Abstract
OBJECTIVE
Glucose-dependent insulinotropic polypeptide (GIP), unlike glucagon-like peptide (GLP)-1, lacks glucose-lowering properties in patients with type 2 diabetes. We designed this study to elucidate the underlying pathophysiology.
RESEARCH DESIGN AND METHODS
Twenty-two insulin-naïve subjects with type 2 diabetes were given either synthetic human GIP (20 ng · kg−1 · min−1) or placebo (normal saline) over 180 min, starting with the first bite of a mixed meal (plus 1 g of acetaminophen) on two separate occasions. Frequent blood samples were obtained over 6 h to determine plasma GIP, GLP-1, glucose, insulin, glucagon, resistin, and acetaminophen levels.
RESULTS
Compared with placebo, GIP induced an early postprandial increase in insulin levels. Intriguingly, GIP also induced an early postprandial augmentation in glucagon, a significant elevation in late postprandial glucose, and a decrease in late postprandial GLP-1 levels. Resistin and acetaminophen levels were comparable in both interventions. By immunocytochemistry, GIP receptors were present on human and mouse α-cells. In αTC1 cell line, GIP induced an increase in intracellular cAMP and glucagon secretion.
CONCLUSIONS
GIP, given to achieve supraphysiological plasma levels, still had an early, short-lived insulinotropic effect in type 2 diabetes. However, with a concomitant increase in glucagon, the glucose-lowering effect was lost. GIP infusion further worsened hyperglycemia postprandially, most likely through its suppressive effect on GLP-1. These findings make it unlikely that GIP or GIP receptor agonists will be useful in treating the hyperglycemia of patients with type 2 diabetes.
In response to glucose and fat in digested food, two enteroendocrine hormones, glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide (GIP), are secreted froml- andk-cells, respectively, in the gut. GLP-1 and GIP play important roles in postprandial glucose homeostasis. In healthy individuals, the potent insulinotropic effects of GLP-1 and GIP account for up to 60% of the insulin secreted postprandially (1). Exogenous GLP-1 acts to improve glycemic control in patients with type 2 diabetes by 1) stimulating insulin secretion in a glucose concentration–dependent manner during the fasted state, 2) suppressing glucagon secretion in the presence of hyperglycemia and euglycemia but not hypoglycemia, and 3) decelerating gastric emptying, leading to a delay in the absorption of ingested nutrients and a dampening of postprandial glucose excursion (2–4). However, it is still unclear which of these properties of exogenous GLP-1 plays a more prominent role in lowering postprandial glucose (5). GIP has not been studied as extensively as GLP-1. Similar to GLP-1, the insulinotropic effect of GIP in healthy humans is glucose concentration dependent under glucose clamp conditions (6). Unlike GLP-1, the administration of GIP in healthy humans was reported to have a dose-dependent glucagonotropic effect during euglycemia and no effect during hyperglycemic clamp conditions (6–8). Also, unlike GLP-1, GIP has no effect on gastric emptying (9).
In patients with type 2 diabetes compared with healthy subjects, the ability of GLP-1 to stimulate insulin was noted to be 71%, while that of GIP was 46%; however, the glucose-lowering effect of GLP-1 was relatively preserved while that of GIP was absent (7,10–12). The underlying pathophysiology associated with this loss of glucose-lowering effect of GIP in humans is not known. Some hypotheses include defective expression of GIP receptors (13), accelerated degradation of GIP receptors (14), and downregulation of GIP signaling (15). It is argued that genetic components or GIP receptor defects do not play a role in the reduced insulinotropic response to GIP because patients with different types of diabetes, such as from chronic pancreatitis, latent autoimmune diabetes in adults, maturity-onset diabetes in the young, and newly diagnosed type 1 diabetes, also have impaired insulin response to GIP, thus suggesting that metabolic abnormalities may be the cause (16).
The underlying pathophysiology associated with this loss of glucose-lowering effect of GIP despite still having some insulinotropic effect in humans is not known. The aims of this study were 1) to ascertain if a dose of GIP designed to elevate plasma GIP levels to fivefold of that observed postmeal might lower blood glucose in patients with type 2 diabetes and 2) to gain insight into the pathophysiology underlying the seeming lack of effects of GIP on glucose homeostasis in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Twenty-two subjects with type 2 diabetes treated with diet alone (n = 2), metformin alone (n = 13), and a metformin/sulfonylurea combination (n = 7) were recruited. These subjects had the following characteristics (means ± SD): 13 female, 9 male; 11 Caucasian, 10 African American, and 1 Hispanic; ages 53 ± 9 years; A1C 7.4 ± 1.5%; BMI 37.4 ± 7.8 kg/m2; the average number of years since diagnosis of diabetes 4.3 ± 4.2 years; and no known complications from diabetes. Participants were excluded if they were on medications that may affect glucose homeostasis or have evidence of hepatic, renal, or hematologic abnormalities. This protocol was approved by the Intramural Research Program of the National Institute on Aging and the institutional review board of the MedStar Research Institute, Baltimore, Maryland.
This was a placebo-controlled crossover study. Participants who consented and met the inclusion criteria based on screening clinical examination and standard hematological and clinical chemistry measurements were studied. They stopped their hypoglycemic medication(s) for 5 days and fasted for 8 h overnight before the visit. If any of their morning fasting blood glucose values were >240 mg/dl during any of these 5 days, they were not allowed to participate in the study. The morning of the study, two intravenous lines were inserted: one for blood sampling and one for delivery of saline (0.9% NaCl with 1% albumin) or human synthetic GIP (20 ng · kg−1 · min−1 with 1% albumin) (Clinalfa, Läufelfingen, Switzerland). The GIP was synthesized in one lot, and the GIP activity was confirmed in the Chinese hamster ovary/GIP receptor transfected cell line, where the EC50for cAMP generation was 250 ± 51 pmol/l. After three baseline blood draws, blood sampling took place over a 6-h period. At time 0, each subject consumed, within 15 min, a standardized mixed meal (440 kcal; 56% carbohydrates, 17% protein, and 27% fat) consisting of one egg, corn flakes with 2% milk, toast with margarine, and a medium banana. The saline or GIP infusion was started with the first bite of the meal and maintained for 3 h. With the first bite, 1 g (tablet form) of acetaminophen was also given, and the appearance rate of acetaminophen in plasma was taken as a measure of gastric emptying (17). Each subject served as his/her own control and returned for the second visit in about 6–12 weeks.
Blood sampling.
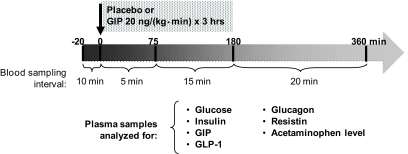
During each study, blood samples were taken at times −20, −10, and 0 min before the intervention, then every 5 min for 75 min, then every 15 min until 180 min, and then every 20 min until 360 min after the start of the intervention for a total of 34 samples per intervention per subject. Blood samples were collected on ice with heparinized syringes into EDTA-coated tubes (1.5 μg/ml blood) containing aprotinin (Trasylol 40 μg/ml blood; Serological Proteins, Kankakee, IL) and a dipeptidyl peptidase 4 (DPP-4) inhibitor (10 μg/ml blood, #DPP4; Linco Research, St. Charles, MO). Right after collection, each sample was centrifuged and the plasma was pipetted into separate aliquots and immediately frozen on dry ice and stored in −80°C until analysis. Each aliquot contained only the amount of plasma needed for one assay analysis; therefore, all the hormone levels were assayed from plasma that was thawed just once. Glucose was analyzed in real time using fresh (not frozen) samples. Study schema is shown in Fig. 1.
FIG. 1.
Each participant took part in two different interventions spaced ∼6–12 weeks apart. Starting with ingestion of a mixed meal, placebo (normal saline) or synthetic human GIP (20 ng · kg−1 · min−1) was administered intravenously for 3 h. At the same time, frequent blood samples were taken for 6 h to measure various factors known to be involved in glucose homeostasis. With the first bite, 1 g of acetaminophen was also given, and the rate of appearance of acetaminophen in plasma was taken as a measure of gastric emptying.
Plasma hormone and biochemical assays.
We quantified plasma glucose levels using a glucose analyzer (Beckman Instruments, Brea, CA). A1C was measured with an automated DiaSTAT analyzer (Bio-Rad Laboratories, Hercules, CA). The plasma hormones were measured by enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA) methods according to the kit manufacturers' instructions: GIP (RIA; Phoenix Pharmaceuticals, Belmont, CA), GLP-1 (ELISA, Linco Research, St. Charles, MO), glucagon (with RIA; Linco Research), insulin (with ELISA; Alpco Diagnostics, Salem, NH), and resistin (with ELISA; Alpco Diagnostics). Acetaminophen levels were measured in the last 10 subjects. The method for measuring acetaminophen levels is summarized in online appendix Section 1 (available at http://diabetes.diabetesjournals.org/cgi/content/full/db08-0958/DC1).
Statistical analysis.
The sample size of 22 in our study would provide a power of >80% and an α = 0.05 to detect a 30% difference in early-phase insulin secretion between placebo and GIP infusion of 20 ng · kg−1 · min−1, based on hyperglycemic clamp study performed in subjects with type 2 diabetes (16). Data from hyperglycemic clamp were used for sample size calculation because no comparable study using mixed meal was found when we designed the study.
Results are reported as means ± SE. All statistical calculations were carried out using GraphPad Prism, version 4.0 (GraphPad Software, San Diego, CA). Values at single time points were compared by paired t test. To account for the variation in baseline values for the hormones between studies (placebo versus GIP), all the values were adjusted for baseline fasting value (t = 0) during the single time point analysis by subtracting every value from its own baseline.
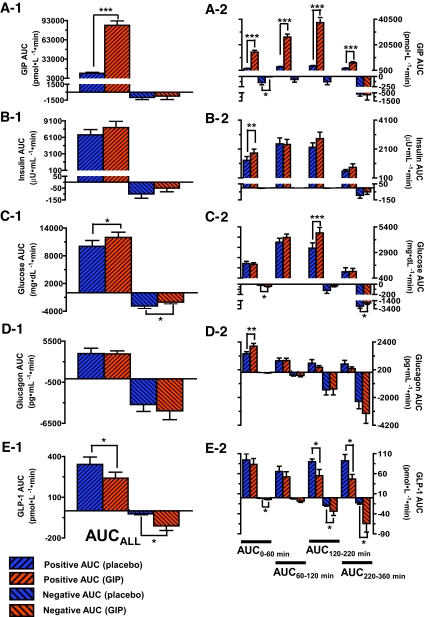
Area under the curve (AUC) was calculated using the trapezoidal rule and compared using a paired t test. With fasting values (t = 0) serving as baseline levels, positive AUCs and negative AUCs corresponded to area above and below baseline levels, respectively. The AUC for each curve, AUCALL (t = 0–360 min), was further divided into different time periods: AUC0–60 (t = 0–60 min), AUC60–120 (t = 60–120 min), AUC120–220 (t = 120–220 min), and AUC220–360 (t = 220–360 min) to better quantify the changes in hormonal responses to placebo versus GIP. NetAUCALL is the sum of positive AUC and negative AUC. Correlation analyses were performed between the netAUCALL for each biochemical parameter (GIP, insulin, glucose, glucagon, GLP-1, and acetaminophen levels) and each of the following measures: age, A1C, BMI, and duration of type 2 diabetes. Correlation analyses were also performed between AUCALL (t = 0–180 min) for acetaminophen levels and initial rise (t = 0–20 min) in insulin and glucose levels for both placebo and GIP infusion. A two-sided P value <0.05 was taken to indicate significant differences.
Animals and cell lines studies: immunostaining and histologic method.
For cryosection, human islets (National Islet Cell Resource Center) and mouse pancreata were fixed in 4% paraformaldehyde, embedded in OCT compound (Tissue-Tek; Electron Microscopy Sciences, Hatfield, PA), frozen, and stored at −80°C. Tissues were then cut with cryostat, yielding sections 7–10 μm thick. Antigen retrieval (Biogenex, San Ramon, CA) was used on all sections. Tissue was incubated with the primary insulin antibody (1:500, guinea pig anti-insulin; Linco Research), primary glucagon antibody (1:100, guinea pig anti-glucagon; Linco Research), or primary GIP receptor antibody (1:200, rabbit anti-GIP receptor; MBL, Woburn, MA). Secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for immunofluorescent detection of primary antibodies were animal IgGs conjuguated to Alexa Fluor 594 and 488 (1:1,000; Molecular Probes, Eugene, OR). Slides were mounted using fluorescence mounting medium (Vector Laboratories, Burlingame, CA). Immunofluorescent pictures were captured using a Zeiss Axiovert 200M confocal microscopy. To detect endogenous GIP receptor in αTC1, the cells were fixed in 4% paraformaldehyde, permeabilized in Triton X-100 (0.25%), blocked in 3% BSA/PBS, incubated with rabbit anti-GIP receptor antibody, and stained with Alexa Fluor–conjugated secondary antibodies. Nuclei were labeled with TOPRO-3 (Molecular Probes).
Intracellular cAMP determination and glucagon secretion.
We assayed intracellular cAMP as described previously (18). Briefly, αTC1 cells grown to 60–70% confluence on six-well plates were washed with Krebs-Ringer phosphate buffer (KRP) and incubated with 1 ml KRP containing 0.1% BSA for 2 h at 37°C in a humidified air incubator. Cells were then incubated in 1 ml KRP supplemented with 0.1% BSA with isobutylmethylxanthine (1 mmol/l) in the presence of GIP. The reaction was stopped by washing the intact cells with ice-cold PBS and cAMP extracted by incubating the cells in 0.1 mol/l HCl for 10 min. After centrifuging at 600 g, the supernatant was directly used in the assay. αTC1 cell samples were diluted 1:1 with 0.1 mol/l HCl, and we assayed all samples using a cyclic AMP (direct) EIA Kit (Assay Designs, Ann Arbor, MI). To check for specificity of GIP action, we incubated cells, as outlined above, and measured glucagon secretion into the medium in the presence of GIP with or without a GIP receptor antagonist GIP (3–42) (New England Peptide, Gardner, MA).
RESULTS
Human study.
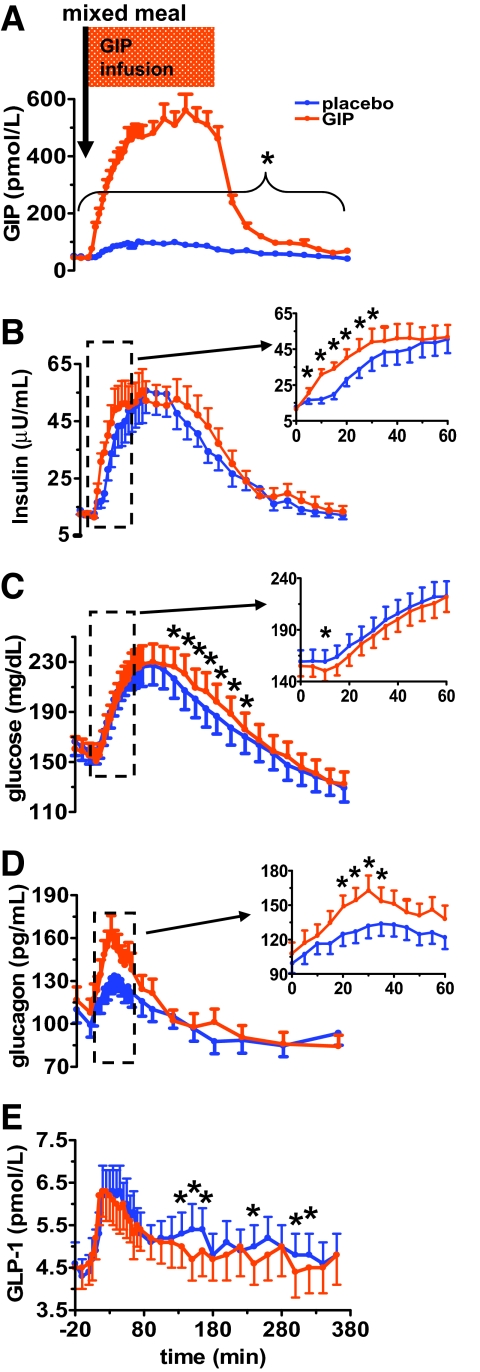
The average fasting values (t = 0) for all plasma glucose and hormones were not statistically different between placebo and GIP intervention: GIPplacebo = 43.3 ± 6.5 pmol/l, GIPGIP = 47.9 ± 10.0 pmol/l, P = 0.408; insulinplacebo = 12.7 ± 1.5 μU/ml, insulinGIP = 11.5 ± 1.1 μU/ml, P = 0.220; glucoseplacebo = 159.3 ± 11.0 mg/dl, glucoseGIP = 155.0 ± 10.0 mg/dl, P = 0.431; glucagonplacebo = 99.1 ± 8.4 ρg/ml, glucagonGIP = 107.7 ± 9.7 ρg/ml, P = 0.054; and GLP-1placebo = 5.1 ± 0.9 pmol/l, GLP-1GIP = 5.4 ± 1.0 pmol/l, P = 0.205. Mixed meal alone induced an increase in fasting GIP levels in placebo from ∼43 ± 6 to 91 ± 10 pmol/l (average from 60 to 180 min). When GIP was infused from 0 to 180 min with a meal, mean GIP levels went from 48 ± 10 pmol/l at baseline to 495 ± 44 pmol/l (average from 60 to 180 min) (Fig. 2A). Upon termination of infusion, GIP levels rapidly decreased and approached fasting levels by the end of the study. As expected, the difference in plasma GIP levels between placebo and GIP infusion was statistically significant (P < 0.001) at all time periods, as calculated by both single time point comparisons and AUC analyses (Fig. 2A andFig. 3A-1– A-2).
FIG. 2.
When compared with placebo, exogenous GIP infusion not only did not lower postprandial glucose but further worsened hyperglycemia during late postprandial period (120–360 min) in patients with type 2 diabetes. GIP infusion at a pharmacologic dose (20 ng · kg−1 · min−1) during a mixed meal is associated with a fivefold increase in plasma GIP levels (A), an early transient increase in plasma insulin (0–60 min) (B), a late postprandial elevation of plasma glucose (120–360 min) (C), a significant early postprandial increase in plasma glucagon (0–60 min) (D), and a significant decrease in late postprandial plasma GLP-1 levels (120–360 min) (E). GIP or placebo infusion was started a time 0 and continued for 180 min. A mixed meal was given at time 0. Data are presented as means ± SE. *Significant (P < 0.05) differences between GIP versus placebo at individual time points relative to baseline at t = 0. Blue circle, placebo; orange circle, GIP.
FIG. 3.
AUCALL (t = 0–360 min) for GIP (A-1), insulin (B-1), glucose (C-1), glucagon (D-1), and GLP-1 (E-1) during placebo (blue) and GIP infusion (orange). With fasting values (t = 0) serving as baseline levels, positive AUC and negative AUC corresponded to area above and below baseline levels, respectively. The AUC for each curve, AUCALL (t = 0–360 min), was further divided into different time periods: AUC0–60 (t = 0–60 min), AUC60–120 (t = 60–120 min), AUC120–220 (t = 120–220 min), and AUC220–360 (t = 220–360 min) to better quantify the changes in response to placebo versus GIP infusion for GIP (A-2), insulin (B-2), glucose (C-2), glucagon (D-2), and GLP-1 (E-2). ***P < 0.001; **P < 0.01; *P < 0.05.
GIP infusion was associated with an early transient increase in plasma insulin and a late postprandial elevation of plasma glucose.
Compared with placebo, GIP induced a statistically significant increase in plasma insulin during the early postprandial period (t = 0–60 min), as noted inFig. 2B and as a larger positive insulin AUC0–60 (P < 0.01) (Fig. 3B-2). However, the increase in insulin level was accompanied by only an early transient decrease in plasma glucose (Fig. 2C), as demonstrated by a greater negative glucose AUC0–60 (P < 0.05) (Fig. 3C-2). Intriguingly, GIP also induced a statistically significant elevation in plasma glucose from 120 to 360 min (Fig. 2C), as noted by a significantly larger positive AUC120–220 (P < 0.001), and less suppression of plasma glucose, as demonstrated by a smaller negative AUC220–360 (P < 0.05) (Fig. 3C-1– C-2). To further understand why an increase in plasma insulin from 0 to 60 min was not associated with a decrease in plasma glucose, and to elucidate the underlying cause of elevation in plasma glucose from 120 to 360 min, we measured plasma glucagon, GLP-1, resistin, and acetaminophen levels (as a measure of gastric emptying).
GIP infusion was associated with a significant increase in early postprandial plasma glucagon and a significant decrease in late postprandial plasma GLP-1 levels.
With GIP administration, a statistically significant augmentation in glucagon secretion was noted between 0 and 60 min (Fig. 2D), as shown by larger positive AUC0–60 (P < 0.01) (Fig. 3D-2). A significant decrease in GLP-1 secretion was noted (Fig. 2E), as measured by both positive and negative AUCALL, and is most prominent in the last postprandial period (120–360 min) (P < 0.05) (Fig. 3E-1– E-2).
GIP infusion had no effect on resistin or gastric emptying.
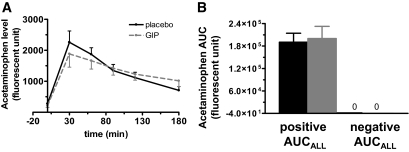
GIP administration had no effect on resistin levels during 3 h of GIP or placebo infusion (data not shown). It also did not affect gastric emptying, as plasma acetaminophen levels were comparable in both groups with no significant difference between the AUCALL (t = 0–180 min) for acetaminophen levels from GIP and placebo infusion (Fig. 4). There was also no correlation between the AUCALL (t = 0–180 min) for acetaminophen levels and the initial rise in insulin or glucose during either placebo or GIP infusion (data not shown). The characteristics of the 10 subjects in which acetaminophen levels were measured did not statistically differ from those of the entire study cohort (data not shown).
FIG. 4.
Acetaminophen level (A), used as a marker of gastric emptying, showed no difference between placebo (black solid line) and GIP infusion (gray dash line), as assessed by AUC of acetaminophen levels (B). ■, placebo; □, GIP.
Correlation analyses were performed to determine whether the observed effects were influenced by factors such as glycemic control, duration of diabetes, BMI, and age. There was no correlation between the netAUCALL for each of the biochemical parameters (GIP, insulin, glucose, glucagon, GLP-1, and acetaminophen levels) and each of the patients' measures (age, A1C, BMI, and duration of type 2 diabetes) (data not shown).
Animal and cell line studies.
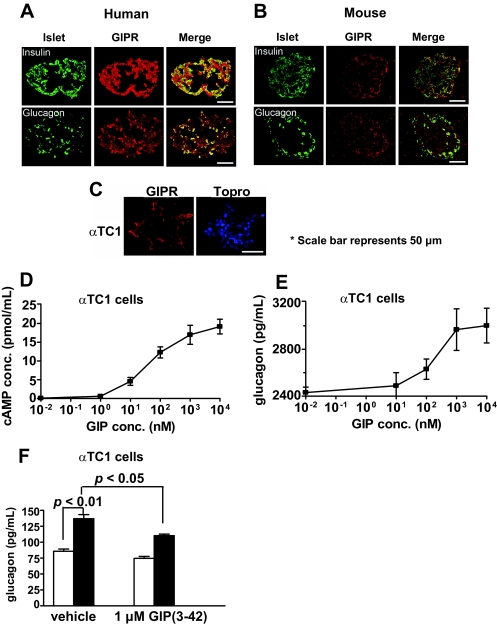
To understand how GIP might induce glucagon secretion, we used in vitro models. First, we looked for the presence of GIP receptors in mouse and human islets and found them to be present in both α- and β-cells (Fig. 5A and B). We also found GIPR in αTC1 cells, an α-cell line (Fig. 5C). Stimulating these cells with GIP led to increased intracellular cAMP and glucagon secretion in a concentration-dependent manner (Fig. 5D and E). Furthermore, in αTC1 cells, GIP-mediated glucagon secretion was diminished in the presence of a GIP receptor antagonist (Fig. 5F).
FIG. 5.
GIP receptors are present on human and mouse islets. Immunofluorescent images show coexpression of insulin with GIPR and coexpression of glucagon with GIPR in human (A) and in mouse (B) islets. C: GIP receptors are present on αTC1 cells as shown on immunoflurescent images. Stimulation of αTC1 cells with GIP led to increased intracellular cAMP levels (D) and glucagon secretion (E) in a concentration-dependent manner. F: In αTC1 cells, GIP-mediated glucagon secretion was diminished in the presence of GIP (3–42), a GIP receptor antagonist. □, vehicle; ■, 1 μmol/l GIP. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
In this study, we sought to determine the role of a supraphysiologic dose of exogenous GIP on postprandial glucose homeostasis in patients with type 2 diabetes in order to understand the pathophysiology underlying the lack of glucose-lowering effect of GIP in type 2 diabetes. We administered a mixed meal to each subject to induce an elevation in blood glucose and started GIP infusion at the first bite of the meal. We showed that in patients with type 2 diabetes, a mixed meal of 440 kcal induced a gradual rise in GIP from fasting levels of ∼43 pmol/l to a peak of ∼91 pmol/l at 60 min followed by a slow decline back to fasting levels. GIP infusion at a dose of 20 ng · kg−1· min−1 increased plasma GIP concentration to ∼500 pmol/l for the duration of the infusion (a fivefold increase relative to placebo, which is comparable with another study where GIP was given at the same dose and by the same route) (16).
GIP infusion induced a moderate, but statistically significant, early insulin response from 0 to 60 min, with no accompanying decrease in plasma glucose during the same time period. The presence of early postprandial insulin response to GIP is supported by the findings of Vilsboll et al. (12), where they showed that in response to GIP infusion during a hyperglycemic clamp, patients with type 2 diabetes still have early insulin response (0–20 min), although the response was delayed and reduced. However a late-phase insulin response was lacking (20–120 min).
We also found that GIP induced a statistically significant increase in glucose levels during the late postprandial period (120–360 min) when the insulin levels were comparable between the placebo and GIP infusion subjects. Therefore, either GIP itself is glucogenic in patients with type 2 diabetes or GIP modulates some other factors or hormones that are glucogenic, or increase insulin resistance, or both. To our knowledge, this observation has not been previously reported.
GIP has been shown in hyperglycemic clamp studies to have no effect on glucagon levels in type 2 diabetes (7,16). In other studies, patients with type 2 diabetes are reported to show a paradoxical rise of glucagon levels after ingestion of carbohydrate or protein (19,20). The present glucagon data following ingestion of a mixed meal with placebo infusion are in agreement with these later observations. With a mixed meal, and therefore a more physiological paradigm, we show that, relative to placebo, GIP infusion caused a statistically significant rise in early postprandial (0–60 min) glucagon levels. This increase in glucagon secretion would explain the lack of glucose-lowering effect of GIP in the early phase even though GIP is insulinotropic and can induce a statistically significant increase in insulin levels.
Using an in vitro cellular system, we were able to show that the elevation in glucagon secretion with GIP infusion can be explained by the presence of GIP receptors on human islets and by the ability of GIP to induce glucagon secretion in vitro. To our knowledge, this is the first time GIP receptors have been shown to be present on human α-cells. Other investigators have detected GIP receptors by immunofluorescence techniques in rat pancreatic islets including both α- and β-cells (21). GIP receptor mRNA expression has also been found in rat pancreatic α-cells, and GIP-stimulated increase in cAMP levels was demonstrated in purified rat α-cells (22). Furthermore, GIP has also been shown to stimulate glucagon release from perfused rat pancreas (23) and human pancreata (24) and to increase plasma glucagon in rats (25).
Obviously, plasma glucagon levels cannot explain why glucose was elevated with GIP infusion during the late postprandial phase (120–360 min) because glucagon levels were already at the level of placebo by this time. We found that the elevation in glucose during late postprandial phase with GIP infusion (ranging from 5 to 14 mg/dl without adjusting for differences in fasting glucose levels between placebo and GIP infusion [with baseline adjustment, the differences in glucose elevation would be 10–19 mg/dl]) may be explained by the suppressive effect of exogenous GIP infusion on GLP-1. The observed reduction in GLP-1 level is small but significant using AUC analysis and may explain the similarly modest but significant increase in blood glucose. GLP-1, in addition to its many other effects, has also been shown to activate hepato-portal glucose sensor and increase hepatic glucose uptake in dogs (26). The lower GLP-1 level with GIP infusion would, in theory and in keeping with published research, lower hepatic glucose uptake and therefore decrease the clearance rate of glucose out of plasma by the liver during postprandial period. To our knowledge, only one other study examined the effect of exogenous GIP infusion on GLP-1 during hyperglycemic clamp experiments in healthy subjects and subjects with type 2 diabetes. No changes in GLP-1 levels during infusion was found (7). GIP doses in that study were 4 and 12 ng · kg−1 · min−1 and were given only during hyperglycemic clamp. Our study used 20 ng · kg−1 · min−1 and physiologic simulation with mixed meals during clamp. Other studies using in situ models of isolated, vascularly perfused rat ileum preparation and isolated, perfused segments of porcine ileum and in vitro studies using GLP-1 release assay based on primary canine intestinall-cells and GLUTag cells line, demonstrated that GIP stimulated GLP-1 secretion (27–30). Taken together, the regulation of GLP-1 secretion is complex where in vitro and in situ models might not be representative of actual human physiology. These data are, however, in line with what happens when DPP-4 inhibitors are used. In that case, the elevated plasma active GIP and GLP-1 levels cause a decrease in incretin secretion (31). How exogenous GIP administration suppresses GLP-1 secretion in humans needs further research.
Several studies have shown that GIP does not affect gastric emptying, and our assessment of gastric emptying using plasma acetaminophen levels is in agreement with them (9,32). Even though acetaminophen level is not an optimal marker to measure gastric emptying, it has been shown to correlate well to the gold standard of gastric emptying measurement using scintigraphy (17). Therefore, unlike GLP-1, which partly modulates postprandial glucose homeostasis through delaying gastric emptying, GIP does not appear to share this property.
In a recent study examining how endogenous incretin receptors control glucose homeostasis using Glp1r−/− and Gipr−/− mice, plasma resistin levels were found to be significantly increased after GIP analog administration in wild-type and Glp1r−/− mice but not in Gipr−/− mice (33). We measured resistin levels in our study to see whether GIP affects resistin levels in humans. Our results showed comparable resistin levels between placebo and GIP infusion subjects. This difference between mice and humans can partly be explained by the findings that human fat cells, unlike those of mice, do not produce resistin (34,35). In humans, resistin is expressed and secreted by bone marrow and peripheral mononuclear cells (36). This also underscores the necessity for human studies to see if rodent findings have physiological significance in humans.
This study has certain limitations. First, a relatively large dose of GIP was infused with only one meal, and a dose-response GIP-glucagon would have been helpful. Second, the group of patients studied was heterogeneous in their clinical characteristics; therefore, there might be unmeasured variability in responses to GIP that are tied to each subject's unique characteristics.
In conclusion, GIP, given at a pharmacological dose with a meal, still has an early, short-lived insulinotropic effect in type 2 diabetes. However, with a concomitant increase in glucagon levels, the glucose-lowering effect was lost. Exogenous GIP infusion further worsened hyperglycemia in the late postprandial stage, most likely through its suppressive effect on GLP-1 secretion. If it is confirmed that the use of GIP and GIP receptor agonists results in changes in glucose homeostasis, as shown here, then it is unlikely that they will be useful as glucose- lowering agents in type 2 diabetes.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
Portions of the data in this study were presented at the American Diabetes Association 67th Scientific Sessions, Chicago, IL, 22–26 June 2007.
We acknowledge the assistance of Liz Bannon and Victoria Collingham in data collection for this study.
Footnotes
Clinical trial reg. no. NCT00239707, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Meier JJ, Nauck MA: Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005; 21: 91– 117 [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH: Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 2002; 87: 1239– 1246 [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W: Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36: 741– 744 [DOI] [PubMed] [Google Scholar]
- 4.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA: Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003; 88: 2719– 2725 [DOI] [PubMed] [Google Scholar]
- 5.Horowitz M, Nauck MA: To be or not to be–an incretin or enterogastrone? Gut 2006; 55: 148– 150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilsboll T, Krarup T, Madsbad S, Holst JJ: Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept 2003; 114: 115– 121 [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W: Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91: 301– 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA: Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003; 46: 798– 801 [DOI] [PubMed] [Google Scholar]
- 9.Meier JJ, Goetze O, Anstipp J, Hagemann D, Holst JJ, Schmidt WE, Gallwitz B, Nauck MA: Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am J Physiol Endocrinol Metab 2004; 286: E621– E625 [DOI] [PubMed] [Google Scholar]
- 10.Krarup T, Saurbrey N, Moody AJ, Kuhl C, Madsbad S: Effect of porcine gastric inhibitory polypeptide on beta-cell function in type I and type II diabetes mellitus. Metabolism 1987; 36: 677– 682 [DOI] [PubMed] [Google Scholar]
- 11.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK: The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept 1994; 51: 63– 74 [DOI] [PubMed] [Google Scholar]
- 12.Vilsboll T, Krarup T, Madsbad S, Holst JJ: Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia 2002; 45: 1111– 1119 [DOI] [PubMed] [Google Scholar]
- 13.Holst JJ, Gromada J, Nauck MA: The pathogenesis of NIDDM involves a defective expression of the GIP receptor. Diabetologia 1997; 40: 984– 986 [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Livak MF, Bernier M, Muller DC, Carlson OD, Elahi D, Maudsley S, Egan JM: Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab 2007; 293: E538– E547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng CC, Boylan MO, Jarboe LA, Usdin TB, Wolfe MM: Chronic desensitization of the glucose-dependent insulinotropic polypeptide receptor in diabetic rats. Am J Physiol 1996; 270: E661– E666 [DOI] [PubMed] [Google Scholar]
- 16.Vilsboll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ: The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab 2003; 88: 4897– 4903 [DOI] [PubMed] [Google Scholar]
- 17.Willems M, Quartero AO, Numans ME: How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci 2001; 46: 2256– 2262 [DOI] [PubMed] [Google Scholar]
- 18.Doyle ME, Greig NH, Holloway HW, Betkey JA, Bernier M, Egan JM: Insertion of an N-terminal 6-aminohexanoic acid after the 7 amino acid position of glucagon-like peptide-1 produces a long-acting hypoglycemic agent. Endocrinology 2001; 142: 4462– 4468 [DOI] [PubMed] [Google Scholar]
- 19.Muller WA, Faloona GR, Aguilar-Parada E, Unger RH: Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970; 283: 109– 115 [DOI] [PubMed] [Google Scholar]
- 20.Buchanan KD, McCarroll AM: Abnormalities of glucagon metabolism in untreated diabetes mellitus. Lancet 1972; 2: 1394– 1395 [DOI] [PubMed] [Google Scholar]
- 21.Lewis JT, Dayanandan B, Habener JF, Kieffer TJ: Glucose-dependent insulinotropic polypeptide confers early phase insulin release to oral glucose in rats: demonstration by a receptor antagonist. Endocrinology 2000; 141: 3710– 3716 [DOI] [PubMed] [Google Scholar]
- 22.Moens K, Heimberg H, Flamez D, Huypens P, Quartier E, Ling Z, Pipeleers D, Gremlich S, Thorens B, Schuit F: Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 1996; 45: 257– 261 [DOI] [PubMed] [Google Scholar]
- 23.Pederson RA, Brown JC: Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology 1978; 103: 610– 615 [DOI] [PubMed] [Google Scholar]
- 24.Brunicardi FC, Druck P, Seymour NE, Sun YS, Elahi D, Andersen DK: Selective neurohormonal interactions in islet cell secretion in the isolated perfused human pancreas. J Surg Res 1990; 48: 273– 278 [DOI] [PubMed] [Google Scholar]
- 25.Rabinovitch A, Dupre J: Effects of the gastric inhibitory polypeptide present in impure pancreozymin-cholecystokinin on plasma insulin and glucagon in the rat. Endocrinology 1974; 94: 1139– 1144 [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD: Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab 2003; 284: E1027– E1036 [DOI] [PubMed] [Google Scholar]
- 27.Herrmann-Rinke C, Voge A, Hess M, Goke B: Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol 1995; 147: 25– 31 [DOI] [PubMed] [Google Scholar]
- 28.Hansen L, Holst JJ: The effects of duodenal peptides on glucagon-like peptide-1 secretion from the ileum: a duodeno-ileal loop? Regul Pept 2002; 110: 39– 45 [DOI] [PubMed] [Google Scholar]
- 29.Damholt AB, Buchan AM, Kofod H: Glucagon-like-peptide-1 secretion from canine L-cells is increased by glucose-dependent-insulinotropic peptide but unaffected by glucose. Endocrinology 1998; 139: 2085– 2091 [DOI] [PubMed] [Google Scholar]
- 30.Brubaker PL, Schloos J, Drucker DJ: Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 1998; 139: 4108– 4114 [DOI] [PubMed] [Google Scholar]
- 31.El-Ouaghlidi A, Rehring E, Holst JJ, Schweizer A, Foley J, Holmes D, Nauck MA: The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab 2007; 92: 4165– 4171 [DOI] [PubMed] [Google Scholar]
- 32.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B: Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996; 97: 92– 103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ: Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 2007; 117: 143– 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagaev I, Smith U: Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 2001; 285: 561– 564 [DOI] [PubMed] [Google Scholar]
- 35.Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M: Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun 2003; 300: 674– 678 [DOI] [PubMed] [Google Scholar]
- 36.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA: Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003; 300: 472– 476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.