Abstract
OBJECTIVE
Regeneration of the insulin-secreting β-cells is a fundamental research goal that could benefit patients with either type 1 or type 2 diabetes. β-Cell proliferation can be acutely stimulated by a variety of stimuli in young rodents. However, it is unknown whether this adaptive β-cell regeneration capacity is retained into old age.
RESEARCH DESIGN AND METHODS
We assessed adaptive β-cell proliferation capacity in adult mice across a wide range of ages with a variety of stimuli: partial pancreatectomy, low-dose administration of the β-cell toxin streptozotocin, and exendin-4, a glucagon-like peptide 1 (GLP-1) agonist. β-Cell proliferation was measured by administration of 5-bromo-2′-deoxyuridine (BrdU) in the drinking water.
RESULTS
Basal β-cell proliferation was severely decreased with advanced age. Partial pancreatectomy greatly stimulated β-cell proliferation in young mice but failed to increase β-cell replication in old mice. Streptozotocin stimulated β-cell replication in young mice but had little effect in old mice. Moreover, administration of GLP-1 agonist exendin-4 stimulated β-cell proliferation in young but not in old mice. Surprisingly, adaptive β-cell proliferation capacity was minimal after 12 months of age, which is early middle age for the adult mouse life span.
CONCLUSIONS
Adaptive β-cell proliferation is severely restricted with advanced age in mice, whether stimulated by partial pancreatectomy, low-dose streptozotocin, or exendin-4. Thus, β-cells in middle-aged mice appear to be largely postmitotic. Young rodents may not faithfully model the regenerative capacity of β-cells in mature adult mice.
β-Cell mass normally grows well into adulthood to provide increased insulin secretion capacity to match the greater insulin requirements of maturity (1,2). β-Cell mass can slowly expand in adult rodents in response to increased insulin requirements (3) or during pregnancy (4). Several mechanisms have been invoked to explain adult β-cell mass expansion, including neogenesis from pancreatic ducts or hematopoietic tissues, replication of specialized β-cell progenitors, and self-renewal by β-cells (5–7). However, the findings of recent studies by several groups (including ours) indicate that normal β-cell growth primarily occurs by self-renewal of mature β-cells—not by replication of specialized progenitors (8–12). This regenerative capacity has prompted speculation that regeneration of β-cell function might someday be possible in adult patients with diabetes (8). However, β-cell regeneration has proven to be an ambitious and elusive goal. Sadly, expansion of bona fide human β-cells has not been convincingly demonstrated (13–15). Why is β-cell regeneration easily stimulated in rodents, yet difficult to achieve in humans? Is rodent β-cell replication regulated in fundamentally different ways compared with that of humans? Or is mammalian β-cell replication limited by unrealized factors? Could age be a factor?
In the past, rodent β-cells were widely assumed to have a very short life span and to require ongoing turnover (3,16). In contrast, we recently discovered that aged mice have very little evidence of β-cell turnover (17). We therefore hypothesized that cell cycle entry of β-cells could be restricted with age. To test this hypothesis, we investigated β-cell regeneration as a function of age in adult mice. Here, we show that adaptive β-cell proliferation capacity is severely restricted with advanced age.
RESEARCH DESIGN AND METHODS
All experiments with mice were performed in the animal facility at The Children's Hospital of Philadelphia according to the guidelines of the Institutional Animal Care and Use Committee (IACUC). The first set of experiments included male F1 hybrid B6129SF1/J mice (stock 101043), obtained at 1 and 8 months of age from The Jackson Laboratory (Bar Harbor, ME). The Jackson B6129SF1/J hybrid is the product of an intercross between C57BL/6J (000664) female mice and 129S1/SvImJ (002448) male mice from The Jackson Laboratory's commercial colonies. The second set of experiments used male F1 hybrid B6129SF1/J mice obtained at 1 month of age from the Taconic Farms (Hudson, NY). The Taconic B6129F1 hybrid model is the product of an intercross between C57BL/6NTac (black 6) female mice and 129S6/SvEvTac (129S6) male mice from Taconic commercial colonies.
Partial pancreatectomy.
Partial pancreatectomy was performed, as previously reported (9). The splenic portion of the pancreas was surgically removed, resulting in an ∼50% pancreatectomy. A sham operation was performed by opening the abdomen but leaving the pancreas intact.
Low-dose streptozotocin administration and exendin-4 treatment.
Mice received five daily injections of low-dose (30 mg/kg) streptozotocin using established protocols (9,18–21). Exendin-4 was administered as previously described (9). Mice were injected daily with 24 nmol/kg body wt in the subcutaneous space daily for 21 days.
Statistics.
All results are reported as means ± SE for equivalent groups. Results were compared with independent Student's t tests (unpaired and two-tailed) reported as P values.
Proliferation analysis.
Mice were continuously labeled with 5-bromo-2′-deoxyuridine (BrdU) for 14 days after partial pancreatectomy or low-dose streptozotocin or for 21 days during exendin-4 treatment before they were killed. BrdU was administered in drinking water at 1 mg/ml as previously described (9). Paraformaldehyde-fixed, paraffin-embedded sections were stained with DAPI/insulin/BrdU or DAPI/insulin/BrdU/Ki67 as previously described (9). Images were acquired from 10–20 islets per animal and condition, which represented 900–4,000 β-cells per animal. To measure Ki67, images were acquired from more than 100–200 islets per animal and condition, which represented 4,000–8,000 β-cells per animal. Acinar cell proliferation analysis was performed by acquiring 10 random images per animal as previously described (17). In all but one group, the results represent the average from four to seven animals (the partial pancreatectomy cohort at 19 months included only three mice). β-Cell proliferation was calculated per day by dividing BrdU-positive β-cells by the labeling period (14 or 21 days) and expressed as percent total per day.
Islet morphometry.
β-Cell area was quantified as previously described (9). β-Cell area was measured in all three groups of the partial pancreatectomy experiment: sham-operated, pancreatectomy-removed (the splenic portion), and residual (the duodenal portion) sections.
RT-PCR.
Partial pancreatectomy or sham operation was performed on mice of various ages. Five days later, islets were isolated from pools of at least four mice and processed into cDNA as previously described (22). Real-time quantitative dual fluorescent–labeled fluorescence resonance energy transfer (FRET) PCR (50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min) was performed with the ABI 7900 real-time PCR thermal cycler (Applied Biosystems, Foster City, CA) to amplify triplicate samples, comparing sample values with islet log10dilution curves. Relative gene product amounts were reported for each gene compared with cyclophillin and confirmed in separate studies with a large panel of control genes (for primer sequences, see supplemental Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1198/DC1). Results are reported from a single experiment with technical repeats averaged and reported as means ± SEM. Representative RT-PCR data were confirmed in an independent experiment with pools of islet mRNAs derived from equivalent groups of mice.
Immunoblotting.
Cells were lysed in Tween-20 buffer. Proteins were resolved on denaturing polyacrylamide gels, electrophoretically transferred to polyvinylidine fluoride (PVDF) membranes (Immobilon-P Transfer Membrane; Millipore, Bedford, MA), and blotted with mouse anti-cyclin D2 ab-4 (Lab Vision, Thermo Fisher Scientific, Fremont, CA) and α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA).
RESULTS
Partial pancreatectomy is well tolerated in mice across a wide range of ages.
To test whether adaptive β-cell proliferation is restricted with age, we chose 50% partial pancreatectomy, a robust model to induce β-cell proliferation (9). Partial pancreatectomy modestly reduces β-cell mass while leaving sufficient β-cell function for normal control of glucose homeostasis. As expected, partial pancreatectomy was well tolerated across a wide range of ages. Blood glucose values and postoperative weight loss were equivalent in pancreatectomized and sham-operated mice (Table 1). The resected portion of pancreas comprised one-half of the total pancreatic weight at each age-group (Table 2). Thus, splenic partial pancreactomy is a reliable and well-tolerated procedure in adult mice of all ages.
TABLE 1.
Partial pancreatectomy and high-dose exendin-4 treatment
| Age (months) | Body weight (g) in The Jackson Laboratory cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham operated (control) |
Partial pancreatectomy |
|||||||
| Initial | Final | Change | Initial | Final | Change | P | ||
| 2 | 30.9 ± 1.1 | 25.6 ± 1.4 | 5.3 ± 0.5 | 30.2 ± 0.9 | 26.0 ± 0.8 | 4.2 ± 0.2 | 0.387 | |
| 8 | 38.1 ± 1.1 | 33.0 ± 0.4 | 5.1 ± 0.7 | 36.9 ± 1.4 | 31.9 ± 1.5 | 5.0 ± 0.4 | 0.307 | |
| 12 | 42.2 ± 2.5 | 35.7 ± 1.7 | 6.5 ± 1.5 | 41.7 ± 2.5 | 33.3 ± 0.9 | 8.0 ± 2.4 | 0.914 | |
| 14 | 45.3 ± 2.6 | 37.1 ± 1.7 | 8.2 ± 1.1 | 36.4 ± 6.6 | 28.2 ± 4.7 | 8.1 ± 2.4 | 0.749 | |
| 19 | 48.9 ± 2.4 | 44.7 ± 2.2 | 4.1 ± 0.9 | 46.9 ± 4.0 | 40.6 ± 3.5 | 4.4 ± 0.7 | 0.923 | |
| Age (months) | Body glucose (mg/dl) in The Jackson Laboratory cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham operated (control) |
Partial pancreatectomy |
|||||||
| Initial | Final | Average | Initial | Final | P | Average | P | |
| 2 | 135 ± 10 | 122 ± 9 | 121 ± 9 | 135 ± 11 | 145 ± 10 | 0.117 | 143 ± 5 | 0.063 |
| 8 | 121 ± 7 | 120 ± 11 | 114 ± 7 | 137 ± 7 | 122 ± 9 | 0.857 | 123 ± 4 | 0.307 |
| 12 | 136 ± 7 | 134 ± 13 | 131 ± 4 | 132 ± 9 | 137 ± 9 | 0.838 | 131 ± 7 | 0.914 |
| 14 | 139 ± 9 | 131 ± 6 | 124 ± 6 | 131 ± 6 | 108 ± 8 | 0.794 | 121 ± 4 | 0.749 |
| 19 | 115 ± 8 | 132 ± 6 | 125 ± 6 | 109 ± 7 | 139 ± 9 | 0.783 | 125 ± 3 | 0.923 |
| Age (months) | Body weight (g) in the Taconic Farms cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham operated (control) |
Partial pancreatectomy |
|||||||
| Initial | Final | Change | Initial | Final | Change | P | ||
| 2 | 31.7 ± 1.1 | 30.6 ± 0.9 | 1.1 ± 0.9 | 29.7 ± 0.4 | 28.4 ± 1.1 | 1.3 ± 1.4 | 0.928 | |
| 19 | 42.4 ± 5.1 | 39.1 ± 5.7 | 3.3 ± 1.1 | 42.0 ± 2.7 | 36.8 ± 1.4 | 5.2 ± 1.7 | 0.401 | |
| Age (months) | Body glucose (mg/dl) in the Taconic Farms cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham operated (control) |
Partial pancreatectomy |
|||||||
| Initial | Final | Average | Initial | Final | P | Average | P | |
| 2 | 134 ± 12 | 126 ± 12 | 128 ± 8 | 127 ± 7 | 132 ± 11 | 0.117 | 131 ± 7 | 0.570 |
| 19 | 94 ± 9 | 100 ± 11 | 98 ± 8 | 86 ± 9 | 103 ± 11 | 0.783 | 92 ± 5 | 0.605 |
Data are means ± SEM for four to seven mice per group except the 19-month-old partial pancreatectomy cohort, which consisted of only three mice. Body weight of mice before and 2 weeks after a sham operation (top left) or partial pancreatectomy (top right). Random-fed blood glucose values for 2 weeks following sham operation or partial pancreatectomy, expressed as average values (middle left) or area under the curve (middle right).
TABLE 2.
β-Cell morphometry
| Age (months) |
|||||
|---|---|---|---|---|---|
| 2 | 8 | 12 | 14 | 19 | |
| Pancreatic weight (mg) | |||||
| Sham operated (control) | 253 ± 13 | 301 ± 19 | ND | 331 ± 6 | 386 ± 24 |
| Partial pancreatectomy | |||||
| Resected (splenic) | 127 ± 5 | 169 ± 15 | ND | 184 ± 3 | 187 ± 9 |
| Remaining (duodenal) | 108 ± 32 | 113 ± 18 | ND | 204 ± 6 | 212 ± 27 |
| β-Cell area (%) | |||||
| Sham operated (control) | 0.61 ± 0.09 | 0.85 ± 0.17 | ND | 1.27 ± 0.28 | 2.04 ± 0.36 |
| Partial pancreatectomy | |||||
| Resected (splenic) | 0.72 ± 0.11 | 1.05 ± 0.13 | ND | 1.90 ± 0.61 | 2.60 ± 0.61 |
| Remaining (duodenal) | 0.60 ± 0.04 | 0.79 ± 0.12 | ND | 1.28 ± 0.20 | 2.07 ± 0.55 |
| β-Cell mass (mg) | |||||
| Sham operated (control) | |||||
| Whole pancreas | 1.57 ± 0.26 | 2.46 ± 0.41 | ND | 4.15 ± 0.89 | 7.89 ± 1.59 |
| Partial pancreatectomy | |||||
| Resected (splenic) | 0.98 ± 0.14 | 1.81 ± 0.31 | ND | 3.48 ± 1.12 | 4.76 ± 1.07 |
| Remaining (duodenal) | 0.78 ± 0.23 | 0.93 ± 0.23 | ND | 2.59 ± 0.36 | 4.72 ± 1.75 |
| Acinar proliferation (% BrdU/total) | |||||
| Sham operated (control) | 0.72 ± 0.08 | 0.57 ± 0.07 | ND | 0.41 ± 0.07 | 0.53 ± 0.11 |
| Partial pancreatectomy | 1.47 ± 0.28 | 1.64 ± 0.14 | ND | 1.62 ± 0.24 | 1.07 ± 0.1 |
| P | 0.04 | 0.0002 | ND | 0.001 | 0.02 |
Data are means ± SEM for four to seven mice per group except the 19-month-old partial pancreatectomy cohort, which consisted of only three mice. Data show pancreatic weight of sham-operated, resected, or remaining pancreas following partial pancreatectomy, expressed as milligrams; β-cell area of sham-operated, resected (splenic portion), or remaining pancreas (duodenal portion) following partial pancreatectomy, expressed as percent total pancreas; β-cell mass of sham-operated, resected, or remaining pancreas following partial pancreatectomy, expressed as percent total pancreas; and acinar proliferation of sham-operated, resected, or remaining pancreas following partial pancreatectomy, expressed as percent total pancreas. ND, not determined.
Partial pancreatectomy is a robust stimulus of β-cell proliferation in young mice.
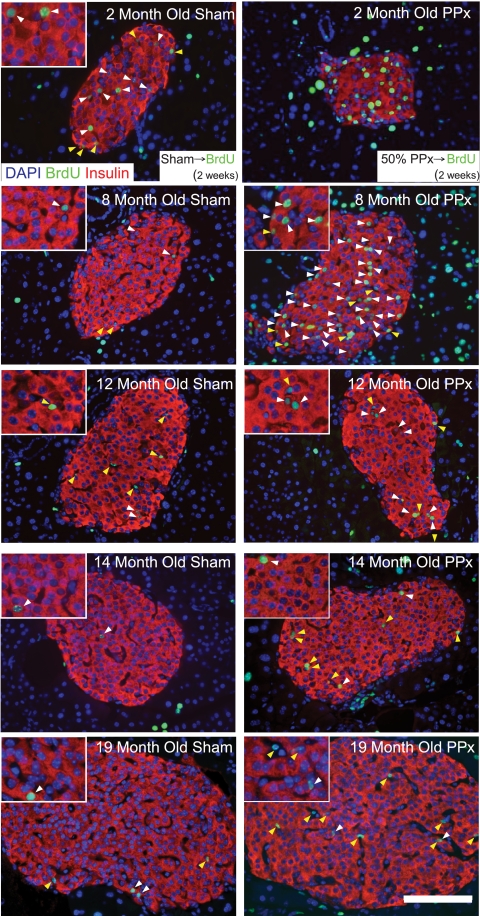
We then tested whether partial pancreatectomy stimulates β-cell regeneration. To detect all proliferation events during acute β-cell regeneration, mice continuously received BrdU in the drinking water for 2 weeks after pancreatectomy and were then killed immediately. Partial pancreatectomy stimulated β-cell proliferation in mice aged 2 months (from 0.18 ± 0.05 to 1.80 ± 0.36% per day after partial pancreatectomy; P = 0.003) (Figs. 1 and 3). Partial pancreatectomy also stimulated β-cell proliferation in mice aged 8 months compared with control mice (from 0.08 ± 0.02 to 1.06 ± 0.39% per day after partial pancreatectomy; P = 0.04) (Figs. 1 and 3). Notably, the change in total number of BrdU-positive β-cells (the absolute increase in β-cell proliferation) was less in mice aged 8 months than in mice aged 2 months. Still, our results indicate that β-cell regeneration capacity is retained well into maturity, even in mice aged 8 months.
FIG. 1.
Partial pancreatectomy (PP)–induced β-cell replication in mice at 2, 8, 12, 14, and 19 months of age. BrdU was administered for 2 weeks after the procedure before the mice were killed. Representative pancreatic β-cell histology of pancreas sections immunostained with antibodies against insulin (red) and BrdU (green) and counterstained with DAPI (blue) and photographed with a 40× objective. White arrows indicate insulin and BrdU copositive cells; yellow arrows denote BrdU-labeled non–insulin-containing cells within the islet. Scale bars: 100 μm in full image and 20 μm within inset. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 3.
Quantitative analysis of β-cell regeneration following partial pancreatectomy, low-dose streptozotocin, or exendin-4 as a function of age in mice. Results are expressed as BrdU-positive β-cells (% total per day) and represent means ± SEM (n = 4–6 animals per group). *P < 0.05; **P < 0.01; ***P < 0.001 sham vs. partial pancreatectomy at various ages. P <0.05 shams at 2 months vs. shams at 12 months, 14 months, or 19 months.
Minimal β-cell proliferation in aged mice after partial pancreatectomy.
To test the hypothesis that β-cell regeneration is restricted with advanced age, we analyzed β-cell proliferation in aged mice after partial pancreatectomy. Surprisingly, partial pancreatectomy failed to stimulate β-cell proliferation in mice aged 19 months (from 0.03 ± 0.01 to 0.03 ± 0.00% per day after partial pancreatectomy; P = 0.84) (Figs. 1 and 3). This result suggests that β-cell regeneration capacity could be very limited in aged mice.
Our data indicate that partial pancreatectomy–induced β-cell regeneration capacity is restricted in mice aged 19 months. To confirm and refine this observation, we repeated our studies with middle-aged mice. Interestingly, mice aged 12 months displayed an intermediate phenotype. Partial pancreatectomy stimulated β-cell proliferation in mice aged 12 months. However, the change in total number of BrdU-positive β-cells (the absolute increase in β-cell proliferation) was very small (from 0.03 ± 0.01 to 0.25 ± 0.09% per day after partial pancreatectomy; P = 0.03) (Figs. 1 and 3). Similar to results obtained in very old mice, partial pancreatectomy failed to substantially increase β-cell proliferation in mice aged 14 months (from 0.02 ± 0.01 to 0.05 ± 0.02% per day after partial pancreatectomy; P = 0.31) (Figs. 1 and 3). Taken together, our results illustrate that partial pancreatectomy–induced β-cell proliferation capacity is retained well into maturity. However, partial pancreatectomy–induced β-cell proliferation capacity is powerfully and abruptly restricted by early middle age (12 months of age represent ∼40% of the typical mouse life span) (23).
We then considered the possibility that partial pancreatectomy might not adequately reduce β-cell mass in aged mice. In particular, we were concerned that unequal distribution of β-cells within the pancreas could influence partial pancreatectomy–mediated β-cell proliferation. If β-cell mass grew more rapidly in the head (duodenal) than in the tail (splenic) with advanced age, removal of the splenic portion of the pancreas might not efficiently reduce β-cell mass in aged mice. We quantified β-cell mass reduction after partial pancreatectomy, measuring β-cell area and β-cell mass in pancreata of sham-operated (the entire pancreas), resected (the splenic portion), and remaining (the duodenal portion) samples in each age cohort. The total β-cell area and β-cell area mass continuously increased with age in control mice (Table 2). β-Cell area and mass also continuously increased with age in resected pancreata. Similarly, β-cell area and mass continuously increased in the remaining pancreata after partial pancreatectomy. Moreover, β-cell area and mass was roughly equivalent in the resected and residual pancreata of each age-group, indicating that partial pancreatectomy reduced β-cell mass by approximately one-half. Thus, splenic partial pancreatectomy reduces β-cell mass by equivalent amounts in young and old mice. As a result, β-cell reduction by partial pancreatectomy should be a robust stimulus of β-cell regeneration in mice across all ages.
Preserved acinar cell regeneration in aged mice.
To investigate whether regeneration of other pancreatic components is also restricted with age, we tested acinar regeneration after partial pancreatectomy. Confirming previous reports, partial pancreatectomy robustly stimulated occasional patches of acinar cell regeneration in very young mice (from 0.72 ± 0.08 to 1.47 ± 0.28% per day after partial pancreatectomy at 2 months of age; P = 0.04) (Table 2) (images not shown). Partial pancreatectomy also stimulated acinar cell regeneration in very old mice aged 19 months (from 0.53 ± 0.11 to 1.07 ± 0.10% per day after partial pancreatectomy; P = 0.02) (Table 2). Similarly, partial pancreatectomy stimulated acinar cell regeneration at 8 months and 14 months of age (Table 2). These results indicate that acinar replication capacity may not be influenced by age. Thus, age-dependent restriction of regeneration capacity of β-cells is not common to all pancreatic components.
Minimal β-cell proliferation in aged mice after low-dose streptozotocin.
We then used the β-cell toxin streptozotocin to further test the hypothesis that β-cell regeneration capacity is restricted with advanced age. Streptozotocin is a robust stimulus of β-cell regeneration when administered in multiple low doses to adult mice (24). Low-dose streptozotocin was well tolerated and did not cause hyperglycemia or extensive weight loss. As expected, streptozotocin administration stimulated β-cell proliferation in mice aged 2 months by 0.36% per day compared with controls (from 0.18 ± 0.05 to 0.55 ± 0.07% per day after streptozotocin; P = 0.003) (Figs. 2 and 3). However, streptozotocin had little effect in aged mice: β-cell proliferation was only slightly increased in mice aged 15 months compared with controls (from 0.02 ± 0.01 to 0.04 ± 0.01% per day after streptozotocin; P = 0.17) (Figs. 2 and 3).
FIG. 2.
Low-dose streptozotocin (Stz) and exendin-4 induced β-cell replication in mice at 2 and 14–15 months of age. BrdU was administered for 2 weeks before the mice were killed. A: Low-dose streptozotocin. B: Exendin-4. Representative pancreatic β-cell histology of pancreas sections immunostained with antibodies against insulin (red) and BrdU (green) and counterstained with DAPI (blue) and photographed with a 40× objective. White arrows indicate insulin and BrdU copositive cells; yellow arrows denote BrdU-labeled non–insulin-containing cells within the islet. Scale bars: 100 μm in full image and 20 μm within inset. (A high-quality digital representation of this figure is available in the online issue.)
Minimal β-cell proliferation in aged mice after exendin-4treatment.
We hypothesized that glucagon-like peptide 1 (GLP-1)–stimulated β-cell proliferation might also be restricted in aged mice. To test this corollary hypothesis, we treated mice with exendin-4, a GLP-1 agonist, using established protocols (9,18–21). Exendin-4 was fairly well tolerated and was not associated with severe weight loss. Exendin-4 robustly stimulated β-cell proliferation in mice aged 2 months (0.76 ± 0.07% per day with exendin-4 treatment; P = 0.00001 compared with sham-operated controls) (Figs. 2 and 3). By contrast, exendin-4 treatment resulted in little β-cell proliferation in 14-month-old mice (0.08 ± 0.03% per day with exendin treatment; P = 0.13 compared with sham-operated controls) (Figs. 2 and 3). Notably, the change in total number of BrdU-positive β-cells' (the absolute increase in β-cell proliferation) response to exendin-4 was ∼10-fold greater in young mice than in old mice. Thus, GLP-1–stimulated β-cell regeneration is also restricted in aged mice.
Basal β-cell proliferation declines in aged mice.
The observation that adaptive β-cell proliferation is severely restricted with advanced age implies that β-cells could undergo cell cycle exit as a function of age (equivalent to cellular quiescence). To further test this hypothesis, we compared basal (nonstimulated) β-cell proliferation rates in control mice across a range of ages. Confirming our previous observations (17), basal β-cell proliferation decreased from 2 to 8 months (from 0.18 ± 0.05 to 0.08 ± 0.02% per day; P = 0.008) (Figs. 1 and 3). Basal β-cell proliferation further decreased by 12 months (to 0.03 ± 0.01% per day; P = 0.03) and remained low in 14- and 19-month-old mice (P = 0.01 at 14 months compared with 2 months; P = 0.03 at 19 months compared with 2 months). Thus, basal β-cell proliferation was fully restricted by 12 months of age, which was rapidly followed by restriction of partial pancreatectomy–induced β-cell regeneration.
Minimal β-cell proliferation in aged mice after partial pancreatectomy in an independent genetic cohort.
Our studies on aging were initially performed with F1 hybrid B6129SF1/J mice from The Jackson Laboratory. We chose this strain because it closely approximates the mixed genetic background of laboratory knockout mice (commonly derived from SV129-derived embryonic stem cells and crossed into c57B6). However, we could not rule out the possibility that our results are unique to the F1 hybrid B6129SF1/J strain from The Jackson Laboratory. Consequently, we performed additional studies on aging in a similar but separate genetic cohort, Taconic Farms F1 hybrid c57 SV129 mice. Because the Jackson and Taconic Farms lines have significantly diverged over the years (25), the Taconic Farms F1 hybrid c57 SV129 mice represent a similar but genetically distinct lineage compared with Jackson F1 hybrid B6129SF1/J mice. Consequently, the Taconic Farms F1 hybrid c57 SV129 mice should also approximate the mixed genetic background of laboratory knockout mice. As previously indicated, partial pancreatectomy was well tolerated in the Taconic cohort at 2 and 19 months of age. Partial pancreatectomy robustly induced β-cell regeneration in young Taconic mice (from 0.24 ± 0.09 to 1.33 ± 0.26% per day after partial pancreatectomy; P = 0.009) (Fig. 4). In contrast, partial pancreatectomy had no effect on β-cell proliferation in aged Taconic mice (from 0.020 ± 0.003 compared with 0.019 ± 0.003% per day after partial pancreatectomy; P = 0.84) (Fig. 4). These results independently confirm our observations in the Jackson cohort and further illustrate that partial pancreatectomy–induced β-cell regeneration becomes severely restricted in aged mice. Moreover, these additional studies indicate that our findings may be broadly applicable to genetically engineered mice, which frequently have a mixed c57 SV129 genetic background.
FIG. 4.
Taconic cohort. Partial pancreatectomy (PP) induced β-cell replication in mice at 2 and 19 months of age. BrdU was administered for 2 weeks after the procedure before the mice were killed. A: Representative pancreatic β-cell histology of pancreas sections immunostained with antibodies against insulin (red) and BrdU (green) and counterstained with DAPI (blue) and photographed with a 40× objective. White arrows indicate insulin and BrdU copositive cells; yellow arrows denote BrdU-labeled non–insulin-containing cells within the islet. Scale bars: 100 μm in full image and 20 μm within inset. B: Quantitative analysis of β-cell regeneration following partial pancreatectomy as a function of age in mice. Results are expressed as percent BrdU-positive β-cells per day and represent means ± SEM (n = 4–6 animals per group). **P < 0.01 sham vs. partial pancreatectomy at 2 months. P <0.05 shams at 2 months vs. shams at 19 months. (A high-quality digital representation of this figure is available in the online issue.)
No evidence for BrdU toxicity to proliferating β-cells.
Our results indicate that partial pancreatectomy–induced β-cell regeneration capacity may be severely restricted with advanced age. However, we were concerned about potential BrdU toxicity, which could theoretically reduce β-cell proliferation. Continuous low-dose BrdU administration in the drinking water is generally well tolerated and does not severely reduce β-cell proliferation compared with short-term infusions of high-dose BrdU (17). However, Hellerstein and colleagues (26) recently reported that continuous BrdU administration reduces proliferation of islet components by ∼25%, as measured by heavy water incorporation into DNA. Consequently, we tested for BrdU-associated toxicity by assessing β-cell proliferation in our cohort, as measured by the presence of Ki67. We administered BrdU in the drinking water or control water to a cohort of 1-month-old mice for 2 weeks, after which they were killed. Reassuringly, Ki67-positive β-cells were equivalent in BrdU-treated and untreated pancreata (2.29 ± 0.31 vs. 2.51 ± 0.25%, respectively; P = 0.59) (Fig. 5). Thus, prolonged infusion of BrdU does not influence β-cell proliferation, as measured by Ki67 expression. This result indicates that limited β-cell regeneration in aged mice cannot be readily explained by BrdU toxicity to proliferating β-cells.
FIG. 5.
Low-dose-BrdU is not toxic to proliferating β-cells. BrdU was continuously administered in the drinking water for 2 weeks to a cohort of mice aged 1 month that were then compared with untreated control mice. A: Representative pancreatic β-cell histology of pancreas sections immunostained with antibodies against insulin (yellow), Ki67 (red), and BrdU (green) and counterstained with DAPI (blue) and photographed with a 40× objective. White arrows indicate insulin and BrdU copositive cells; red arrows denote insulin and Ki67 copositive cells. Scale bars: 100 μm in full image and 20 μm within inset. B: Quantitative analysis of β-cell proliferation as measured by Ki67 incorporation. Continuous BrdU treatment does not slow β-cell proliferation. Results are expressed as percent Ki67-positive β-cells and represent means ± SEM (n = 5 animals per group). (A high-quality digital representation of this figure is available in the online issue.)
Aging islets exhibit gene expression program changes consistent with reduced cell cycle entry.
To further characterize the role of aging in adaptive β-cell proliferation capacity, we compared gene expression in islets from young (2 months) and aged (14 months) mice. Cyclin D2, the most abundant cyclin in islets, did not change expression with age (supplementalFig. 1A). Similarly, cyclin D2 protein expression was unaltered in aged islets compared with young islets (supplementalFig. 1B). Although expression of many cyclins did not change, a few of the weakly expressing cyclins such as D3, E1, and E2 actually increased with age (supplementalFig. 1A). Notably, islets from aged mice had greater basal expression of cell cycle inhibitors, including p21Cip1, p27Kip1, p16Ink4a, p15Ink4b, p15Ink4b, and Rb (supplemental Fig. 1A). p16Ink4a is differentially expressed in aging tissues and has been shown to restrict islet growth (27,28). Thus, differential expression of negative regulators of cell cycle such as p16Ink4a or p15Ink4b could potentially explain the age-dependent decline in adaptive β-cell proliferation capacity. Taken together, these results confirm the observation that partial pancreatectomy–stimulated β-cell proliferation is severely restricted in islets from aged mice.
DISCUSSION
We observe that β-cell regeneration is severely and abruptly restricted by middle age in our cohort of mice. Fifty percent partial pancreatectomy stimulated a massive amount of β-cell proliferation in young mice. However, partial pancreatectomy had little effect on β-cell proliferation in aged mice. Similarly, the β-cell toxin streptozotocin greatly increased β-cell replication in young mice but failed to stimulate β-cell regeneration in aged mice. Moreover, β-cell proliferation was stimulated by exendin-4 in young but not in aged mice. Taken together, these results reveal that adaptive β-cell proliferation is severely restricted with advanced age.
In this study, we advance the hypothesis that age is a major factor limiting human β-cell regeneration. Basal β-cell proliferation was severely reduced as a function of age in our mice, consistent with our previous observations (17). We further this observation to show that adaptive β-cell proliferation is also severely restricted with age. In support of this concept, basal replication rates in human pancreata and cultured human islets decline with donor age (29–31). Similarly, islets from young donors have been reported to perform better when transplanted into type 1 diabetic patients (32). Notably, type 2 diabetes is typically a disease of the elderly, most commonly diagnosed during middle age or beyond. Similarly, gestational diabetes mellitus is much more frequent with advanced maternal age (33,34). This would imply that human patients of advanced age could have little regenerative capacity to increase β-cell mass. Indeed, there is indirect evidence to support this concept of an age-dependent decline in β-cell regeneration. For instance, increased rates of diabetes have been reported in patients after shock-wave lithotripsy for renal stones (35). Similarly, minimal β-cell regeneration is observed in patients after partial pancreatectomy (15). Thus, β-cell regeneration could be constrained in advanced age in humans, similar to that in the aged rodents in our study. Restricted β-cell mass expansion could have severe consequences in elderly patients with type 2 diabetes, limiting compensatory β-cell mass expansion to cope with increased insulin requirements. Interestingly, several of the recently discovered risk loci for type 2 diabetes have been implicated in cell cycle control of β-cells and could theoretically influence adult β-cell mass or alter the timing of cell cycle exit of adult β-cells (36–39).
Discrepancies between rodent and human β-cell regeneration capacity have confounded diabetes researchers for many years. Regeneration of β-cell function in experimental animal models has been widely observed in rodents but remains elusive and controversial in humans (13,14). Notably, islets from human cadaveric donors are typically in the 4th–6th decade of life and are therefore much more mature (32). Because our results in rodents indicate that β-cell regeneration capacity declines with age, we hypothesize that young rodents may not faithfully model the regenerative capacity of mature adult human β-cells.
Our studies reveal that the regenerative capacity of adult β-cells becomes limited by early middle age (12 months of age [∼40% of the mouse life span]) (23). As such, aging β-cells may not be comparable with hematopoietic stem cells, which gradually lose replicative capacity during the normal aging process (40). Under this schema, β-cells could have a developmental program that allows them to replicate early in adulthood to match insulin secretion capacity to peripheral insulin requirements. β-Cell replication might then become fully restricted when adult insulin requirements are established in middle age.
Supplementary Material
Acknowledgments
This work was supported by a research grant from the Juvenile Diabetes Research Foundation International (to J.A.K.). Additional support was provided by the National Institutes of Health (grants K08-DK064101, R03-DK078546, and R01-DK081469), a March of Dimes Basil O'Connor Starter Scholar Research Award, a Lawson Wilkins Pediatric Endocrine Society Clinical Scholar Award, a Charles H. Hood Foundation Child Health Research Grant, funds from The Children's Hospital of Philadelphia, and a University of Pennsylvania Diabetes and Endocrinology Research Center pilot and feasibility grant (DK19525).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying original article, p. 1312.
REFERENCES
- 1.Heit JJ, Karnik SK, Kim SK: Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol 2006; 22: 311– 338 [DOI] [PubMed] [Google Scholar]
- 2.Ackermann AM, Gannon M: Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 2007; 38: 193– 206 [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S: β-Cell turnover: its assessment and implications. Diabetes 2001; 50( Suppl. 1): S20– S24 [DOI] [PubMed] [Google Scholar]
- 4.Parsons JA, Brelje TC, Sorenson RL: Adaptation of islets to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992; 130: 1459– 1466 [DOI] [PubMed] [Google Scholar]
- 5.Levine F, Itkin-Ansari P: beta-cell Regeneration: neogenesis, replication or both? J Mol Med 2008; 86: 247– 258 [DOI] [PubMed] [Google Scholar]
- 6.Xu X, D'Hoker J, Stange G, Bonne S, De Lu N, Xiao X, De Casteele MV, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008; 132: 197– 207 [DOI] [PubMed] [Google Scholar]
- 7.Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T: Weighing up beta-cell mass in mice and humans: self-renewal, progenitors or stem cells? Mol Cell Endocrinol 2008; 288: 79– 85 [DOI] [PubMed] [Google Scholar]
- 8.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41– 46 [DOI] [PubMed] [Google Scholar]
- 9.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007; 12: 817– 826 [DOI] [PubMed] [Google Scholar]
- 10.Brennand K, Huangfu D, Melton D: All beta cells contribute equally to islet growth and maintenance. PLoS Biol 2007; 5: e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117: 2553– 2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA: Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 2007; 117: 971– 977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharfmann R: Expanding human beta cells. Diabetologia 2008; 51: 692– 693 [DOI] [PubMed] [Google Scholar]
- 14.Meier JJ: Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia 2008; 51: 703– 713 [DOI] [PubMed] [Google Scholar]
- 15.Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ: Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 2008; 57: 142– 149 [DOI] [PubMed] [Google Scholar]
- 16.Finegood DT, Scaglia L, Bonner-Weir S: Dynamics of β-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 1995; 44: 249– 256 [DOI] [PubMed] [Google Scholar]
- 17.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA: Very slow turnover of β-cells in aged adult mice. Diabetes 2005; 54: 2557– 2567 [DOI] [PubMed] [Google Scholar]
- 18.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM: Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999; 42: 45– 50 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ: beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 2005; 54: 482– 491 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ: Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003; 278: 471– 478 [DOI] [PubMed] [Google Scholar]
- 21.De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA: Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 2003; 52: 365– 371 [DOI] [PubMed] [Google Scholar]
- 22.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF: Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005; 25: 3752– 3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DE, Archer JR: Genetic differences in effects of food restriction on aging in mice. J Nutr 1987; 117: 376– 382 [DOI] [PubMed] [Google Scholar]
- 24.Like AA, Rossini AA: Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976; 193: 415– 417 [DOI] [PubMed] [Google Scholar]
- 25.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG: Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav 2004; 3: 149– 157 [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Turner S, Tsang E, Stark J, Turner H, Mahsut A, Keifer K, Goldfinger M, Hellerstein MK: Measurement of pancreatic islet cell proliferation by heavy water labeling. Am J Physiol Endocrinol Metab 2007; 293: E1459– E1464 [DOI] [PubMed] [Google Scholar]
- 27.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE: Ink4a/Arf expression is a biomarker of aging. J Clin Invest 2004; 114: 1299– 1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE: p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 2006; 443: 453– 457 [DOI] [PubMed] [Google Scholar]
- 29.Tyrberg B, Eizirik DL, Hellerstrom C, Pipeleers DG, Andersson A: Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology 1996; 137: 5694– 5699 [DOI] [PubMed] [Google Scholar]
- 30.Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY: Aging correlates with decreased β-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes 2006; 55: 2455– 2462 [DOI] [PubMed] [Google Scholar]
- 31.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC: β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008; 57: 1584– 1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihm SH, Matsumoto I, Sawada T, Nakano M, Zhang HJ, Ansite JD, Sutherland DE, Hering BJ: Effect of donor age on function of isolated human islets. Diabetes 2006; 55: 1361– 1368 [DOI] [PubMed] [Google Scholar]
- 33.Mestman JH: Outcome of diabetes screening in pregnancy and perinatal morbidity in infants of mothers with mild impairment in glucose tolerance. Diabetes Care 1980; 3: 447– 452 [DOI] [PubMed] [Google Scholar]
- 34.Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, Francis MM, Jain JK: Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA 2002; 288: 2320– 2323 [DOI] [PubMed] [Google Scholar]
- 35.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW: Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol 2006; 175: 1742– 1747 [DOI] [PubMed] [Google Scholar]
- 36.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K: A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 2007; 39: 770– 775 [DOI] [PubMed] [Google Scholar]
- 37.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341– 1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S: Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007; 316: 1331– 1336 [DOI] [PubMed] [Google Scholar]
- 40.Geiger H, Van Zant G: The aging of lympho-hematopoietic stem cells. Nat Immunol 2002; 3: 329– 333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.