Abstract
OBJECTIVE
Fetal malnutrition may predispose to type 2 diabetes through gene programming and developmental changes. Previous studies showed that these effects may be modulated by genetic variation. Genome-wide association studies discovered and replicated a number of type 2 diabetes–associated genes. We investigated the effects of such well-studied polymorphisms and their interactions with fetal malnutrition on type 2 diabetes risk and related phenotypes in the Dutch Famine Birth Cohort.
RESEARCH DESIGN AND METHODS
The rs7754840 (CDKAL1), rs10811661 (CDKN2AB), rs1111875 (HHEX), rs4402960 (IGF2BP2), rs5219 (KCNJ11), rs13266634 (SLC30A8), and rs7903146 (TCF7L2) polymorphisms were genotyped in 772 participants of the Dutch Famine Birth Cohort Study (n = 328 exposed, n = 444 unexposed). Logistic and linear regression models served to analyze their interactions with prenatal exposure to famine on type 2 diabetes, impaired glucose tolerance (IGT), and area under the curves (AUCs) for glucose and insulin during oral glucose tolerance testing (OGTT).
RESULTS
In the total population, the TCF7L2 and IGF2BP2 variants most strongly associated with increased risk for type 2 diabetes/IGT and increased AUC for glucose, while the CDKAL1 polymorphism associated with decreased AUC for insulin. The IGF2BP2 polymorphism showed an interaction with prenatal exposure to famine on AUC for glucose (β = −9.2 [95% CI −16.2 to −2.1], P = 0.009).
CONCLUSIONS
The IGF2BP2 variant showed a nominal interaction with exposure to famine in utero, decreasing OGTT AUCs for glucose. This may provide a clue that modulation of the consequences of fetal environment depends on an individual's genetic background.
The fetal origins hypothesis (1,2) states that malnutrition during fetal development predisposes to adverse health outcomes, such as type 2 diabetes. According to the hypothesis, fetal adaptation to a low-caloric intrauterine environment may involve programming to optimize the use of restricted nutrient supply. Programming may lead to altered gene expression profiles and eventually to disease later in life. In addition, adverse intrauterine circumstances may divert nutrients to critical organs such as the brain at the expense of organs such as the pancreas, liver, or muscles (3).
Type 2 diabetes and defective insulin secretion show high heritabilities (4). Genetic variation is likely to interact with the fetal response to an extreme nutritional situation. We found that the effects of the Pro12Ala polymorphism of the peroxisome proliferator–activated receptor (PPAR)γ2 gene depend on prenatal exposure to famine (5), and several studies (6–8) have shown interactions of genes with size at birth, a marker of fetal environment. Such interactions may have consequences for development and function. Previously, the PPARγ, TCF7L2, and KCNJ11 genes were identified by linkage and candidate gene studies as type 2 diabetes risk loci (9–11). Recently, genome-wide association studies have identified and replicated new genetic variants associated with type 2 diabetes (12–15). Notably, a number of these variants are thought to be involved in the development and function of critical organs for glucose metabolism, such as the pancreatic β-cell (16–18).
Based on the findings described above, we hypothesized that these genes interact with fetal exposure to famine. In the Dutch Famine Birth Cohort, we investigated the effects of genetic variation in these loci on type 2 diabetes, impaired glucose tolerance (IGT), oral glucose tolerance testing (OGTT), and their interaction with fetal malnutrition.
RESEARCH DESIGN AND METHODS
The Dutch Famine Birth Cohort is composed of individuals born as term singletons around the time of the Dutch famine during World War II in the Wilhelmina Gasthuis in Amsterdam. Details of the study have been described elsewhere (19). In brief, a total of 2,414 singletons were born between 1 November 1943 and 28 February 1947. Members of the cohort living in the Netherlands at 1 September 2002 were invited (n = 1,423). Of these, 810 agreed to participate. The study was approved by the local medical ethics committee and was conducted in accordance with the declaration of Helsinki. All participants gave their written informed consent.
Exposure to famine.
Prenatal exposure to famine was defined as a daily food ration of the mother <1,000 calories during any 13-week period of gestation, based on the official daily food rations for the general population aged ≥21 years. Based on these data, individuals born between 7 January 1945 and 8 December 1945 were exposed to famine in utero. Initially, we defined three exposure groups of 16 weeks, dividing the period of exposure in late, mid-, and early gestation. However, this led to small groups in which meaningful genetic analyses were impossible. Therefore, we combined the exposed groups for the interaction analyses.
Study parameters.
Trained research nurses conducted measurements and interviews as previously described (19). In brief, information on medical history, lifestyle, and medication was derived from a standardized interview. Information on the mother, the pregnancy, and size at birth was derived from medical birth records.
The present study was limited to measures of glucose metabolism. Pre-existent diabetes was defined as the use of oral or injected antidiabetes medications. Individuals with pre-existent diabetes were excluded from OGTT. OGTT was performed after an overnight fast with a standard load of 75 g of glucose at t = 0. Blood samples were collected after 0, 30, 60, and 120 min for measurement of plasma glucose and insulin concentrations. Plasma glucose was measured by a standardized enzymatic photometric assay on a Modulator P analyzer (Roche, Basel, Switzerland). Plasma insulin was measured by immunoluminometric assay on an Immulite 2000 Analyzer (Diagnostic Product Corporation, Los Angeles, CA). IGT was defined as a 120-min glucose level between 7.8 and 11.0 mmol/l. Diabetes based on OGTT was defined as a 120-min glucose level >11.0 mmol/l.
Genotyping.
Genomic DNA was extracted from fasting blood samples. The rs7754840 (CDKAL1), rs10811661 (CDKN2AB), rs1111875 (HHEX), rs4402960 (IGF2BP2), rs5219 (KCNJ11), rs13266634 (SLC30A8), and rs7903146 (TCF7L2) polymorphisms were genotyped with Taqman allelic discrimination assays. The assays were designed and optimalized by Applied Biosystems (Foster City, CA). The analyses were performed as described previously (20). Assays were run on 90 blood bank samples to test for adequate cluster separation. Genotypes were determined in 2 ng genomic DNA. Reactions were performed on the Taqman Prism 7900HT platform. Success rates for genotyping ranged from 86.1% (IGF2BP2) to 96.7%. The duplicate mismatch rate for the IGF2BP2 assay was 0. Genotyping for at least one polymorphism was successful in 772 subjects.
Statistical methods.
The primary end point was the area under the curve (AUC) for glucose and insulin during OGTT. A secondary combined end point of type 2 diabetes and IGT consisted of pre-existent type 2 diabetes and type 2 diabetes and IGT identified at OGTT.
Continuous variables are expressed as means ± SD. Comparisons between groups were performed with ANOVA and χ2tests for normally distributed continuous and categorical variables, respectively. Logarithmic transformation was performed on variables that were not normally distributed. Allele frequencies were estimated by gene counting. Hardy-Weinberg equilibrium was tested by χ2testing.
The AUCs during OGTT were calculated by the trapezoidal rule (21): {[15 × log (gluc0min)] + [30 × log (gluc30min)] + [45 × log (gluc60min)] + [30 × log (gluc120min)]/120} and {[15 × log (ins0min)] + [30 × log (ins30min)] + [45 × log (ins60min)] + [30 × log (ins120min)]/120} for glucose and insulin, respectively. Binary logistic and linear regression models served to investigate the associations of the genetic variants with type 2 diabetes/IGT risk and AUC for glucose and insulin. The additive model of inheritance was assumed.
Genotype by prenatal famine exposure interactions were tested by creating interaction terms for each genetic variant (coded 0, 1, or 2 for carrying the risk allele) with the exposure group (coded 0 and 1 for unexposed and exposed subjects, respectively). Unexposed subjects were the subjects born before or conceived after the Dutch famine. Genotype by birth weight interaction models were created by taking the product of birth-weight (continuous) and the genetic variant (coded 0, 1, and 2). All models were adjusted for sex. Subsequently, models were adjusted for BMI. Additionally, waist circumference and the mother's parity and weight at the last antenatal visit were added. Models investigating famine were additionally adjusted for birth weight and vice versa. We adjusted for multiple testing by means of Bonferroni adjustment. We had 80% power to detect interactions of β = 7.8 with gene and environmental β = 3.0 (minor allele frequency 30%, α = 0.05).
RESULTS
General characteristics and population genetics.
General and maternal characteristics of 772 genotyped individuals divided into the famine exposure groups are shown in Table 1. Minor allele frequencies were 0.31, 0.19, 0.35, 0.29, 0.36, 0.29, and 0.30 for the CDKAL1, CDKN2A/B, HHEX, IGF2BP2, KCNJ11, SLC30A8, and TCF7L2 polymorphisms, respectively. All polymorphisms were in Hardy-Weinberg equilibrium (χ2< 3.3; 2 df; P> 0.07 for all SNPs). A total of 94 individuals with type 2 diabetes (62 prevalent and 32 based on OGTT) and 100 individuals with IGT were included in the analyses on type 2 diabetes/IGT. For the analyses on the AUCs for glucose and insulin during OGTT, 102 individuals were excluded from the total population because of pre-existent diabetes, nonfasting before the test, or incomplete results.
TABLE 1.
General and maternal characteristics according to time of pregnancy when exposed to the Dutch famine
| All | Born before | Late gestation | Mid-gestation | Early gestation | Conceived after | P* | |
|---|---|---|---|---|---|---|---|
| n | 772 | 233 | 140 | 117 | 71 | 211 | |
| Characteristics at age 58 years | |||||||
| % men | 45.9 | 46.8 | 44.3 | 39.3 | 42.3 | 50.7 | NS |
| Age (years) | 58.3 ± 0.9 | 59.2 | 58.5 | 58.2 | 58.0 | 57.4 | NS |
| BMI (kg/m2) | 28.6 ± 4.8 | 28.7 | 28.3 | 28.1 | 28.0 | 29.0 | NS |
| Waist circumference (cm) | 92.7 ± 8.9 | 93.3 | 92.9 | 90.8 | 91.9 | 93.2 | NS |
| Currently smoking (%) | 24.1 | 20.6 | 27.9 | 25.9 | 31.0 | 23.1 | NS |
| Birth characteristics | |||||||
| Gestational age (days) | 285 ± 11.1 | 284 | 283 | 285 | 289 | 285 | NS |
| Birth weight (g) | 3,359 ± 469 | 3,396 | 3,187 | 3,204 | 3,500 | 3,471 | <0.001 |
| Maternal characteristics | |||||||
| Age at delivery (years) | 28.9 ± 6.4 | 28.7 | 31.1 | 28.8 | 27.2 | 28.4 | NS |
| Primiparous (%) | 33.7 | 36.1 | 20.0 | 33.3 | 42.3 | 37.4 | 0.04 |
| Weight gain (third trimester) | 2.9 ± 2.9 | 2.8 | 0.0 | 4.3 | 5.0 | 3.4 | 0.01 |
| Weight at last antenatal visit | 66.4 ± 8.7 | 66.4 | 62.8 | 63.6 | 69.0 | 69.3 | <0.001 |
Data are means ± SD.
*P value for comparison between exposed and nonexposed. NS, not significant.
Associations and interactions with prenatal exposure to famine on type 2 diabetes/IGT and AUCs for glucose and insulin.
Table 2 shows odds ratios (ORs) of the genetic variants for the composite outcome type 2 diabetes/IGT and the β-coefficients for AUCs for glucose and insulin during the OGTT. The TCF7L2 and IGF2BP2 variants were associated with increased type 2 diabetes/IGT risk (TCF7L2: OR 1.39 [95% CI 1.08–1.79], IGF2BP2: 1.43 [1.11–1.85]) and increased AUC for glucose (TCF7L2: β = 4.5 [1.0–8.1], IGF2BP2: β = 3.6 [0.1–7.1]). The CDKAL1 variant associated with a decreased AUC for insulin (β = −8.2 [−16.1 to −0.41]), which became less strong after adjustment for BMI. None of the other results changed considerably after additional adjustments in multivariate models. None of the polymorphisms had a significant effect on birth weight or were related to prenatal famine exposure (data not shown).
TABLE 2.
Associations with DM/IGT and AUC for glucose and insulin in the total population
| Gene variant | Major/minor allele | OR1 diabetes/IGT (95% CI) | OR2 diabetes/IGT (95% CI) | β1 AUC glucose | β2 AUC glucose | β1 AUC insulin | β2 AUC insulin |
|---|---|---|---|---|---|---|---|
| CDKAL1 rs7754840 | G/C | 1.11 (0.87–1.42) | 1.16 (0.90–1.49) | 0.04 (−3.3 to 3.4) | 0.7 (−2.6 to 4.0) | −8.2 (−16.1 to −0.41) | −6.0 (−13.3 to 1.3) |
| CDKN2A/B rs10811661 | T/C | 1.08 (0.81–1.44) | 1.03 (0.76–1.39) | −0.004 (−4.0 to 4.0) | −0.4 (−4.2 to 3.5) | −0.22 (−9.4 to 8.9) | −1.4 (−9.9 to 7.1) |
| HHEX rs1111875 | C/T | 1.09 (0.86–1.36) | 0.99 (0.79–1.27) | −2.1 (−5.2 to 1.0) | −2.7 (−5.8 to 0.3) | 2.43 (−4.8 to 9.62) | 0.1 (−6.7 to 6.8) |
| IGF2BP2 rs4402960 | G/T | 1.43 (1.11–1.85) | 1.42 (1.09–1.84) | 3.6 (0.1–7.1) | 3.2 (−0.2 to 6.5) | 3.16 (−4.9 to 11.2) | 1.66 (−5.9 to 9.2) |
| KCNJ11 rs5219 | AG (341) | 0.97 (0.76–1.23) | 0.99 (0.77–1.27) | −1.4 (−4.7 to 1.8) | −1.4 (−4.5 to 1.8) | 2.46 (−5.1 to 10.0) | 2.9 (−4.1 to 9.9) |
| SLC30A8 rs13266634 | C/T (299) | 0.88 (0.68–1.13) | 0.91 (0.70–1.18) | 0.3 (−3.1 to 3.8) | 0.6 (−2.7 to 3.9) | 4.26 (−3.7 to 12.2) | 4.8 (−2.6 to 12.1) |
| TCF7L2 rs7903146 | C/T (316) | 1.39 (1.08–1.79) | 1.37 (1.06–1.78) | 4.5 (1.0–8.1) | 4.6 (1.2–8.0) | −5.5 (−13.5 to 2.52) | −5.3 (−12.9 to 2.1) |
OR1 and β1: adjusted for sex. OR2 and β2: adjusted for sex and BMI.
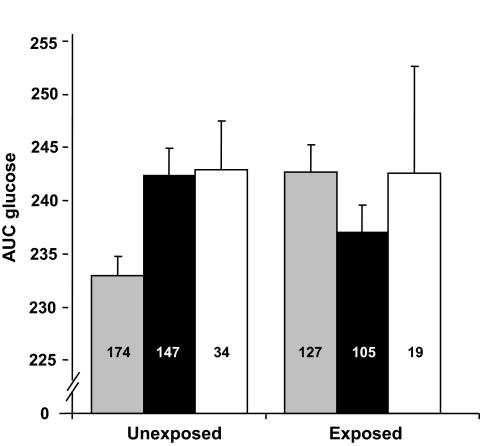
Table 3 shows the interactions between genetic variants and exposure to famine in utero. The IGF2BP2 showed a significant interaction on AUC glucose (β interaction −9.2 [−16.2 to −2.1], P = 0.009).Figure 1 shows the effects of the IGF2BP2 polymorphism on AUC for glucose in exposed and unexposed subjects. After Bonferroni correction for 21 tests, this was no longer significant. None of the polymorphisms showed a significant interaction with birth weight (data not shown). Additional adjustments in multivariate models did not change the results.
TABLE 3.
Interactions of genetic variants with prenatal exposure to famine
| Gene variant | OR interaction DM/IGT(95% CI)* | P for interaction | β interaction AUC glucose (95% CI)* | P for interaction | β interaction AUC insulin(95% CI)* | P for interaction |
|---|---|---|---|---|---|---|
| CDKAL1-rs7754840 | 1.16 (0.70–1.90) | 0.57 | 2.20 (−4.7 to 9.1) | 0.66 | 7.7 (−8.2 to 23.7) | 0.37 |
| CDKN2A/B-rs10811661 | 1.37 (0.75–2.51) | 0.31 | 3.46 (−4.8 to 11.7) | 0.42 | 11.6 (−7.4 to 30.6) | 0.24 |
| HHEX-rs1111875 | 1.61 (1.01–2.58) | 0.04 | 5.2 (−1.2 to 11.5) | 0.08 | −4.7 (−19.4 to 10.0) | 0.55 |
| IGF2BP2-rs4402960 | 0.82 (0.49–1.37) | 0.45 | −9.2 (−16.2 to −2.1) | 0.009 | 0.49 (−15.9 to 16.8) | 0.94 |
| KCNJ11-rs5219 | 1.22 (0.75–1.98) | 0.41 | −2.9 (−9.7 to 3.9) | 0.42 | −6.0 (−21.6 to 9.7) | 0.42 |
| SLC30A8-rs13266634 | 1.38 (0.82–2.32) | 0.22 | 2.0 (−5.0 to 8.9) | 0.62 | −7.1 (−23.0 to 8.9) | 0.40 |
| TCF7L2-rs7903146 | 1.12 (0.68–1.85) | 0.66 | −0.1 (−7.2 to 7.1) | 0.93 | −0.8 (−15.5 to 17.0) | 0.86 |
*OR and β for the interaction term: All models were adjusted for sex. P values for AUC for glucose and insulin calculated after logarithmic transformation. DM, type 2 diabetes.
FIG. 1.
The effect of the IGF2BP2 genotypes on AUC glucose according to prenatal exposure to famine. Number of subjects shown inside of each bar, SEs of the mean shown on top of each bar.  , GG; ■, GT; □, TT.
, GG; ■, GT; □, TT.
DISCUSSION
In the present study, we investigated interactions of type 2 diabetes–associated polymorphisms in a population exposed to famine in utero. The IGF2BP2 polymorphism showed a nominally significant interaction with prenatal exposure to famine on AUC for glucose during OGTT.
The Dutch Famine Birth Cohort provides a unique opportunity to directly investigate the interactions of fetal malnutrition with type 2 diabetes and related measurements. Most studies on the association of early nutrition with type 2 diabetes and related parameters have used birth weight as a marker of fetal nutrition and growth. Unfortunately, a consequent limitation is the size of our study population, which limits the power to detect small effects. This especially holds for the categorical outcome type 2 diabetes/IGT. Nonetheless, effects found in the population largely corresponded to previous literature, except for the direction of the effect of the HHEX polymorphism, for which we have no explanation. This effect disappeared after adjustment for BMI. Due to power limitations, we were unable to investigate the interactions with timing of exposure to famine during gestation (late, mid-, or early gestational exposure to famine).
Several studies have identified interactions of size at birth and genetic variants on type 2 diabetes risk and measurements of glucose metabolism (6–8). Previously, we reported an interaction between a PPARγ gene variant and fetal malnutrition (5). Now, we observed a nominally significant interaction of the IGF2BP2 polymorphisms with exposure to famine on glucose levels during OGTT. The effect on type 2 diabetes/IGT risk was in the same direction but not significant. The presence of both the IGF2BP2 risk allele and exposure to famine associated with lower AUC for glucose, which seems counterintuitive considering the main effects of the two factors, which are both associated with increased type 2 diabetes risk. However, a similar interaction was observed in two other studies on the effect of a genetic variant in the ACE gene (6,7). Subjects carrying the ACE risk allele were relatively protected from the effect of low birth weight on insulin resistance (6,7). These findings together with the current finding on IGF2BP2 may be explained by overefficient modulating effects of risk alleles during fetal development. This would imply that the IGF2BP2 variant, being part of an important developmental pathway, confers a relative resistance to the detrimental consequences of fetal malnutrition on glucose tolerance at an adult age.
IGF2BP2, also referred to as IMP-2, is an mRNA-binding protein that posttranslationally regulates IGF2, a fetal growth factor, during several developmental stages (22). IGF2 plays a critical role during placental and fetal development (23,24). IGFBPs have a tissue-specific expression pattern, with IGF2BP2 being expressed in fetal lung, kidney, thymus, and placenta and having its highest expression in fetal liver (22). Interactions between genetic variation in IGF2BP2 and fetal malnutrition may be exerted through this IGF2 developmental pathway. However, such explanations are fully speculative, and it is important to stress that our observation was no longer significant after correcting for multiple testing and should therefore be interpreted with caution. Nonetheless, it may suggest that consequences of the fetal environment depend on an individual's genetic background. Interactions of other variants investigated may unfortunately not have become apparent due to the limited power of our study.
None of the polymorphisms was associated with birth weight. This is not in line with the fetal insulin hypothesis, which proposes that the same genetic factors would alter both intrauterine growth and adult glucose metabolism (25). The TCF7L2 gene has consistently been associated with birth weight (26). Unfortunately, we do not have maternal genotypes available. Mother-child pair analyses suggest that the TCF7L2 variant effect on birth weight is a reflection of its presence in the mother (26). In conclusion, genetic variants involved in fetal development, like IGF2BP2, may influence the response to fetal malnutrition and its consequences in the adult hypercaloric environment.
Acknowledgments
The study was financially supported by a Netherlands Heart Foundation Grant (NHS2003B165).
No potential conflicts of interest relevant to this article were reported.
Footnotes
REFERENCES
- 1.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS: Fetal nutrition and cardiovascular disease in adult life. Lancet 1993; 341: 938– 941 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Eriksson JG, Forsen T, Osmond C: Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235– 1239 [DOI] [PubMed] [Google Scholar]
- 3.Hill DJ, Duvillie B: Pancreatic development and adult diabetes. Pediatr Res 2000; 48: 269– 274 [DOI] [PubMed] [Google Scholar]
- 4.Matsuda A, Kuzuya T: Relationship between obesity and concordance rate for type 2 (non-insulin-dependent) diabetes mellitus among twins. Diabetes Res Clin Pract 1994; 26: 137– 143 [DOI] [PubMed] [Google Scholar]
- 5.de Rooij SR, Painter RC, Phillips DI, Osmond C, Tanck MW, Defesche JC, Bossuyt PM, Michels RP, Bleker OP, Roseboom TJ: The effects of the Pro12Ala polymorphism of the peroxisome proliferator–activated receptor-γ2 gene on glucose/insulin metabolism interact with prenatal exposure to famine. Diabetes Care 2006; 29: 1052– 1057 [DOI] [PubMed] [Google Scholar]
- 6.Cambien F, Leger J, Mallet C, Levy-Marchal C, Collin D, Czernichow P: Angiotensin I–converting enzyme gene polymorphism modulates the consequences of in utero growth retardation on plasma insulin in young adults. Diabetes 1998; 47: 470– 475 [DOI] [PubMed] [Google Scholar]
- 7.Kajantie E, Rautanen A, Kere J, Andersson S, Yliharsila H, Osmond C, Barker DJ, Forsen T, Eriksson J: The effects of the ACE gene insertion/deletion polymorphism on glucose tolerance and insulin secretion in elderly people are modified by birth weight. J Clin Endocrinol Metab 2004; 89: 5738– 5741 [DOI] [PubMed] [Google Scholar]
- 8.Kubaszek A, Markkanen A, Eriksson JG, Forsen T, Osmond C, Barker DJ, Laakso M: The association of the K121Q polymorphism of the plasma cell glycoprotein-1 gene with type 2 diabetes and hypertension depends on size at birth. J Clin Endocrinol Metab 2004; 89: 2044– 2047 [DOI] [PubMed] [Google Scholar]
- 9.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES: The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000; 26: 76– 80 [DOI] [PubMed] [Google Scholar]
- 10.Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, McCarthy MI, Hattersley AT, Frayling TM: Large-scale association studies of variants in genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003; 52: 568– 572 [DOI] [PubMed] [Google Scholar]
- 11.Reynisdottir I, Thorleifsson G, Benediktsson R, Sigurdsson G, Emilsson V, Einarsdottir AS, Hjorleifsdottir EE, Orlygsdottir GT, Bjornsdottir GT, Saemundsdottir J, Halldorsson S, Hrafnkelsdottir S, Sigurjonsdottir SB, Steinsdottir S, Martin M, Kochan JP, Rhees BK, Grant SF, Frigge ML, Kong A, Gudnason V, Stefansson K, Gulcher JR: Localization of a susceptibility gene for type 2 diabetes to chromosome 5q34–q35.2. Am J Hum Genet 2003; 73: 323– 335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson BK, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S: Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007; 316: 1331– 1336 [DOI] [PubMed] [Google Scholar]
- 13.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341– 1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007; 445: 881– 885 [DOI] [PubMed] [Google Scholar]
- 15.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grarup N, Rose CS, Andersson EA, Andersen G, Nielsen AL, Albrechtsen A, Clausen JO, Rasmussen SS, Jorgensen T, Sandbaek A, Lauritzen T, Schmitz O, Hansen T, Pedersen O: Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes 2007; 56: 3105– 3111 [DOI] [PubMed] [Google Scholar]
- 17.Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M: Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic β-cell function. Diabetes 2007; 56: 3101– 3104 [DOI] [PubMed] [Google Scholar]
- 18.Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, Schafer SA, Kirchhoff K, Fritsche A, Haring HU: Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS ONE 2007; 2: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP: Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998; 351: 173– 177 [DOI] [PubMed] [Google Scholar]
- 20.Fang Y, van Meurs JB, d'Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG: Promoter and 3′-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the Rotterdam Study. Am J Hum Genet 2005; 77: 807– 823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittaker ET, Robinson G: The Trapezoidal and parabolic rules. In The Calculus of Observations: A Treatise on Numerical Mathematics 4th ed.Dover, New York, 1967, p. 156– 158 [Google Scholar]
- 22.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC: A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999; 19: 1262– 1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunger DB, Petry CJ, Ong KK: Genetic variations and normal fetal growth. Horm Res 2006; 65Suppl. 3: 34– 40 [DOI] [PubMed] [Google Scholar]
- 24.Gicquel C, Le BY: Hormonal regulation of fetal growth. Horm Res 2006; 65Suppl. 3: 28– 33 [DOI] [PubMed] [Google Scholar]
- 25.Hattersley AT, Tooke JE: The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 1999; 353: 1789– 1792 [DOI] [PubMed] [Google Scholar]
- 26.Freathy RM, Weedon MN, Bennett A, Hypponen E, Relton CL, Knight B, Shields B, Parnell KS, Groves CJ, Ring SM, Pembrey ME, Ben-Shlomo Y, Strachan DP, Power C, Jarvelin MR, McCarthy MI, Davey SG, Hattersley AT, Frayling TM: Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet 2007; 80: 1150– 1161 [DOI] [PMC free article] [PubMed] [Google Scholar]