Abstract
OBJECTIVE
Genome-wide association studies have identified several variants within the MTNR1B locus that are associated with fasting plasma glucose (FPG) and type 2 diabetes. We refined the association signal by direct genotyping and examined for associations of the variant displaying the most independent effect on FPG with isolated impaired fasting glycemia (i-IFG), isolated impaired glucose tolerance (i-IGT), type 2 diabetes, and measures of insulin release and peripheral and hepatic insulin sensitivity.
RESEARCH DESIGN AND METHODS
We examined European-descent participants in the Inter99 study (n = 5,553), in a sample of young healthy Danes (n = 372), in Danish twins (n = 77 elderly and n = 97 young), in additional Danish type 2 diabetic patients (n = 1,626) and control subjects (n = 505), in the Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) study (n = 4,656), in the North Finland Birth Cohort 86 (n = 5,258), and in the Haguenau study (n = 1,461).
RESULTS
The MTNR1B intronic variant, rs10830963, carried most of the effect on FPG and showed the strongest association with FPG (combined P = 5.3 × 10−31) and type 2 diabetes. The rs10830963 G-allele increased the risk of i-IFG (odds ratio [OR] 1.64, P = 5.5 × 10−11) but not i-IGT. The G-allele was associated with a decreased insulin release after oral and intravenous glucose challenges (P < 0.01) but not after injection of tolbutamide. In elderly twins, the G-allele associated with hepatic insulin resistance (P = 0.017).
CONCLUSIONS
The G-allele of MTNR1B rs10830963 increases risk of type 2 diabetes through a state of i-IFG and not through i-IGT. The same allele associates with estimates of β-cell dysfunction and hepatic insulin resistance.
The neurohormone melatonin is the main secretory product of the pineal gland and is mainly involved in the regulation of circadian rhythms. Melatonin has been proposed to influence both insulin secretion and endogenous glucose production (1,2). Interestingly, melatonin secretion is reported to be impaired in type 2 diabetic patients (2,3).
The effect of melatonin is mediated by the membrane receptors melatonin receptor 1 (MT1; encoded by MTNR1A) and melatonin receptor 2 (MT2; encoded by MTNR1B), which both belong to the seven-transmembrane G protein–coupled receptor superfamily (4). MTNR1B is abundantly expressed in the retina and the suprachiasmatic nucleus and the circadian rhythm control center (5), and the association with glucose homeostasis might be mediated indirectly through the brain. However, MTNR1B is also expressed in human pancreatic islets (6,7) and pancreatic β-cells (8), and MTNR1B mRNA expression is upregulated in pancreatic tissue of type 2 diabetic patients compared with control subjects (7), suggesting a putative direct role of melatonin on β-cell function.
In a recent genome-wide association study involving 2,151 nondiabetic French individuals, we identified rs1387153, located 28 kb upstream of the 5′ region of MTNR1B at chromosome 11q21-q22 as a modulator of fasting plasma glucose (FPG) (8). Additional analyses in 16,094 Europeans confirmed that the rs1387153 T-allele associated with both increased FPG and risk of type 2 diabetes (8). Concurrently, similar findings were reported (9,10). Using in silico imputation from the HapMap study in their overall meta-analysis, Prokopenko et al. (9) also suggested that the MTNR1B intronic variant, rs10830963, which is in substantial linkage disequilibrium (LD) with rs1387153 (r2 = 0.7), shows the strongest signal to FPG.
In the present study, we initially aimed to refine the association signal within the 62-Kb LD block that includes MTNR1B. This was done by direct genotyping of additional single-nucleotide polymorphisms (SNPs) in LD (r2> 0.70) with rs1387153 (including rs10830963) in large European populations. We then evaluated the association of the SNP displaying the most associated and independent signal with isolated impaired fasting glycemia (i-IFG) and isolated impaired glucose tolerance (i-IGT). Finally, we examined for potential associations of the marker SNP with glucose- and tolbutamide-stimulated insulin release and measures of both peripheral and hepatic insulin sensitivity.
RESEARCH DESIGN AND METHODS
Study participants: DESIR.
Associations between genotype and quantitative metabolic traits were studied in the Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort, which is a longitudinal French general population that was fully described elsewhere (11,12). Among them, 3,411 individuals had been followed for the incidence of IFG and type 2 diabetes during 9 years (n = 517 incident cases), and 4,589 had been analyzed for the association with A1C level. Clinical characteristics are found in an online-only appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08–1660/DC1, supplementary Table 1.
NFBC86.
Associations between genotype and quantitative metabolic traits were studied in the Northern Finland 1986 Birth Cohort (NFBC86), which is a prospective 1-year birth cohort including all Finnish Caucasian mothers with children whose expected date of birth fell between 1 July 1985 and 30 June 1986 in the two northernmost provinces of Finland (13). Clinical characteristics are found in the online appendix, supplementary Table 1.
Haguenau.
Associations between genotype and quantitative metabolic traits were studied in the Haguenau community-based cohort of young adults that investigates long-term consequences of being born small for gestational age and was fully described elsewhere (14). At a mean age of 22 years, participants under overnight fasting conditions underwent a medical examination to assess anthropometric and clinical parameters, including an oral glucose tolerance test (OGTT) with a glucose load of 75 g, and further blood samples were taken after 30 and 120 min for measurement of plasma glucose. Clinical characteristics are given in the online appendix, supplementary Table 1.
Inter99.
Associations between genotype and quantitative metabolic traits were studied in the population-based Inter99 sample of middle-aged people (clinicaltrials.gov reg. no. NCT00289237) (15,16). Individuals with normal glucose tolerance (NGT; n = 4,400), i-IFG (n = 485), i-IGT (n = 464), or both IFG and IGT (IFG/IGT, n = 201) were investigated for associations between genotype and quantitative metabolic traits. Patients in the Inter99 sample with type 2 diabetes (n = 322) were not included in the present analysis of quantitative traits. Clinical characteristics are shown in online appendix supplementary Table 2.
Young healthy Danes.
Studies of quantitative metabolic traits were performed in 372 young healthy Danes characterized by a combined intravenous glucose tolerance test (IVGTT) and intravenous tolbutamide challenge recruited at the Research Centre for Prevention and Health (17) (online appendix supplementary Table 2).
The twin study.
Studies of quantitative metabolic traits were further performed in two population-based samples of elderly (monozygotic n = 38, dizygotic n = 39) and young (monozygotic n = 58, dizygotic n = 40) twins characterized by an IVGTT followed by a 2-h hyperinsulinemic-euglycemic clamp (18) (online appendix Table 2).
TABLE 2.
Quantitative metabolic traits during an OGTT in the Danish population-based Inter99 sample of 5,553 middle-aged and nondiabetic individuals stratified according to the MTNR1B rs10830963 genotype
| rs10830963 | CC | CG | GG | Effect per allele (95% CI) | Padd |
|---|---|---|---|---|---|
| n (men/women) | 2,950 (1,410/1,540) | 2,167 (1,101/1,066) | 436 (211/225) | ||
| Age (years) | 46 ± 8 | 46 ± 8 | 46 ± 8 | ||
| BMI (kg/m2) | 26.1 ± 4.5 | 25.9 ± 4.3 | 26 ± 4.1 | −0.1 (−0.3 to 0.08) | 0.26 |
| Plasma glucose (mmol/l) | |||||
| Fasting | 5.41 ± 0.5 | 5.48 ± 0.51 | 5.59 ± 0.54 | 0.09 (0.07 to 1.1) | 1 × 10−21 |
| 30 min post-OGTT | 8.48 ± 1.67 | 8.62 ± 1.75 | 8.83 ± 1.63 | 0.2 (0.1 to 0.2) | 2 × 10−7 |
| 120 min post-OGTT | 6.01 ± 1.55 | 5.83 ± 1.51 | 6.1 ± 1.56 | −0.03 (−0.09 to 0.03) | 0.35 |
| Serum insulin (pmol/l) | |||||
| Fasting | 34 (24–50) | 33 (23–49) | 35 (24–52) | 0.3 (−2.4 to 1.9) | 0.81 |
| 30 min post-OGTT | 251 (181–359) | 240 (171–343) | 243 (174–351) | −2.4 (−4.5 to −0.3) | 0.025 |
| 120 min post-OGTT | 155 (98–247) | 147 (88–234) | 162 (105–286) | 0.5 (−2.5 to 3.5) | 0.75 |
| Insulinogenic index | 75 (47–127) | 71 (44–117) | 68 (46–106) | −5.0 (−8.0 to −1.9) | 0.0016 |
| BIGTT-AIR | 1,699 (1,342–2,160) | 1,576 (1,258–2,007) | 1,515 (1,223–1,909) | −6.2 (−8.0 to −4.5) | 4.3 × 10−12 |
| BIGTT-Si | 9.4 ± 4.0 | 9.6 ± 5.9 | 9.0 ± 3.9 | −0.8 (−0.25 to 0.09) | 0.37 |
| HOMA-IR | 8.1 (5.5–12.2) | 8.0 (5.4–12.2) | 8.6 (6.0–13.4) | 1.3 (−0.9 to 3.6) | 0.23 |
| A1C (%) | 5.78 ± 0.4 | 5.82 ± 0.41 | 5.82 ± 0.38 | 0.03 (0.02 to 0.05) | 0.00011 |
Data are means ± SD or medians (interquartile range). Type 2 diabetic patients have been excluded from the present quantitative trait analyses. Values of serum insulin, homeostasis model assessment of insulin resistance (HOMA-IR), insulinogenic index, and BIGTT-AIR were logarithmically transformed before statistical analysis, and their effect sizes are presented as the increase/decrease in percent. Calculated P values and effect sizes were adjusted for age (BIGTT-AIR and BIGTT-Si); age and sex (BMI); or age, sex, and BMI (all other traits) assuming an additive genetic model. Calculations of HOMA-IR and the insulinogenic index are described in the online appendix. BIGTT-AIR and BIGTT-Siare calculated as described elsewhere (21).
All participants from Inter99, the young healthy Danish study population, and the twin study were of Danish nationality and European descent.
IFG, IGT, and type 2 diabetic case subjects.
The Danish case-control studies of IFG, IGT, and type 2 diabetes included all unrelated IFG, IGT, and type 2 diabetic case subjects and all NGT control subjects from the Inter99 cohort as well as samples recruited from the outpatient clinic at the Steno Diabetes Center (NGT = 505, type 2 diabetes = 1,626). The French case-control study of type 2 diabetes included 2,622 unrelated type 2 diabetic patients ascertained from the French type 2 diabetic family cohort recruited in the CNRS-UMR8090 Unit (Lille, France), the Endocrinology-Diabetology Department of the Corbeil-Essonnes Hospital (Evry, France), and the DESIR cohort. A total of 4,343 DESIR participants were included as control subjects.
Control subjects had normal fasting glycemia and were glucose tolerant after a World Health Organization–standardized OGTT. Type 2 diabetes, IFG, and IGT were defined according to the World Health Organization 1999 criteria (19).
Ethics.
In the mentioned study samples, informed written consent was obtained from all study participants before any investigations. All studies were approved by the local ethics committees and were in accordance with the principles of the Helsinki Declaration II.
Biochemical and anthropometrical measurements.
Methods and conditions for biochemical and anthropometrical measurements are described in the online appendix.
Genotyping.
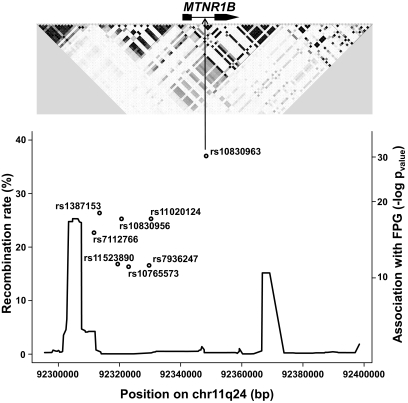
The recombination rate based on HapMap data defines a region that includes MTNR1B and its 5′ region flanked by two recombination hot spots (Fig. 1). In DESIR, NFBC86, and Haguenau, we genotyped all common SNPs (HapMap data, n = 7) in high LD (r2 > 0.70) with rs1387153: six are located in the 5′ region of MTNR1B (rs7112766 [LD with rs1387153 r2 = 0.86], rs11523890 [r2 = 0.82], rs10830956 [r2 = 1.0], rs10765573 [r2 = 0.82], rs7936247 [r2 = 0.84], and rs11020124 [r2 = 0.94]) and one SNP rs10830963 (r2 = 0.70) is located in the only intron of MTNR1B (Fig. 1). Genotyping of all SNPs was performed using Taqman allelic discrimination. Inter99, the young Danish sample set and twin populations were genotyped for rs10830963. Genotype success rate was at least 96%, and no deviation (P≥ 0.05) from Hardy-Weinberg equilibrium was observed in any of the examined populations.
FIG. 1.
Genomic context and recombination rate of the eight associated SNPs at the MTNR1B locus with FPG. The upper panel shows the LD structure of the 621-kb LD block in the CEU population from HapMap phase II using the Haploview software. The lower panel shows the association magnitude (−log P value) with FPG of the eight SNPs and the recombination rate (%) at the MTNR1B locus.
Statistical analysis.
We analyzed the effect of all eight SNPs on FPG (and in the case of rs10830963 also for additional metabolic quantitative traits) in nondiabetic subjects, using linear regression models under the additive model adjusted for age, sex, and BMI. The estimates of each SNP effect on FPG and their standard errors for each separate analysis were combined in the meta-analyses using the weighted inverse normal method. The overall effect and its confidence interval were estimated using the inverse variance method implemented in the meta.summaries function of the R rmeta package. The effect of each SNP on diabetes status was tested in a logistic regression model applying the R mgcv package, adjusted for age, sex, and BMI. In the DESIR participants, we assessed the effect of each SNP on IFG or diabetes incidence with Cox proportional hazards regression models, adjusted for sex and BMI. Survival time was age at diagnosis or censoring. The statistical analyses in the DESIR, NFBC86, and Haguenau study samples were performed with R (version 2.7.2), combined with the survival rmeta and mgcv packages.
To examine the impact of rs10830963 in comparison to the effects of each of the other associated SNPs, we analyzed in each population separately four haplotypes using the THESIAS genetic model program (20) (online appendix supplementary Table 4). This method estimates likelihood of specified models for haplotype effects, under the general linear model framework, and allows testing of constrained models. This analysis aimed at testing two-SNP models while accounting for the high correlation between SNPs (unlike a plain two-loci regression model).
RESULTS
SNP rs10830963 shows the strongest and most independent effect on FPG.
We analyzed the association between all seven genotyped SNPs (Fig. 1; see details in research design and methods) and FPG in nondiabetic participants from the DESIR (mean age 47 years), NFBC86 (mean age 16 years), and Haguenau (mean age 22 years) cohorts. Applying an additive genetic model adjusted for age, sex, and BMI, the rs10830963 G-allele showed the strongest association with increased FPG in each study taken individually and in the combined meta-analyses (n = 11,375; β = 0.07 mmol/l, 95% CI 0.06–0.08; combined P = 5.3 × 10−31) (Fig. 1 and online appendix supplementary Table 3). In the combined sample, the inclusion of rs10830963 in the regression models of the other SNPs annulled their effect on FPG (P> 0.12). By constraining the effect of rs10830963, we found significant differences betweenmodels of haplotype analyses. In contrast, when we constrained the effects of any of the others SNPs, we found no significant differences (online appendix supplementary Table 4). These data confirm an indispensable effect of rs10830963 in the association with FPG.
G-allele of rs10830963 associates not only with increased risk of diabetes but also with impaired fasting glycemia.
In the Danish case-control study, involving 1,948 type 2 diabetic case subjects and 4,905 glucose-tolerant control subjects, the rs10830963 G-allele of MTNR1B was significantly associated with increased risk of type 2 diabetes (odds ratio [OR] 1.23, 95% CI 1.10–1.37, P = 0.00036) (Table 1).
TABLE 1.
Association studies of MTNR1B rs10830963 in the Danish population-based Inter99 sample, in individuals sampled at Steno Diabetes Center, and in the French DESIR cohort in relation to glucose tolerance status
| Glucose tolerant | i-IFG | i-IGT | Both IFG and IGT | Type 2 diabetes | |
|---|---|---|---|---|---|
| n(men/women) | 4,905 (2,277/2,628) | 485 (356/129) | 464 (196/268) | 201 (130/71) | 1,948 (1,200/748) |
| CC | 2,644 (53.9%) | 196 (40.4%) | 282 (60.8%) | 106 (52.7%) | 1,002 (51.4%) |
| CG | 1,913 (39%) | 230 (47.4%) | 151 (32.5%) | 66 (32.8%) | 776 (39.8%) |
| GG | 348 (7.1%) | 59 (12.2%) | 31 (6.7%) | 29 (14.4%) | 170 (8.7%) |
| G-allele frequency (%) | 26.6 | 36.0 | 23.0 | 30.8 | 28.6 |
| OR per G-allele (95% CI) | 1.64 (1.41–1.89) | 0.85 (0.72–1.00) | 1.35 (1.08–1.69) | 1.23 (1.10–1.37) | |
| Padd | 5.5 × 10−11 | 0.051 | 0.0085 | 0.00036 | |
| French DESIR cohort (n = 3,411) | Incidence of IFG (n = 334) | Incidence of type 2 diabetes (n = 183) | |||
| Hazard ratio (95% CI) | 1.24 (1.06–1.45) | 1.02 (0.80–1.25) | |||
| Padd | 0.007 | 0.87 | |||
In the Danish study populations, data are n (%), number of subjects stratified according to MTNR1B rs10830963, G-allele frequency in %, and OR (95% CI) per allele. P values compare genotype distribution between case subjects (i-IFG, i-IGT, both IFG and IGT, and type 2 diabetes), and glucose-tolerant control subject applying an additive genetic model while adjusting for age, sex, and BMI. In the French DESIR cohort, the incidence of IFG or diabetes was defined as fasting plasma glucose ≥6.1 mmol/l or treatment with antidiabetic agents after a 9-year follow-up period.
In the French case-control samples, the same allele also showed the strongest association with increased risk of type 2 diabetes in studies of 2,622 case subjects and 4,343 nondiabetic control subjects (OR 1.19, 95% CI 1.08–1.32; P = 0.00048) (online appendix supplementary Table 5).
Interestingly, in the Inter99 population-based sample of middle-aged Danes, the same G-allele associated with increased risk of having i-IFG (OR 1.64, 95% CI 1.43–1.90, P = 5.5−11) and increased risk of combined IFG/IGT (OR 1.35, 95% CI 1.08–1.69, P = 0.0085; Table 1). In addition, prospective data from the DESIR cohort demonstrated that the G-allele of rs10830963 predicted the incidence of IFG after a 9-year period (hazard ratio 1.24, 95% CI 1.06–1.45; P = 0.007) (Table 1).
rs10830963 impacts on glycemia, insulin release, and hepatic insulin sensitivity.
The impact of MTNR1B rs10830963 on diabetes-related quantitative traits was investigated after an OGTT in two population-based cohorts: the Inter99 study (mean age 46 years) and the Haguenau study. Assuming an additive genetic model, carriers of the minor G-allele of rs10830963 demonstrated an increased plasma glucose level 30 min post-OGTT in Inter99 and Haguenau (β = 0.2 mmol/l, 95% CI 0.1–0.2, P = 2 × 10−7 [Table 2]; and β = 0.2 mmol/l, 95% CI [0.09–0.3], P = 4.1 × 10−4 [online appendix supplementary Table 6], respectively).
In addition, an allele-dependent increase in A1C level was found for carriers of the minor G-allele of rs10830963 in the Inter99 and DESIR cohorts (β = 0.03%, 95% CI 0.02–0.05, P = 0.00011 [Table 2]; and β = 0.03%, 95% CI 0.02–0.05, P = 0.00027 [data not shown], respectively).
In the Inter99 study population, carriers of the minor G-allele of rs10830963 showed a decreased serum insulin level 30 min post-OGTT (2.4%, 95% CI 0.3–4.6, P = 0.025) and a decreased insulin release as assessed by the insulinogenic index (5.0%, 95% CI 1.9–8.0, P = 0.0016) and BIGTT–acute insulin response (AIR) (6.2%, 95% CI 4.5–8.0, P = 4.3 × 10−12) (Table 2). No associations with surrogate measures of insulin resistance were found.
In the Haguenau cohort, these findings were substantiated: decreased serum insulin 30 min post-OGTT (β = 5.8%, 95% CI 1.0–10.4; P = 0.02) and decreased insulinogenic index (β = 4.4%, 95% CI 1.5–7.3; P = 0.003; online appendix supplementary Table 6).
In the study of 372 young healthy Danes (mean age 25 years), carriers of the rs10830963 G-allele showed an impairment of acute serum insulin response (P = 0.0022) as well as a decreased disposition index (P = 0.021) after an intravenous glucose load (Table 3). We found no association of the genotype with the Bergman minimal model–derived insulin sensitivity index. After the IVGTT, each individual had an intravenous injection of tolbutamide to elicit a secondary β-cell response. No difference in the tolbutamide-induced insulin response was shown when stratifying the individuals according to the rs10830963 genotype (P = 0.60) (Table 3).
TABLE 3.
Quantitative metabolic traits during an IVGTT in the population-based sample of 372 young healthy Danes stratified according to the MTNR1B rs10830963 genotype
| rs10830963 | CC | CG | GG | Effect per allele (95% CI) | Padd |
|---|---|---|---|---|---|
| n(men/women) | 200 (104/96) | 146 (72/74) | 26 (8/18) | ||
| Age (years) | 25 ± 3 | 26 ± 4 | 26 ± 4 | ||
| BMI (kg/m2) | 23.2 ± 3.5 | 23.9 ± 4.0 | 24.9 ± 3.8 | 0.7 (0.1 to 1.3) | 0.016 |
| Fasting plasma glucose (mmol/l) | 4.92 ± 0.43 | 5.06 ± 0.47 | 5.17 ± 0.77 | 0.1 (0.07 to 0.2) | 0.00015 |
| Fasting serum insulin (pmol/l) | 30 (23–45) | 30 (23–46) | 37 (29–51) | −0.006 (−0.09 to 0.07) | 0.97 |
| Acute serum insulin response 0–8 min (pmol/l × min) | 2,098 (1,297–3,018) | 1,642 (1,135–2,548) | 2,172 (1,387–2,621) | −0.7 (−1.1 to −0.2) | 0.0022 |
| Acute serum insulin response 0–10 min after tolbutamide injection (pmol/l × min) | 1,399 (765–2,248) | 1,376 (870–2,022) | 2,036 (1,357–2,588) | 0.1 (−0.3 to 0.5) | 0.60 |
| Insulin sensitivity index (10−5× (min) × pmol/l)−1) | 13.5 (9.0–19.3) | 13.3 (9.0–20.3) | 11.1 (7.5–14.8) | 0.02 (−0.05 to 0.09) | 0.65 |
| Disposition index | 28,760 (16,810–41,470) | 24,030 (16,480–33,730) | 23,900 (15,470–27,910) | −1.3 (−2.3 to −0.2) | 0.021 |
Data are means ± SD (age, BMI, and plasma glucose) or medians (interquartile range). Values of fasting serum insulin, acute serum insulin response 0–8 min, acute serum insulin response 0–10 min after tolbutamide injection, insulin sensitivity index, and disposition index were transformed using cube root before statistical analysis, and their effect sizes are presented as the increase/decrease on a cubic scale. Calculated P values and effect sizes were adjusted for age, sex, and BMI (except for BMI) assuming an additive model. Acute serum insulin response 0–10 min after tolbutamide injection was in addition adjusted for plasma glucose values before the injection of tolbutamide. Acute serum insulin response 0–8 min, acute serum insulin response 0–10 min after tolbutamide injection, insulin sensitivity index, and disposition index are calculated as described elsewhere (17) and in the online appendix.
In a population of 77 elderly Danish twins (mean age 62 years), who underwent an IVGTT and a 2-h hyperinsulinemic-euglycemic clamp, the association between the minor G-allele of MTNR1B rs10830963 and decreased acute plasma insulin response was confirmed (P = 0.0077) (Table 4). In addition, the G-allele carriers had decreased plasma insulin levels 30 min after an IVGTT (P = 0.035). The MTNR1B genotype was not associated with clamp-derived measures of peripheral insulin sensitivity (Rd); however, carriers of the G-allele were less likely to decrease their hepatic glucose production during insulin infusion (P = 0.017) (Table 4). We failed to show any significant associations of MTNR1B genotype to measures of insulin sensitivity and hepatic glucose production in the sample of young twins (mean age 28 years) (data not shown).
TABLE 4.
Quantitative metabolic traits during an IVGTT followed by a hyperinsulinemic-euglycemic clamp in the population-based sample of 77 elderly Danish twins stratified according to MTNR1B rs10830963 genotype
| rs10830963 | CC | CG | GG | Effect per allele (95% CI) | Padd |
|---|---|---|---|---|---|
| n (men/women) | 38 (20/18) | 36 (13/23) | 3 (0/3) | ||
| Age (years) | 62 ± 2 | 62 ± 2 | 62 ± 2 | ||
| BMI (kg/m2) | 27.5 ± 4.2 | 25.3 ± 4.5 | 22.0 ± 0.9 | −2.5 (−4.4 to −0.6) | 0.015 |
| Plasma glucose (mmol/l) | |||||
| Fasting | 5.8 ± 1.2 | 5.5 ± 1.1 | 6.3 ± 1.2 | −0.06 (−0.4 to 0.3) | 0.72 |
| 10 min post-IVGTT | 14.2 ± 1.2 | 13.9 ± 1.1 | 15.0 ± 0.5 | 0.3 (−0.1 to 0.8) | 0.15 |
| 30 min post-IVGTT | 11.2 ± 1.3 | 11.1 ± 1.1 | 12.0 ± 0.7 | 0.5 (0.003 to 1.1) | 0.055 |
| Plasma insulin (pmol/l) | |||||
| Fasting | 34 (19–52) | 24 (16–34) | 19 (19–21) | −0.03 (−0.2 to 0.2) | 0.74 |
| 10 min post-IVGTT | 165 (111–288) | 94 (67–125) | 86 (37–98) | −0.3 (−0.7 to 0.03) | 0.075 |
| 30 min post-IVGTT | 108 (71–170) | 75 (55–96) | 63 (29–82) | −0.3 (−0.5 to −0.03) | 0.035 |
| Acute serum insulin response 0–10 min (pmol/l × min) | 1,802 (1,389–3,724) | 1,144 (739–1,690) | 855 (99–1,433) | −0.5 (−0.9 to −0.2) | 0.0077 |
| Basal (mg × kg−1× min−1) | |||||
| Hepatic glucose production | 3.1 ± 0.4 | 3.0 ± 0.3 | 3.2 ± 0.8 | −0.1 (−0.3 to 0.03) | 0.13 |
| Clamp (mg × kg−1× min−1) | |||||
| Glucose disposal rate (Rd) | 9.0 ± 3.3 | 9.8 ± 2.7 | 15.7 ± 3.6 | 1.0 (−0.4 to 2.2) | 0.18 |
| Hepatic glucose production | 1.5 ± 0.7 | 1.7 ± 0.5 | 2.9 ± 1.7 | 0.4 (0.07 to 0.6) | 0.017 |
Data are means ± SD or medians (interquartile range) (values of plasma insulin and acute serum insulin response). Hepatic glucose production and glucose disposal rates are expressed as mg · kg body mass−1 · min−1in the fasting state and during insulin infusion. Values of plasma insulin were logarithmically transformed before statistical analysis, and their effect sizes are presented as the increase/decrease in percent. P values and effect sizes were calculated using a mixed model adjusted for sex, age, and BMI. The additive model includes a random-effects term for twin pair membership and a fixed-effects term for zygosity. Acute serum insulin response 0–10 min, hepatic glucose production, and glucose disposal rate are calculated as described elsewhere (18) and in the online appendix.
DISCUSSION
We and others have recently demonstrated that the MTNR1B is a genetic determinant of FPG and type 2 diabetes (8–10). Our present refinement of the association signal based on direct genotyping of data in more than 11,300 individuals within the LD block that includes MTNR1B supports the conclusion from the in silico search by Prokopenko et al. (9) that the intronic variant rs10830963 may be the source of the association signal on FPG and type 2 diabetes.
Of novelty, the G-allele of rs10830963 is associated with increased risk of i-IFG in a population-based sample of middle-aged Danes. Also, associations were found between the G-allele and the combined IFG/IGT phenotype as well as with overt type 2 diabetes. In contrast, the G-allele of MTNR1B rs10830963 tended to decrease the risk of i-IGT, suggesting that the minor allele of rs10830963 predisposes to type 2 diabetes via a state of i-IFG and not through increased postprandial plasma glucose levels as is a major feature of individuals with i-IGT. This interpretation of results is supported by the finding that the G-allele of rs10830963 was not associated with any estimates of insulin resistance, which is a well-known pathophysiological feature of individuals with i-IGT, but not in individuals with i-IFG (22).
To further evaluate the underlying metabolic phenotype, we examined quantitative traits related to glucose homeostasis in the Inter99 and the Haguenau cohorts. In both of these population samples, we showed associations between the G-allele and decreased oral glucose–stimulated serum insulin release, indicating that carriers of the diabetogenic risk allele in MTNR1B may have pancreatic β-cell impairments relative to their level of plasma glucose.
These results were further substantiated in a subset of young healthy Danes, where the same allele of MTNR1B rs10830963 was associated with a decreased acute serum insulin response as well as a decreased disposition index after an intravenous glucose load.
The results are in line with recent results (10,23) emphasizing the effect of the variant in pancreatic β-cell function. After intravenous injection of tolbutamide, which stimulates insulin release directly through the ATP-sensitive potassium channel, the insulin response of the risk allele carriers was indistinguishable from that of the wild-type carriers. These findings may be related to a stimulus-dependent effect of the variant, suggesting an allele-specific glucose insensitivity of the pancreatic β-cells localized upstream of the ATP-sensitive potassium channel. Obviously, this hypothesis needs further testing in a clinical-physiological setting.
The study of elderly Danish twins undergoing an IVGTT and a hyperinsulinemic-euglycemic clamp confirmed the association between the MTNR1B risk allele and an impaired first-phase insulin release after an intravenous glucose challenge. In addition, we demonstrated a reduced suppression of hepatic glucose production during the hyperinsulinemic-euglycemic clamp documenting hepatic, but not peripheral, insulin resistance. This is consistent with the results and indirect suggestions in another recent study reporting insulin resistance when indirectly assessed during oral glucose ingestion in MTNR1B risk allele carriers (23). It has previously been proposed that abnormal diurnal rhythms in endogenous glucose production are a major contributor to fasting hyperglycemia in type 2 diabetes (2). The extent to which hepatic insulin resistance contributes to the elevated FPG level in carriers of the risk alleles is uncertain. However, in a population of young twins, we observed a tendency toward elevated basal hepatic glucose production (P = 0.06, data not shown) in the absence of hepatic insulin resistance during clamps. The twin data also suggest that age may play a role for the phenotypic presentation of a disproportionately elevated hepatic glucose production in carriers of the risk allele of MTNR1B. Yet, we recognize that the results from the present twin studies may be chance findings due to the limited size of the study populations, the amount of tests made, and the lack of correction for multiple hypothesis testing.
Surprisingly, we observed differences in BMI when stratifying according to rs10830963 among young healthy Danes and among elderly Danish twins. Because the results occur in opposite directions and since we do not demonstrate any difference in BMI in the much larger Inter99 study, we argue, however, that these findings may be due to random chance, and consequently, we adjust for BMI to correct for these differences between the genotype groups.
Rs10830963 is located at mid-distance from exon 1 (+5.6 Kb) and exon 2 (−5.9 Kb) of MTNR1B in a nonconserved sequence across species (http://www.bx.psu.edu/miller_lab). Lyssenko et al. (10) have reported an increased MTNR1B expression in human pancreatic islets in rs10830963 G-allele carriers, although the effect was only significant in individuals older than 45 years. In silico analyses do not predict any potential consequence of the change from C to G at this position on mRNA splicing (http://www.tigr.org/tdb/GeneSplicer/gene_spl.html). Nonetheless, additional studies are necessary to fully understand the molecular and physiological impact of rs10830963, or any unidentified rare and potentially causal variants in high LD with rs10830963.
In summary, the refinement at the MTNR1B locus shows that the intronic rs10830963 is the variant that exhibits the tightest associations with FPG and type 2 diabetes risk. Importantly, the G-allele of rs10830963 confers risk of diabetes through a stage of i-IFG and a decrease of oral and intravenous glucose-stimulated insulin release, suggesting a pancreatic β-cell dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
Research in the laboratory of O.P. was supported by grants from the Lundbeck Foundation Centre of Applied Medical Genomics for Personalized Disease Prediction, Prevention and Care (LUCAMP); the Danish Medical Research Council, University of Copenhagen, and the European Union (InterAct, grant LSHMCT-2006-037197; EUGENE2, grant LSHM-CT-2004-512013; EXGENESIS: LSHM-CT-2004-005272); and the Danish Diabetes Association. Research in the laboratory of P.F. was supported in part by anALFEDIAM-Les Laboratoires Servier grant, the “Conseil Regional Nord-Pas-de-Calais: Fonds européen de développement économique et régional,” and the British Medical Research Council. N.B.-N.'s position is supported by a grant from the ANR (Agence Nationale pour la Recherche: ANR-06 PHYSIO-037-02). A.B. is partly funded by a research fellowship from the Conseil Régional du Nord Pas de Calais (France). We acknowledge funding to P.F. by the European Union (Integrated Project EURODIA LSHM-CT-2006-518153 in the Framework Programme 6 [FP06] of the European Community).
Research in the laboratory of O.P. was also supported by Novo Nordisk. K.B.-J. is the director and professor of the Steno Diabetes Center (which is owned by Novo Nordisk, Bagsvaerd, Denmark), owns stock shares in Novo Nordisk, and has received honorarium from Novo Nordisk, Bristol-Myers Squibb, Novartis, Pfizer, Hermedico, and AstraZeneca. O.B.P. is employed by the Steno Diabetes Center, which is owned by Novo Nordisk, Bagsvaerd, Denmark. No other potential conflicts of interest relevant to this article were reported.
We thank A. Forman, I.-L. Wantzin and M. Stendal for technical assistance, A.L. Nielsen for data management, M. Kristensen for scientific administration, and G. Lademann for secretarial support. We thank M. Deweirder and F. Allegaert for DNA extraction of part of the cohorts studied. We thank L. Peltonen, A.-L. Hartikainen, and A. Ruokonen, who are part of the NFBC86, which is supported by the European Commission.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Van Cauter E: Putative roles of melatonin in glucose regulation. Therapie 1998; 53: 467– 472 [PubMed] [Google Scholar]
- 2.Radziuk J, Pye S: Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes: suprachiasmatic deficit or limit cycle behaviour? Diabetologia 2006; 49: 1619– 1628 [DOI] [PubMed] [Google Scholar]
- 3.Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Muhlbauer E: Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res 2006; 40: 135– 143 [DOI] [PubMed] [Google Scholar]
- 4.von Gall C, Stehle JH, Weaver DR: Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 2002; 309: 151– 162 [DOI] [PubMed] [Google Scholar]
- 5.Peschke E: Melatonin, endocrine pancreas and diabetes. J Pineal Res 2008; 44: 26– 40 [DOI] [PubMed] [Google Scholar]
- 6.Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ: Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res 2008; 44: 273– 279 [DOI] [PubMed] [Google Scholar]
- 7.Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Muhlbauer E: Melatonin and type 2 diabetes: a possible link? J Pineal Res 2007; 42: 350– 358 [DOI] [PubMed] [Google Scholar]
- 8.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P: A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 2009; 41: 89– 94 [DOI] [PubMed] [Google Scholar]
- 9.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR: Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009; 41: 77– 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L: Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009; 41: 82– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkau B: [An epidemiologic survey from a network of French Health Examination Centres, (D.E.S.I.R.): epidemiologic data on the insulin resistance syndrome]. Rev Epidemiol Sante Publique 1996; 44: 373– 375 [PubMed] [Google Scholar]
- 12.Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, Marchand M, Hartikainen AL, Sovio U, De Graeve F, Rung J, Vaxillaire M, Tichet J, Marre M, Balkau B, Weill J, Elliott P, Jarvelin MR, Meyre D, Polychronakos C, Dina C, Sladek R, Froguel P: A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 2008; 320: 1085– 1088 [DOI] [PubMed] [Google Scholar]
- 13.Jarvelin MR, Elliott P, Kleinschmidt I, Martuzzi M, Grundy C, Hartikainen AL, Rantakallio P: Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr Perinat Epidemiol 1997; 11: 298– 312 [DOI] [PubMed] [Google Scholar]
- 14.Jaquet D, Collin D, Levy-Marchal C, Czernichow P: Adult height distribution in subjects born small for gestational age. Horm Res 2004; 62: 92– 96 [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen T, Borch-Johnsen K, Thomsen TF, Ibsen H, Glumer C, Pisinger C: A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil 2003; 10: 377– 386 [DOI] [PubMed] [Google Scholar]
- 16.Glumer C, Jorgensen T, Borch-Johnsen K: Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003; 26: 2335– 2340 [DOI] [PubMed] [Google Scholar]
- 17.Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, Pedersen O: Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians: analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest 1996; 98: 1195– 1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A: Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes 2005; 54: 275– 283 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Geneva, World Health Org, 1999 [Google Scholar]
- 20.Tregouet DA, Tiret L: Cox proportional hazards survival regression in haplotype-based association analysis using the Stochastic-EM algorithm. Eur J Hum Genet 2004; 12: 971– 974 [DOI] [PubMed] [Google Scholar]
- 21.Hansen T, Drivsholm T, Urhammer SA, Palacios RT, Volund A, Borch-Johnsen K, Pedersen O: The BIGTT test: a novel test for simultaneous measurement of pancreatic β-cell function, insulin sensitivity, and glucose tolerance. Diabetes Care 2007; 30: 257– 262 [DOI] [PubMed] [Google Scholar]
- 22.Faerch K, Vaag A, Holst JJ, Glumer C, Pedersen O, Borch-Johnsen K: Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 2008; 51: 853– 861 [DOI] [PubMed] [Google Scholar]
- 23.Staiger H, Machicao F, Schäfer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Häring HU, Fritsche A: Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS ONE 2008; 3: e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.