) genes and response to exercise training regarding stimulation of fATPase and dynamic insulin sensitivity (OGIS) in RESP and NRES
| G/G | G/A | A/A | P values | |
|---|---|---|---|---|
| NDUFB6 rs540467 | ||||
| fATPase | ||||
| RESP | 14 | 4 | 1 | 0.02* |
| NRES | 5 | 9 | 1 | |
| OGIS | ||||
| RESP | 16 | 7 | 1 | 0.05* |
| NRES | 3 | 6 | 1 |
| Gly/Gly | Gly/Ser | Ser/Ser | P values | |
|---|---|---|---|---|
| PPARGC1A rs8192678 | ||||
| fATPase | ||||
| RESP | 9 | 10 | 0 | 0.24 |
| NRES | 7 | 6 | 2 | |
| OGIS | ||||
| RESP | 11 | 11 | 2 | 0.64 |
| NRES | 5 | 5 | 0 |
χ2tests were performed to analyze associations between genotypes and response to exercise for fATPase and OGIS.
*P value indicating significance for a dominant model. NRES, not responding to exercise with stimulation of fATPase; RESP, responding to exercise with stimulation of fATPase.
DISCUSSION
At baseline, relatives and control subjects did not differ in parameters commonly interfering with insulin sensitivity and fATPase including age, fat mass, physical activity, and aerobic capacity. Nevertheless, relatives were slightly less insulin sensitive based on OGIS, which corresponds to M values of ∼10 mg · kg−1 · min−1. The small difference in OGIS is in line with the variability of insulin sensitivity in overweight individuals (26), healthy humans, and type 2 diabetic relatives (16) obtained with clamp tests. Severely insulin-resistant offspring of type 2 diabetic mothers exhibit increases in plasma FFAs, body fat mass, IMCLs, and HCLs, along with impaired insulin-stimulated glucose transport/phosphorylation (11). Such individuals have ∼30% lower fATPase along with ∼40% lower muscle mitochondrial density than healthy humans. In our study, fATPase was similar to that of the present and previous control subjects (4,5,8), suggesting that their ATP production suffices for nonexercising conditions. This finding is in agreement with unchanged mRNA and protein expression of PGC-1α, regulators of mitochondrial biogenesis, and their downstream effectors in insulin-resistant relatives (11). However, comparable baseline fATPase values between relatives and control subjects do not allow conclusions on differences in mitochondrial number/function but may simply reflect normal basal metabolic rates in both groups. Although overall mitochondrial function can be reduced in relatives (11), it was found to be normal in patients with type 2 diabetes at baseline (7,8,27,28) and was only reduced upon insulin stimulation (7,8). Several groups have further shown dissociation between the level of markers for mitochondrial content and in vitro oxidative capacity. Some researchers (29,30) but not others (6,9) found differences in mitochondrial function adjusted for mitochondrial mass.
This study demonstrates that short-term exercise training 1) uniformly raises myocellular basal metabolic rate as assessed with fATPase independently of insulin sensitivity in healthy humans and 2) does not affect mean fATPase in relatives but 3) identifies subgroups of relatives with different exercise responses. In some relatives, these conditions unmask a variation to increase ATP production, reflecting altered adaptation of the basal metabolic rate to short-term exercise training. In the face of normal fasting glucose and FFAs, hereditary factors seem to play an important role, as this variation in response to exercise training was also seen in carriers of the A allele of the NDUFB6 SNP, rs540467, which has been associated with impaired insulin action and type 2 diabetes risk (12).
Several mechanisms could explain the rise in fTAPase in control subjects and in responders. Whereas long-term exercise training possibly stimulates fat oxidation (18,19) and electron transport chain activity (31) through increased mitochondrial size/number (19,32), it is unlikely that mitochondrial biogenesis contributed to the rise in fATPase after a short duration of training. Of note, it is conceivable that even if mitochondrial capacity is impaired, putative regulators of oxidative phosphorylation could increase and maintain the balance between ATP demand and supply and thereby baseline fATPase. We detected a variation occurring despite normal basalfATPase in insulin-sensitive relatives. In nonresponders, the decreased fATPase affected adaptation of the myocellular basal metabolic rate and could therefore reflect reduced need or sufficient capability to supply ATP to meet energy demands or result from impaired oxidative ATP production (33).
This study also shows that IMCLs were not different from baseline at 48 h after 1 week of three bouts of exercise. Interestingly, nonresponders had higher plasma FFAs, suggesting that increased lipid availability could contribute to impaired fATPase (5), although this might alternatively lead to increased mitochondrial function and biogenesis as demonstrated in rodent models (34,35). Fat oxidation may rise regardless of insulin sensitivity even after 1 week of exercise training because of increased expression of enzymes involved in lipid metabolism (36). At 48 h after one bout of exercise, insulin-stimulated glucose disposal (16,26) and IMCLs can be increased or unchanged (17,37). Within 12 h after moderate- to high-intensity exercise, whole-body oxygen consumption returns to preexercise levels (38,39), whereas both IMCL resynthesis and lipid oxidation continued to rise up to >40 h (38,39). The postexercise rise in fATPase would thereby result from increased ATP demand for augmented lipid turnover. Of note, a portion of the Pi/ATP exchange may be catalyzed by glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase. Although the contribution of anaerobic glycolysis to muscular ATP production is relevant during initial states of exercise and short-time high-intensity exercise, it ceases in the absence of muscle contraction and during aerobic resting conditions (40,41). Of note, despite the known negative relationship between HCLs and insulin sensitivity at baseline (14), the insulin sensitivity improved after exercise training despite no reductions in IMCLs and HCLs. This is an important finding of the study, pointing to independent regulation of insulin sensitivity and ectopic lipids by short-term exercise training.
The absent fATPase response in nonresponders was recorded at 48 h after the last bout of exercise, when insulin secretion, G6P, pH, and IMCLs were not altered in all groups. Thus, acute effects occurring within the first 24 h after exercise such as stimulation of intramyocellular glucose transport/phosphorylation (42) and IMCL depletion (26,37) probably did not contribute to fATPase alterations. Of note, exercise training slightly but significantly stimulated insulin sensitivity in both groups in agreement with previous studies in relatives of type 2 diabetic patients (16). Insulin-resistant relatives responded to 6-week of endurance training with increased insulin sensitivity and substrate oxidation (10,16). On the other hand, combined diet and training for 2 weeks was required to improve insulin sensitivity and IMCLs in type 2 diabetes (43). However, in the present study, insulin sensitivity failed to explain the fATPase response, suggesting that effects of short-term exercise training on mitochondrial function and insulin sensitivity can be dissociated in skeletal muscle. Although one cannot exclude the possibility that mitochondrial oxidation rises before changes in insulin sensitivity in healthy humans, previous studies measuring muscular metabolites and enzyme activities reported that lactate, phosphocreatine hydrolysis, and glycogen depletion are reduced after 5 days of training, whereas maximal succinate dehydrogenase activity as a surrogate of mitochondrial function increased only after 31 days (44). As insulin action was impaired in endurance-trained individuals who stop exercise training, despite unchanged oxidative capacity (45), mechanisms other than oxidative capacity such as muscle glycogen synthesis probably contribute to changes in OGIS. At 48 h after one bout of exercising, overweight insulin-resistant relatives improved their insulin-stimulated glucose disposal associated with increased glycogen synthesis (16). This result suggests that increased glycogen storage mainly accounts for improved insulin action after exercise (46). Because our patients were not consuming a hypercaloric diet, their glycogen stores were probably not replenished to preexercise levels, which is known to be an important modulator of insulin sensitivity for at least 72 h after the last exercise session (46).
The A allele carriers of the NDUFB6 SNP, rs540467, showed a variation in the response to exercise in line with an inherited difference. NDUFB6 is among the genes encoding the respiratory chain, which show lower muscular expression in type 2 diabetic patients than in control subjects (47). This SNP was also associated with resistance to an increase in OGIS after exercise training in line with a reported relationship with glucose disposal and risk of type 2 diabetes (12). The present study further supports the important role for this gene in ATP production in humans in vivo but cannot indicate whether these adaptations were related to changes in mitochondrial number, localization, or function. In this context it is of note that activation of peroxisome proliferator–activated receptors might improve insulin sensitivity by transcriptional control of mitochondrial function because of enhanced fatty acid oxidation and regulation of aerobic capacity. The Botnia study identified a common variant in the peroxisome proliferator-activated receptor-γ gene (P12A) as one of the best predictors of future development of type 2 diabetes (48). Recent data provide evidence that even within 300 min after one bout of exercise expression and activation of PGC-1α, AMP-dependent protein kinase phosphorylation, nuclear respiratory factor-1, and cytochrome c oxidase are increased in lean but not in insulin-resistant obese humans (49). Although previous studies suggested roles for PPARGC1A in oxidative phosphorylation, insulin action, Vo2max, and exercise response (12,13,47,49), in the present study we did not find such associations for the PPARGC1A Gly482Ser gene variant probably because of lack of statistical power. Also, longer duration of training might be needed to reveal the effects of this gene variant, as PPARGC1A Gly482Ser predicts endurance capacity (50).
The present study has some limitations. First, quantitative differences from previous studies might be due to the exercise protocols, degree of insulin resistance, or the individual exercise capacity. To control for variations in exercise capacity, participants were exercising at 90% of their RCP, the highest work load at which oxidative phosphorylation is adequate for energy demand (51). Second, minor changes in amount and timing of caloric intake relative to exercise could have modulated OGIS and fATPase (52), but unchanged caloric intake and identical timing of meals render this possibility unlikely. Third, glucose ingestion during the OGTT before MRS measurements could have differentially affected fATPase. However, the absence of differences in plasma glucose between groups at 150 min of the OGTT and the start of MRS after a further 2 hours and fATPase does not support such an effect. Fourth, fATPase could have been affected by alterations of ATP concentrations, which can increase up to 18 h after exercise (53). Such a rise in ATP would have led to lower fATPase values, thereby underestimating the actual ATP synthase flux. However, the observation of unchanged ATP/PDE ratios and the timing of measurement at 48 h after the last bout of exercise do not support alterations of ATP levels. Moreover, exchange rates, k1, which do not depend on substrate concentrations, behaved similarly to fATPase between and within groups before and after training, underlining the fact that the observed differences reflect variation in ATP synthase flux. Finally, no muscle biopsies were taken so that neither mass-adjusted mitochondrial function nor possible changes in the expression of transcriptional modulators could be assessed. Unchanged plasma RBP-4 and visfatin do not exclude altered expression/activity of these factors.
In conclusion, training-induced increases of insulin sensitivity and ATP synthesis indicate an important role of early adaptation of basal metabolic rate in healthy humans. Conversely, some relatives do not stimulate ATP production in response to short-term exercise training nor do they have improved insulin sensitivity, and these same individuals carry a risk polymorphism in the NDUFB6 gene.
Supplementary Material
Acknowledgments
This study was supported by grants from the European Foundation for the Study of Diabetes (EFSD; Novo Nordisk and GlaxoSmithKline Grants), Austrian Science Foundation (P15656), Austrian National Bank (OENB 11459), and Hochschuljubiläumsstiftung Vienna, by unrestricted grants from Novo Nordisk and Baxter to M.R., by grants from the Swedish Research Council and the Wallenberg Foundation to L.G., by a Research Grant Award by the Austrian Diabetes Association to G.K.-B., and a grant from Regione Veneto (Biotech DGR 2702/10-09-04) to G.P.
L.G. has been a consultant for and served on advisory boards for sanofi-aventis, GlaxoSmithKline, Novartis, Merck, Tethys Bioscience, and Xoma and received lecture fees from Lilly and Novartis. No other potential conflicts of interest relevant to this article were reported.
We acknowledge the cooperation of Attila Brehm, Hannes Wondratsch, Herbert Dworak, and Peter Nowotny. We are also grateful to Wilfried Grossmann, Faculty of Computer Science, University of Vienna, Vienna, Austria, for his expert advice and critical analysis of the statistical evaluation of the data and to Margareta Svensson for extracting DNA.
Footnotes
The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Clinical trial reg. no. NCT00710008, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, Groop L: Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1989; 321: 337– 343 [DOI] [PubMed] [Google Scholar]
- 2.Roden M, Shulman GI: Applications of NMR spectroscopy to study muscle glycogen metabolism in man. Annu Rev Med 1999; 50: 277– 290 [DOI] [PubMed] [Google Scholar]
- 3.Szendroedi J, Roden M: Mitochondrial fitness and insulin sensitivity in humans. Diabetologia 2008; 51: 2155– 2167 [DOI] [PubMed] [Google Scholar]
- 4.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI: Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003; 300: 1140– 1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M: Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006; 55: 136– 140 [PubMed] [Google Scholar]
- 6.Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002; 51: 2944– 2950 [DOI] [PubMed] [Google Scholar]
- 7.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS: Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 2003; 100: 7996– 8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M: Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007; 4: e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F: Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007; 50: 790– 796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostergard T, Andersen JL, Nyholm B, Lund S, Nair KS, Saltin B, Schmitz O: Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 2006; 290: E998– E1005 [DOI] [PubMed] [Google Scholar]
- 11.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI: Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005; 115: 3587– 3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling C, Poulsen P, Simonsson S, Ronn T, Holmkvist J, Almgren P, Hagert P, Nilsson E, Mabey AG, Nilsson P, Vaag A, Groop L: Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin Invest 2007; 117: 3427– 3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling C, Wegner L, Andersen G, Almgren P, Hansen T, Pedersen O, Groop L, Vaag A, Poulsen P: Impact of the peroxisome proliferator activated receptor-γ coactivator-1β (PGC-1β) Ala203Pro polymorphism on in vivo metabolism, PGC-1β expression and fibre type composition in human skeletal muscle. Diabetologia 2007; 50: 1615– 1620 [DOI] [PubMed] [Google Scholar]
- 14.Roden M: Mechanisms of disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2006; 2: 335– 348 [DOI] [PubMed] [Google Scholar]
- 15.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO: Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 1992; 72: 1780– 1786 [DOI] [PubMed] [Google Scholar]
- 16.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI: Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996; 335: 1357– 1362 [DOI] [PubMed] [Google Scholar]
- 17.Kiens B: Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 2006; 86: 205– 243 [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO, Coyle EF: Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 1984; 56: 831– 838 [DOI] [PubMed] [Google Scholar]
- 19.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE: Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 2005; 288: E818– E825 [DOI] [PubMed] [Google Scholar]
- 20.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ: A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001; 24: 539– 548 [DOI] [PubMed] [Google Scholar]
- 21.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT: Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967; 46: 323– 335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newcomer BR, Boska MD: Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 1997; 20: 336– 346 [DOI] [PubMed] [Google Scholar]
- 23.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI: Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999; 42: 113– 116 [DOI] [PubMed] [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE: A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36: 936– 942 [DOI] [PubMed] [Google Scholar]
- 25.Beaver WL, Wasserman K, Whipp BJ: A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020– 2027 [DOI] [PubMed] [Google Scholar]
- 26.Jamurtas AZ, Theocharis V, Koukoulis G, Stakias N, Fatouros IG, Kouretas D, Koutedakis Y: The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur J Appl Physiol 2006; 97: 122– 126 [DOI] [PubMed] [Google Scholar]
- 27.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P: Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 2007; 50: 113– 120 [DOI] [PubMed] [Google Scholar]
- 28.Trenell MI, Hollingsworth KG, Lim EL, Taylor R: Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care 2008; 31: 1644– 1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K: Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007; 56: 1592– 1599 [DOI] [PubMed] [Google Scholar]
- 30.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE: Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005; 54: 8– 14 [DOI] [PubMed] [Google Scholar]
- 31.Holloszy JO, Oscai LB, Don IJ, Mole PA: Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 1970; 40: 1368– 1373 [DOI] [PubMed] [Google Scholar]
- 32.Toledo FG, Watkins S, Kelley DE: Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 2006; 91: 3224– 3227 [DOI] [PubMed] [Google Scholar]
- 33.Weiss RG, Gerstenblith G, Bottomley PA: ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 2005; 102: 808– 813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO: High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 2008; 105: 7815– 7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ: Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007; 56: 2085– 2092 [DOI] [PubMed] [Google Scholar]
- 36.Putman CT, Jones NL, Hultman E, Hollidge-Horvat MG, Bonen A, McConachie DR, Heigenhauser GJ: Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol 1998; 275: E132– E139 [DOI] [PubMed] [Google Scholar]
- 37.Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI: Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab 2000; 85: 748– 754 [DOI] [PubMed] [Google Scholar]
- 38.Kiens B, Richter EA: Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol 1998; 275: E332– E337 [DOI] [PubMed] [Google Scholar]
- 39.Schenk S, Horowitz JF: Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 2007; 117: 1690– 1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quistorff B, Johansen L, Sahlin K: Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 1993; 291 ( Pt. 3): 681– 686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA: In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 2006; 577: 353– 367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price TB, Perseghin G, Duleba A, Chen W, Chase J, Rothman DL, Shulman RG, Shulman GI: NMR studies of muscle glycogen synthesis in insulin-resistant offspring of parents with non-insulin-dependent diabetes mellitus immediately after glycogen-depleting exercise. Proc Natl Acad Sci USA 1996; 93: 5329– 5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R: Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191– 3196 [DOI] [PubMed] [Google Scholar]
- 44.Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM: Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol 1996; 270: E265– E272 [DOI] [PubMed] [Google Scholar]
- 45.Vukovich MD, Arciero PJ, Kohrt WM, Racette SB, Hansen PA, Holloszy JO: Changes in insulin action and GLUT-4 with 6 days of inactivity in endurance runners. J Appl Physiol 1996; 80: 240– 244 [DOI] [PubMed] [Google Scholar]
- 46.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO: Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol 1989; 256: E494– E499 [DOI] [PubMed] [Google Scholar]
- 47.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267– 273 [DOI] [PubMed] [Google Scholar]
- 48.Lyssenko V, Almgren P, Anevski D, Orho-Melander M, Sjogren M, Saloranta C, Tuomi T, Groop L: Genetic prediction of future type 2 diabetes. PLoS Med 2005; 2: e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ: Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 2008; 294: E607– E614 [DOI] [PubMed] [Google Scholar]
- 50.Lucia A, Gomez-Gallego F, Barroso I, Rabadan M, Bandres F, San Juan AF, Chicharro JL, Ekelund U, Brage S, Earnest CP, Wareham NJ, Franks PW: PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J Appl Physiol 2005; 99: 344– 348 [DOI] [PubMed] [Google Scholar]
- 51.Billat VL, Sirvent P, Py G, Koralsztein JP, Mercier J: The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med 2003; 33: 407– 426 [DOI] [PubMed] [Google Scholar]
- 52.Stephens BR, Braun B: Impact of nutrient intake timing on the metabolic response to exercise. Nutr Rev 2008; 66: 473– 476 [DOI] [PubMed] [Google Scholar]
- 53.Kimber NE, Heigenhauser GJ, Spriet LL, Dyck DJ: Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J Physiol 2003; 548: 919– 927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.