Abstract
OBJECTIVE
Heterozygous activating mutations of glucokinase have been reported to cause hypoglycemia attributable to hyperinsulinism in a limited number of families. We report three children with de novo glucokinase hyperinsulinism mutations who displayed a spectrum of clinical phenotypes corresponding to marked differences in enzyme kinetics.
RESEARCH DESIGN AND METHODS
Mutations were directly sequenced, and mutants were expressed as glutathionyl S-transferase–glucokinase fusion proteins. Kinetic analysis of the enzymes included determinations of stability, activity index, the response to glucokinase activator drug, and the effect of glucokinase regulatory protein.
RESULTS
Child 1 had an ins454A mutation, child 2 a W99L mutation, and child 3 an M197I mutation. Diazoxide treatment was effective in child 3 but ineffective in child 1 and only partially effective in child 2. Expression of the mutant glucokinase ins454A, W99L, and M197I enzymes revealed a continuum of high relative activity indexes in the three children (26, 8.9, and 3.1, respectively; wild type = 1.0). Allosteric responses to inhibition by glucokinase regulatory protein and activation by the drug RO0281675 were impaired by the ins454A but unaffected by the M197I mutation. Estimated thresholds for glucose-stimulated insulin release were more severely reduced by the ins454A than the M197I mutation and intermediate in the W99L mutation (1.1, 3.5, and 2.2 mmol/l, respectively; wild type = 5.0 mmol/l).
CONCLUSIONS
These results confirm the potency of glucokinase as the pancreatic β-cell glucose sensor, and they demonstrate that responsiveness to diazoxide varies with genotype in glucokinase hyperinsulinism resulting in hypoglycemia, which can be more difficult to control than previously believed.
Hypoglycemia in infants with congenital hyperinsulinism has been associated with mutations that affect the regulation of insulin secretion by all three major classes of metabolic fuels: glucose, amino acids, and fatty acids (1–6). The most common of these disorders is caused by recessive mutations of the β-cell ATP-sensitive K+(KATP) channel; these mutations cause severe neonatal hypoglycemia that does not respond to medical therapy with diazoxide, a KATP channel agonist, and often requires near-total pancreatectomy (7,8). Other genetic forms of congenital hyperinsulinism, such as dominant mutations of glutamate dehydrogenase, cause less severe disease, with hypoglycemia that may not be recognized until childhood or even adult life and that responds well to diazoxide therapy (4,9–11). In 1998, the first case of hyperinsulinism caused by a dominant gain-of-function mutation of glucokinase was reported (12). This remains one of the rarest forms of hyperinsulinism, and information on its clinical and biochemical manifestations is limited because only a few cases have been reported subsequently (13–19). Most of these cases have been identified because of family histories of hypoglycemia with dominant patterns of transmission, and most affected individuals were reported to have relatively mild disease that could be managed medically with diazoxide.
Glucokinase catalyzes the first step in glucose metabolism in pancreatic β-cells and liver (20). It exists as a monomer in three conformations that control catalytic function: a closed form, an open form, and a super open form (21). Transitions between these conformations are controlled by glucose concentration, giving a sigmoidal enzyme activity curve, as well as by allosteric modulators. Binding of novel glucokinase activator molecules, such as RO0281675, to the allosteric site increases glucokinase activity, resulting in both augmented hepatic glucose uptake and lowering of the β-cell threshold for glucose-stimulated insulin release (22). In the liver, glucokinase enzyme activity is inhibited by binding of glucokinase regulatory protein, which also leads to nuclear sequestration of the enzyme (23).
Glucokinase serves a critical physiological function as the β-cell glucose sensor. It determines the glucose threshold for insulin release because of the low affinity of the enzyme for its substrate, glucose (half-maximal activity, S0.5, occurs at 7.5 mmol/l glucose). Heterozygous mutations that reduce enzyme activity cause a subtype of maturity-onset diabetes of the young 2 (MODY2), whereas, as noted above, heterozygous activating mutations cause hypoglycemia. Expression of these activating mutations shows increased affinity for glucose with elevations of calculated enzyme activity indexes and lower calculated glucose thresholds for insulin release (24).
Based on the initial cases reported, glucokinase hyperinsulinism has been assumed to be a mild form of hypoglycemia that can easily be managed medically. However, one reported case with a more severe clinical phenotype of uncontrollable hypoglycemia suggests that the range of manifestations of glucokinase hyperinsulinism may be greater than has been appreciated (14). The purpose of this report is to describe three children with hyperinsulinism caused by de novo glucokinase mutations who exhibit marked differences in responsiveness to medical therapy that correlate with differences in enzyme activity indexes.
RESEARCH DESIGN AND METHODS
All clinical studies were approved by the institutional review board of the Children's Hospital of Philadelphia. Patient tests were performed in the Clinical Translational Research Center of the Children's Hospital of Philadelphia. Protein sensitivity and acute insulin response tests to calcium, leucine, and glucose were carried out as previously described (25,26). Insulin assays were performed using an enzyme-linked immunosorbent assay kit (Mercodia; Alpco Diagnostics, Salem, NH).
Identification of glucokinase gene mutations.
Genomic DNA from peripheral lymphocytes was amplified by PCR using published primer sequences for glucokinase (Genbank accession no. M88011) (27). Products were sequenced using a BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) and analyzed using Sequencher 3.1 (Gene Codes, Ann Arbor, MI).
Biochemical characterization of ins454A, W99L, and M197I mutants.
Recombinant human islet wild-type glucokinase, the three patient mutants (ins454A, W99L, and ins454A), known instability mutant E300K, and a series of designed mutations (M197A, D, E, F, K, L, T, and V) were generated using methods previously described (28). Briefly, the mutations were cloned for expression as glucokinase fusion proteins containing a COOH-terminal glutathionyl S-transferase (GST). GST-glucokinase was produced in Escherichia coli and then purified from crude extracts to near homogeneity by affinity chromatography using glutathione-agarose (Sigma, St. Louis, MO). Characterization of GST cleaved enzymes was also performed by cleavage of GST with Factor Xa. The product was then purified again using glutathione-agarose and Factor Xa with benzamidine Sepharose 6B to yield the cleaved proteins.
Kinetic analysis of the expressed forms of glucokinase was performed using the protocols developed previously (13,16), both in the presence and absence of the glucokinase activator compound RO0281675. The activity index, an expression of the proposed enzyme's in situ phosphorylation capacity, and the theoretical threshold for glucose-stimulated insulin release were calculated as previously described (13,20). Kinetic analysis was also performed in the presence of recombinant human glucokinase regulatory protein, a competitive inhibitor of glucokinase, with and without sorbitol-6-phosphate, a structural analog of fructose-6-phosphate that enhances glucokinase regulatory protein inhibition (16). For this purpose the assay was performed with glucose at 3 mmol/l for wild type and the glucose concentration adjusted based on S0.5 of the different mutants in both the presence and the absence of 10 μmol/l sorbitol-6-phosphate.
The stability of mutant and wild-type GST-glucokinase to low glucose and high temperature was tested as previously described by spectrophotometric enzyme assay (16,29). The stability of W99L was measured using tryptophan fluorescence by comparing the quantum (photonic) yield of wild-type glucokinase to the mutant glucokinase protein solution (30).
Structural analysis.
Structural modeling of M197I, W99L, ins454A, and published activating mutations was performed using the structure of the super-open form of glucokinase and closed form determined by Kamata et al. (21). Modeling was performed using a SwissPdb viewer (31).
Patients.
Child 1 is a 17-year-old male subject who had a large-for-gestational-age birth weight of 4.8 kg at term. Hypoglycemia was detected in the first hour after delivery, and high rates of intravenous glucose infusion were required to control blood glucose levels (18 mg · kg−1 · min−1; normal <5–6). Plasma insulin levels at times of hypoglycemia were found to be elevated (8–15 μU/ml; normal <3). He was not fully responsive to diazoxide, defined as being able to fast >12 h with blood glucose of >70 mg/dl. However, treatment with a high dose of diazoxide (20 mg · kg−1 · day−1) and feedings every 4 h prevented symptoms of hypoglycemia, although plasma glucose levels remained largely between 2.8 and 3.3 mmol/l (50–60 mg/dl). There were no family members with hypoglycemia.
At 1 year of age, he was referred to the Children's Hospital of Philadelphia because of persistently low plasma glucose values of 2.2–3.3 mmol/l (40–60 mg/dl). A trial of octreotide gave only transient improvement in control of hypoglycemia (32). Because of persistent hypoglycemia at 23 months of age, a 95% pancreatectomy was performed; however, this failed to control hypoglycemia. Diazoxide treatment was retried, but plasma glucose fell to 3.3 mmol/l (60 mg/dl) after only 5 h of fasting. Treatment consisted of feedings given frequently, day and night. Despite the low levels of plasma glucose, hypoglycemic symptoms rarely occurred, mainly triggered by exercise or high-carbohydrate feedings. His parents report that his hypoglycemia appeared to improve after age 11 years, and nighttime feedings were stopped. However, they also report that he now has occasional hyperglycemia after meals, suggestive of mild glucose intolerance. Currently, child 1 has normal school performance.
Child 2 is a boy with a normal term birth weight of 3.2 kg. Hypoglycemia was not recognized until age 6 years, when he became unarousable a few hours after eating and was found to have a blood glucose of 48 mg/dl (2.7 mmol/l). There was no family history of hypoglycemia. Treatment with 10 mg · kg−1 · day−1 of diazoxide failed to control hypoglycemia. He was suspected to have an insulinoma because of the apparent new onset of hypoglycemia. Endoscopic ultrasound suggested a lesion in the head of the pancreas that was found to be a lymph node at surgery; biopsies of the neck and body of the pancreas were normal. Octreotide failed to control hypoglycemia. Successful control of hypoglycemia has required a combination of diazoxide (15 mg · kg−1 · day−1) plus a low rate of continuous dextrose overnight by gastrostomy tube (2 mg · kg−1 · min−1). School performance was considered to be normal.
Child 3 is a 16-year-old boy with a large-for-date birth weight of 4.9 kg at 41 weeks of gestation. Hypoglycemia was detected in the first hours after birth, requiring treatment with intravenous dextrose at 12 mg · kg−1 · min−1. There was no history of hypoglycemia in the parents or in two older siblings. Hyperinsulinemic hypoglycemia was documented but was considered to have resolved after 2 months of treatment with prednisone. There was no further suspicion of hypoglycemia until 14 years of age, when he suffered two brief seizures, each occurring 3 h after breakfast. Plasma glucose was found to be 2.2 mmol/l (40 mg/dl). Hyperinsulinemic hypoglycemia was diagnosed. A high dose of diazoxide at 900 mg (15 mg · kg−1 · day−1) was required to maintain fasting plasma glucose levels >3.9 mmol/l (>70 mg/dl). School performance was considered normal, although not on a par with his siblings.
RESULTS
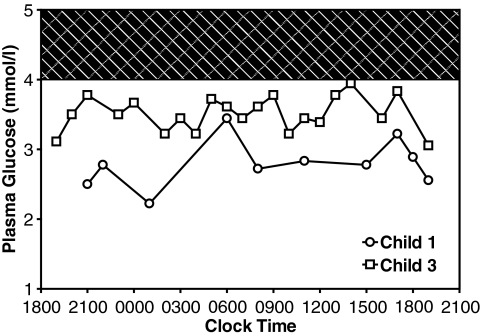
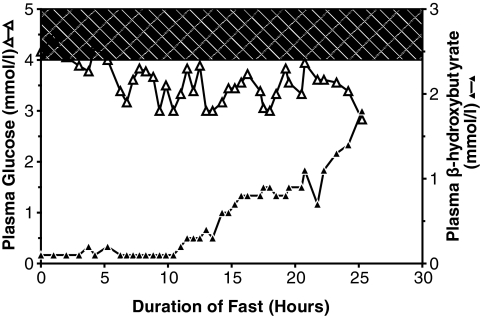
Figure 1 shows the diurnal profile of plasma glucose levels in child 1 and child 3 (at ages 4 and 15 years, respectively). Glucose values ranged between narrow limits, rarely rising into the normal range even after meals, but also rarely falling very low, even during 12 h of overnight fasting (child 3). Child 1, whose hypoglycemia was more difficult to manage, had significantly lower mean plasma glucose levels (mean ± SE) than child 3 (50 ± 2 vs. 63 ± 1 mg/dl, respectively; P < 0.0001). As shown in Fig. 2, child 2 fasted for 24 h with a stable plasma glucose ranging from 3 to 4 mmol/l (54–72 mg/dl) before dropping to 2.8 mmol/l (50 mg/dl). During the fast he developed a significant ketonemic response, albeit lower than normally seen at this level of blood glucose (β-hydroxybutyrate >2.5 mmol/l).
FIG. 1.
Diurnal patterns of plasma glucose concentrations in child 1 and child 3. Plasma glucose was measured before meals and after overnight fasting conditions. Shaded area indicates the normal range of plasma glucose (4.0–5.0 mmol/l, 72–90 mg/dl). Child 1 was 4 years old, 2 years after near-total pancreatectomy; child 3 was 15 years old. Neither child was on medical therapy. Mean glucose was 50 ± 2 mg/dl in child 1 and 63 ± 1 mg/dl in child 3. P < 0.0001 between children.
FIG. 2.
Plasma glucose and β-hydroxybutyrate responses to fasting in child 2. Mean plasma glucose was 64 ± 1 mg/dl. β-Hydroxybutyrate values increased from 0.1 to 1.8 mmol/l, whereas fasting plasma glucose levels decreased. At the end of the fast, plasma C-peptide was suppressed (0.16 nmol/l, normal 0.26–1.32 nmol/l). Glucose response to glucagon stimulation was 36 mg/dl. Reference normals when fasting blood glucose <50 mg/dl: β-hydroxybutyrate >2.5 mmol/l, and glycemic response to glucagon >30 mg/dl. △, plasma glucose (SureStep bedside meter); ▲, β-hydroxybutyrate (Precision Xtra bedside meter).
Table 1 shows the responses in child 1 and child 3 to stimulation tests of insulin secretion. Both children showed exaggerated acute insulin responses to intravenous glucose. In child 3, acute insulin responses to calcium and leucine were normal, in contrast to the hyperresponsiveness to calcium and leucine seen in patients with KATP channel or glutamate dehydrogenase mutations, respectively. In further contrast to patients with KATP or glutamate dehydrogenase hyperinsulinism, hypoglycemia was not provoked by oral protein tolerance tests in either child 1 or child 3. All three children had normal serum concentrations of cholesterol and triglyceride.
TABLE 1.
Acute insulin response to insulin secretagogues and plasma lipid concentrations
| Child 1 | Child 2 | Child 3 | Normal | |
|---|---|---|---|---|
| Glucose AIR (pmol/l) | 483 | — | 474 | 318 ± 228 |
| Calcium AIR (pmol/l) | — | — | −24.0 | 0.6 ± 11.4 |
| Leucine AIR (pmol/l) | — | — | 30.0 | 12.0 ± 66 |
| Cholesterol (mmol/l) | 2.9 | 4.3 | 4.3 | 2.8–5.8 |
| Triglycerides (mmol/l) | 0.29 | 0.61 | 1.7 | 0.4–1.9 |
Review of the surgical specimen of resected pancreas from child 1 showed normal islet distribution, shape, and size from head to tail. There were occasional large endocrine cell nuclei that occupied an area at least three times as large as the surrounding endocrine cell nuclei, similar to the histological features seen in recessive KATP hyperinsulinism. The surgical biopsies from child 2 showed normal pancreas.
Genetic mutation analysis.
In child 1, mutation analysis of genomic DNA identified a heterozygous novel missense mutation, ins454A (nt 13631-364 ins CGG). In child 2, a glucokinase mutation was suspected because of the stability of his hypoglycemia (Fig. 2); he was found to have a heterozygous novel W99L missense mutation (nt 296 G>T). In child 3, a mutation in glucokinase was also suspected because of the stability of hypoglycemia; he had a heterozygous novel M197I mutation (nt 591 G>T). The mutations were not detected in any of the children's parents and were not found in 100 normal chromosomes. No mutations were found in other hyperinsulinism genes, including ABCC8, KCNJ11, and GLUD1.
Biochemical analysis of expressed ins454A, W99L, and M197I glucokinase mutations.
Table 2 shows the enzymatic activities of the purified mutant forms of glucokinase. The ins454A mutation resulted in a markedly increased affinity for glucose (decreased glucose S0.5). The glucose S0.5 values in child 2 and child 3 were reduced to a lesser degree. The turnover rate, kcat, of the ins454A mutation was similar to wild-type glucokinase; however, kcat for the GST-glucokinase fusion M197I mutant was ∼60% of wild type, and kcat for W99L was increased compared with wild type. Hill numbers for all three mutations were only slightly reduced compared with wild type. Kinetic variables for the GST-glucokinase fusion proteins were similar to their cleaved counterparts (data not shown). For all mutations, the calculated enzyme activity index was increased compared with wild type, consistent with a gain of glucokinase enzyme function. This was especially true for child 1, whose ins454A mutation resulted in an activity index >25 times normal, consistent with his poorer response to diazoxide therapy compared with child 2 and child 3.
TABLE 2.
Kinetic characteristics of glucokinase ins454A, W99L, and M197I
| Mutants | Yield (mg/l) | kcat(sec−1) | Glucose S0.5(mmol/l) | ATP Km(mmol/l) | nH(Hill number) | Activity index | Activation by GKA (fold) | EC50for GKA (μmol/l) |
|---|---|---|---|---|---|---|---|---|
| ins454A | 10.9 ± 3.1 | 53.2 ± 2.9 | 1.1 ± 0.1 | 0.3 | 1.2 | 39.5 ± 1.7 | No activation | N/A |
| W99L | 19.9 ± 1.7 | 85.6 ± 3.1 | 2.9 ± 0.1 | 0.4 | 1.6 | 13.3 ± 0.8 | 11.8 | 4.3 |
| M197I | 62.1 ± 0.3 | 38.1 ± 4.5 | 2.6 ± 0.2 | 1.5 | 1.6 | 4.8 ± 0.3 | 11.9 | 4.9 |
| M197L | 34.5 | 54.5 | 5.4 | 1.0 | 1.4 | 3.8 | 13.9 | 6.2 |
| M197V | 25.1 | 44.7 | 2.6 | 0.5 | 1.7 | 8.0 | 13.1 | 3.5 |
| M197A | 36.4 | 53.3 | 3.2 | 0.3 | 1.7 | 7.0 | 14.7 | 3.8 |
| M197T | 26.7 | 53.9 | 4.1 | 0.5 | 1.6 | 4.4 | 18.3 | 5.5 |
| M197F | 40.3 | 21.2 | 54.6 | 0.2 | 1.3 | 0.1 | 8.6 | 6.1 |
| M197K | 36.4 | 3.78 | 86.7 | 0.2 | 1.1 | 0.03 | 13.6 | 9.3 |
| M197D | 27.7 | 10.3 | 197.0 | 0.3 | 1.1 | 0.03 | 6.0 | 2.8 |
| M197E | 6.82 | 21.0 | 56.2 | 1.3 | 0.2 | 0.1 | 9.0 | 2.7 |
| Wild type | 43.4 ± 3.8 | 62.3 ± 4.8 | 7.6 ± 0.2 | 0.4 | 1.7 | 1.5 ± 0.1 | 15.8 ± 0.6 | 6.9 ± 0.4 |
Kinetic data of purified expressed GST-tagged glucokinase with hyperinsulinism-causing ins454A (child 1), W99L (child 2), and M197I (child 3) mutations. EC50relates to the activity index. GKA, glucokinase activator; EC50, half-maximal effective concentration.
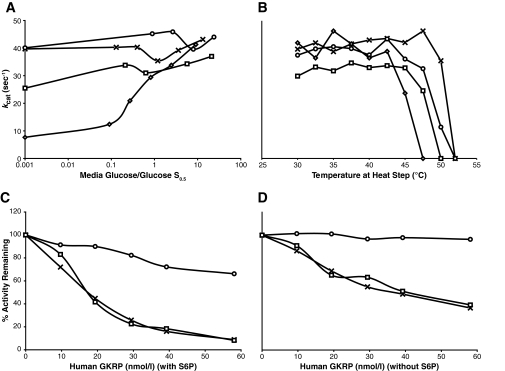
Because enzyme stability can potentially affect glucokinase activity, we examined the effect of incubating ins454A and M197I mutant enzymes under conditions of low glucose and high temperature (Fig. 3A and B). The stability of enzyme activity of the two mutants after incubation at low glucose concentrations was similar to that of wild-type glucokinase and was much greater than that of the known diabetes-associated instability mutant E300K (Fig. 3A). The thermal stability of the ins454A and M197I mutant proteins was intermediate between that of wild-type and E300K glucokinase (Fig. 3B). The thermal stability of W99L was investigated by tryptophan fluorescence under similar glucose conditions (30) and was also intermediate between that of wild type and the E300K instability mutant (data not shown).
FIG. 3.
Thermal stability of GST-tagged wild-type and mutant glucokinase (A and B). Response of GST-glucokinase mutants to inhibition with glucokinase regulatory protein (GKRP) (C and D). GST-glucokinase levels were 22 nmol/l in all tests. A: Effect of glucose concentration on expressed GST-tagged glucokinase with ins454A and M197I hyperinsulinism mutations and the known E300K instability mutant. For M197I, the studies were not extended below the glucose S0.5 of 2.6 mmol/l. B: Effect of temperature on mutant glucokinase enzyme activities. For these assays, enzymes were incubated for 30 min with glucose levels at their respective S0.5. C and D: Inhibition of glucokinase activity by glucokinase regulatory protein measured in the presence and absence of sorbitol-6-phosphate (S6P), respectively. ○, ins454A; □, M197I; ◇, E300K; X, wild type.
As shown in Table 2, the ins454A GST-glucokinase mutant was unresponsive to the glucokinase activator compound RO0281675, suggesting that its activity was already maximal. The response of the W99L and M197I GST-glucokinase mutants to the activator was similar to wild type. The latter finding may be attributable to the M197I mutation being 19 Å away from the activator site and therefore unlikely to interfere with activator binding. Another plausible explanation is the moderate degree of enzyme activation leaving room for further activation by the glucokinase activator, as in the case of W99L.
Figure 3C and D shows the response of two of the mutant glucokinase enzymes to inhibition by hepatic glucokinase regulatory protein. W99L and M197I GST-glucokinase mutants had similar responses to glucokinase regulatory protein as wild type (W99L results not shown). The ins454A mutant showed little response to inhibition by glucokinase regulatory protein.
Biochemical analysis of additional mutants at residue 197.
As shown in Table 2, to evaluate the function of the M197 residue, eight additional mutations were designed that substituted a range of amino acids from hydrophobic to hydrophilic at this site. Generally, substitution of hydrophobic residues for the normal methionine resulted in increased affinity for glucose and increased enzyme activity indexes, whereas changes to hydrophilic or amphipathic residues reduced enzyme activity.
DISCUSSION
The results of these studies demonstrate that congenital hyperinsulinism in these three children was caused by three novel activating mutations of glucokinase, ins454A, W99L, and M197I. Expression of the mutations demonstrated changes in glucokinase kinetics consistent with increased enzyme activity. Increases in calculated glucokinase activity indexes ranged from 25 times normal in child 1, with the ins454A mutation, to three times normal in child 3, with the M197I change. These differences in enzyme activation correlated with the more severe diazoxide-unresponsive hypoglycemia of child 1; the intermediate, partially diazoxide-responsive hypoglycemia of child 2; and the milder diazoxide-responsive hypoglycemia of child 3.
As shown in Fig. 4A, the mutations at positions 99, 197, and 454 of glucokinase are located on the reverse side of the enzyme, opposite the catalytic cleft. The ins454A mutation is adjacent to three previously reported glucokinase activating mutations (V455M, A456V, and V452L), whereas the W99L mutation is located at the same position as a previously reported W99R activating mutation (Table 3 summarizes known glucokinase hyperinsulinism mutations). Most of the 11 glucokinase activating mutations that have been identified occur in or close to the region of the enzyme that interacts with allosteric activator compounds, such as RO0281675, and with the inhibitory glucokinase regulatory protein. Heredia et al. (33,34) have shown that some of these mutations (T65I and A456V) increase glucose binding to the enzyme, whereas the others (W99R, Y214C, and V455M) facilitate enzyme isomerization into the active form. The two mutations that enhance glucose binding also abrogate interaction with glucokinase regulatory protein, suggesting that these mutations cause increased glucokinase activity by favoring maintenance of the closed, active form of the enzyme and preventing opening of the catalytic cleft into the inactive form of the enzyme. The ins454A glucokinase mutation in child 1 appears to fit into this category of mutations, which makes the enzyme unresponsive to both inhibition by glucokinase regulatory protein and allosteric activation by RO0281675. Loss of inhibition by glucokinase regulatory protein does not seem to be a major determinant of clinical phenotype (34) because the Y214C mutation retains sensitivity to glucokinase regulatory protein but was reported to have diazoxide-unresponsive hypoglycemia similar to ins454A (Table 3).
FIG. 4.
A: Location of glucokinase mutations in the active (closed) form of the enzyme crystallographic structure. B: Location of the M197I residue tucked within a hydrophobic pocket of the active conformation of glucokinase. C: Location of the M197I residue projecting into the hydrophilic space of the inactive conformation of the crystal.
TABLE 3.
Comparison of clinical and biochemical features of published glucokinase activating mutants associated with hyperinsulinism
| V455M | A456V | T65I | W99R | Y214C | G68V | S64Y | V452L | ins454A | W99L | M197I | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of families | 1 | 2 | 1 | 1 | — | 1 | — | — | — | — | — |
| de novo case subjects | — | — | — | 1 | 1 | — | 1 | 1 | 1 | 1 | 1 |
| Total cases (n = 29) | 5 | 5 | 2 | 3 | 1 | 8 | 1 | 1 | 1 | 1 | 1 |
| Birth weight (kg) | 2.9–4.1 | 2.4–3.8 | 3.1 | 3.1, 4.0 | 4.4 | 1.9–3.7 | 4.3 | 5.9 | 4.9 | 3.2 | 4.9 |
| Age at diagnosis | |||||||||||
| Neonatal (n = 8) | — | 1 | 1 | 2 | 1 | — | 1 | 1 | 1 | — | — |
| Childhood (n = 7) | 2 | 1 | — | — | — | 3 | — | — | — | 1 | — |
| Adolescence (n = 4) | 1 | — | — | — | — | 2 | — | — | — | — | 1 |
| Adulthood (n = 10) | 2 | 3 | 1 | 1 | — | 3 | — | — | — | — | — |
| Severity of hypoglycemia | |||||||||||
| Mild or untreated (n = 12) | 1 | 3 | — | 1 | — | 7 | — | — | — | — | — |
| Diazoxide treated (n = 13) | 4 | 2 | 1 | 2 | — | — | 1 | 1 | — | 1 | 1 |
| Diazoxide and octreotide treated (n = 1) | — | — | — | — | — | 1 | — | — | — | — | — |
| Required surgery (n = 3) | — | — | 1 | — | 1 | — | — | — | 1 | — | — |
| Pretreatment plasma glucose (normal > 3.9 mmol/l) | 1.3–2.5 | 2.1–3.5 | 2.2–3.0 | 2.0–3.5, 2.1 | 0.1–2.6 | 1.6–3.3 | 2.0 | 2.6–3.3 | 1.7–2.8 | 2.6–3.7 | 2.6–3.6 |
| Response to diazoxide | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Partial | Yes |
| S0.5(normal = 7.55 mmol/l)* | 3.0 | 2.0 | 1.8 | 4.5 | 1.2 | 1.9 | 1.5 | 2.6 | 1.1 | 2.9 | 2.6 |
| Relative activity index* | 5.2 | 17 | 3.1 | 4.1 | 130 | 16 | 22 | 11 | 26 | 8.9 | 3.1 |
| Original clinical report reference no. | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | — | — | — |
*Values from published reports.
The novel M197I amino acid substitution reported here has a unique location that is remote from the other glucokinase activating mutations. As shown in Fig. 4B and C, the M197I mutation alters a methionine residue that moves in and out of a hydrophobic pocket during the transition between the active and inactive forms of glucokinase. The importance of this hydrophobic lock-and-key interaction is demonstrated by the series of designed mutations of M197 (Table 2). Substitutions with isoleucine or other hydrophobic amino acids retained or enhanced glucokinase activity. However, substitutions with hydrophilic amino acids essentially inactivated the enzyme. These observations have been confirmed in a recent report by Pal and Miller (35). The M197I mutation indicates that activating mutations of glucokinase need not be restricted to the allosteric domain, where all previous defects have been located, and suggests that additional mutation sites are likely to be identified in patients with glucokinase hyperinsulinism.
Glucokinase mutations have been found infrequently in mutation analysis of patients with congenital hyperinsulinism, accounting for only 5 of 167 patients in recently reported series (18) and only 3 of 212 cases we have analyzed.Table 3 summarizes the major features of hyperinsulinism caused by previously reported activating mutations of glucokinase and the three additional mutations described in this report. Familial cases account for 76% of the 29 known patients. This contrasts with the high proportion of de novo cases in glutamate dehydrogenase hyperinsulinism and other dominant disorders, suggesting that many cases are not identified in the absence of a family history of hypoglycemia. Only 3 of the 29 patients had surgery, suggesting a relatively mild hypoglycemia phenotype, especially in the familial cases. Many of the familial cases escaped recognition of their hypoglycemia disorder until beyond the neonatal period or even into adult life. In all of the familial cases, treatment with the β-cell KATP channel agonist diazoxide was reported to have been successful in controlling hypoglycemia. Child 3 (M197I mutation) fits this milder, diazoxide-responsive phenotype, although it should be noted that unusually high doses of diazoxide were needed to maintain even low-normal levels of plasma glucose. However, similar to child 2 (W99L), incomplete responsiveness to diazoxide was also apparent in some of the reported cases in the six families with the mild hypoglycemia phenotype (W99R, A456V).
In contrast, children with Y214C and ins454A mutations had a much more severe form of hypoglycemia. Our child 1 with the ins454A mutations could not be controlled on diazoxide, even at a very high dose of 20 mg · kg−1 · day−1. In the case of the Y214C mutation, diazoxide responsiveness is unknown because only a low dose was tried. Both of these children required surgery and continued to have poorly controlled hypoglycemia, despite near-total pancreatectomy. These cases make it apparent that the clinical manifestations of hyperinsulinism in some glucokinase mutations can be as severe and as unresponsive to diazoxide treatment as in children with hyperinsulinism caused by recessive mutations of the KATP channel subunits sulfonylurea receptor 1 and Kir6.2.
The observations in the present three cases identify clinical features that may be useful for distinguishing glucokinase hyperinsulinism. A notable feature in the cases presented here was the remarkable stability of their hypoglycemia (Figs. 1 and 2), consistent with a resetting of the threshold for insulin release at a value lower than normal. This contrasts with other forms of hyperinsulinism in infants, where blood glucose concentrations can fall without interruption to extremely low values. Insulin responses to secretagogues may also help to distinguish glucokinase hyperinsulinism from other disorders. The glucokinase activating mutations in our three cases are not associated with hyperresponsiveness to calcium (as seen in KATP hyperinsulinism) or leucine (as seen in glutamate dehydrogenase hyperinsulinism), did not predispose to protein-induced hypoglycemia (as seen in KATP and glutamate dehydrogenase hyperinsulinism), but are accompanied by increased acute insulin response to glucose (which is blunted in KATP hyperinsulinism).
Information about islet morphologic abnormalities in patients with glucokinase hyperinsulinism remains limited. In child 1, a small proportion of islet cells showed nucleomegaly similar to, but to a lesser extent than, that seen in diffuse KATP hyperinsulinism. The increase in islet size described by Cuesta-Munoz et al. (14) in a child with a Y214C glucokinase mutation was not apparent in our child. No abnormalities were noted in the pancreas of the mother of the child with the T65I mutation (16). Although further study may identify specific histological features, current information suggests that it may not be easy to distinguish glucokinase hyperinsulinism from KATP hyperinsulinism or, possibly, from normal pancreas.
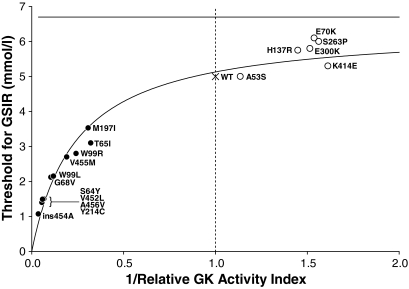
Figure 5 shows the effects of different glucokinase activating mutations on predicted glucose threshold for insulin release, based on the relative activity indexes of the expressed proteins in vitro. Cases reported to be diazoxide unresponsive (ins454A and Y214C) have very low glucose thresholds, as does the A456V mutation, which in one patient was not completely responsive to diazoxide. In contrast, the better responses to diazoxide in child 2 and child 3 correlate with higher calculated glucose thresholds. It should be noted that the range of plasma glucose levels in our three patients and in the reports of other glucokinase hyperinsulinism cases (Table 3) tends to be higher than their predicted glucose thresholds (Fig. 5). This may partly reflect the effects of counterregulatory responses to hypoglycemia. An additional potential problem in correlating data on glucokinase kinetics with clinical features in patients is that some of the changes in enzyme properties exert opposing effects. One example of this phenomenon is the V62M mutation, which has been associated with MODY2 diabetes (36). When expressed in vitro, this mutation has an increased activity index consistent with causing hypoglycemia, rather than diabetes. The increased instability of this mutant form of glucokinase may counterbalance its enhanced activity and explain why the mutation results in a net loss of function in vivo (36). Similarly, although the ins454A, W99L, and M197I mutations have increased activity indexes, they also have slightly reduced stability. Moreover, the impact of the reduced affinity of M197I for ATP in vivo is uncertain. Given these problems, efforts to understand the in vivo and in vitro phenotypes of glucokinase hyperinsulinism mutations will require that accurate data be obtained on the clinical features of affected individuals. For example, these data should especially include careful documentation of the ability of diazoxide treatment to completely normalize plasma glucose levels and accurate estimates of the in vivo glucose “set point,” as illustrated in Figs. 1 and 2.
FIG. 5.
Calculated thresholds for glucose-stimulated insulin release (GSIR) in activating and inactivating mutations of glucokinase. Thresholds are plotted against the inverse of the mutant enzyme activity index relative to wild-type (WT) enzyme. Because relative expression of the glucokinase forms is affected by enzyme affinity for glucose and the ambient glucose concentration, the wild-type enzyme dominates the estimated threshold for the heterozygous inactivating defects, but the mutant enzyme dominates the threshold for activating mutations. Thus, the threshold for inactivating mutations plateaus at ∼7 mmol/l, whereas the calculated threshold for severe activating mutations approaches zero as the relative activity increases. For purposes of consistency, all kinetic data in the figure are from the laboratory of F.M.M. Threshold and activity indexes were calculated per Gloyn et al. (24). ●, glucokinase hyperinsulinism mutations; ○, MODY2 mutations; X, wild type. GK, glucokinase.
In summary, these three cases of congenital hyperinsulinism caused by activating mutations of glucokinase emphasize the key role that this enzyme plays in setting the glucose threshold of the pancreatic islets. These children had de novo mutations, which made them difficult to recognize. Clues to their diagnosis included persistent, but stable, hypoglycemia and exaggerated insulin responses to intravenous glucose stimulation. These cases indicate that the spectrum of hyperinsulinism attributable to glucokinase activating mutations can range from mild and intermediate cases, which can be managed medically with diazoxide, to severe cases that are diazoxide unresponsiveand may require additional treatment, includingnear-total pancreatectomy, to control hypoglycemia. These cases also illustrate the potentials and limitations of new approaches to develop glucokinase activator drugs for the treatment of type 2 diabetes.
Acknowledgments
These studies were supported in part by National Center for Research Resources Clinical and Translational Science Award Grant UL1RR024134 and National Institutes of Health (NIH) grants R01DK53012, R01DK56268 (to C.A.S.), and R01DK22122 (to F.M.M.). A.B.S. was supported by NIH Grant T32DK63688 (to C.A.S.)
J.G. and R.T. were employed by Roche during the research phase of this manuscript. J.G. is employed by Roche and is engaged in preclinical research of antidiabetic drugs. R.T. is no longer employed by Roche. No other potential conflicts of interest relevant to this article were reported.
The assistance of the Diabetes and Endocrinology Research Center, Jae Kwagh, the Clinical and Translational Research Center (CTRC), and the nurses of the CTRC and the Hyperinsulinism Center are gratefully acknowledged. We thank Dr. David G. Stokes for his helpful suggestions in editing this manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Stanley CA, Baker L: Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics 1976; 57: 702– 711 [PubMed] [Google Scholar]
- 2.Stanley CA: Hyperinsulinism in infants and children. Pediatr Clin North Am 1997; 44: 363– 374 [DOI] [PubMed] [Google Scholar]
- 3.Glaser B, Thornton PS, Herold K, Stanley CA: Clinical and molecular heterogeneity of familial hyperinsulinism (Letter). J Pediatr 1998; 133: 801– 802 [DOI] [PubMed] [Google Scholar]
- 4.Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M: Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 1998; 338: 1352– 1357 [DOI] [PubMed] [Google Scholar]
- 5.Kelly A, Ng D, Ferry RJ, Grimberg A, Koo-McCoy S, Thornton PS, Stanley CA: Acute insulin responses to leucine in children with the hyperinsulinism/hyperammonemia syndrome. J Clin Endocrinol Metab 2001; 86: 3724– 3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM: A signaling role of glutamine in insulin secretion. J Biol Chem 2004; 279: 13393– 13401 [DOI] [PubMed] [Google Scholar]
- 7.Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, Landau H, Stanley CA, Thornton PS, Clement JP, Bryan J, Aguilar-Bryan L, Permutt MA: Mutations in the sulfonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet 1996; 5: 1813– 1822 [DOI] [PubMed] [Google Scholar]
- 8.Nestorowicz A, Inagaki N, Gonoi T, Schoor KP, Wilson BA, Glaser B, Landau H, Stanley CA, Thornton PS, Seino S: A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes 1997; 46: 1743– 1748 [DOI] [PubMed] [Google Scholar]
- 9.Thornton PS, MacMullen C, Ganguly A, Ruchelli E, Steinkrauss L, Crane A, Aguilar-Bryan L, Stanley CA: Clinical and molecular characterization of a dominant form of congenital hyperinsulinism caused by a mutation in the high-affinity sulfonylurea receptor. Diabetes 2003; 52: 2403– 2410 [DOI] [PubMed] [Google Scholar]
- 10.Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL: A novel KCNJ11 mutation associated with congenital hyperinsulinism reduces the intrinsic open probability of beta-cell ATP-sensitive potassium channels. J Biol Chem 2006; 281: 3006– 3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton PT, Eaton S, Aynsley-Green A, Edginton M, Hussain K, Krywawych S, Datta V, Malingre HE, Berger R, van den Berg IE: Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest 2001; 108: 457– 465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, Stanley CA, Thornton PS, Permutt MA, Matschinsky FM, Herold KC: Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 1998; 338: 226– 230 [DOI] [PubMed] [Google Scholar]
- 13.Christesen HB, Jacobsen BB, Odili S, Buettger C, Cuesta-Munoz A, Hansen T, Brusgaard K, Massa O, Magnuson MA, Shiota C, Matschinsky FM, Barbetti F: The second activating glucokinase mutation (A456V): implications for glucose homeostasis and diabetes therapy. Diabetes 2002; 51: 1240– 1246 [DOI] [PubMed] [Google Scholar]
- 14.Cuesta-Munoz AL, Huopio H, Otonkoski T, Gomez-Zumaquero JM, Nanto-Salonen K, Rahier J, Lopez-Enriquez S, Garcia-Gimeno MA, Sanz P, Soriguer FC, Laakso M: Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes 2004; 53: 2164– 2168 [DOI] [PubMed] [Google Scholar]
- 15.Dullaart RP, Hoogenberg K, Rouwe CW, Stulp BK: Family with autosomal dominant hyperinsulinism associated with A456V mutation in the glucokinase gene. J Intern Med 2004; 255: 143– 145 [DOI] [PubMed] [Google Scholar]
- 16.Gloyn AL, Noordam K, Willemsen MA, Ellard S, Lam WW, Campbell IW, Midgley P, Shiota C, Buettger C, Magnuson MA, Matschinsky FM, Hattersley AT: Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes 2003; 52: 2433– 2440 [DOI] [PubMed] [Google Scholar]
- 17.Wabitsch M, Lahr G, Van de Bunt M, Marchant C, Lindner M, von Puttkamer J, Fenneberg A, Debatin KM, Klein R, Ellard S, Clark A, Gloyn AL: Heterogeneity in disease severity in a family with a novel G68V GCK activating mutation causing persistent hyperinsulinaemic hypoglycaemia of infancy. Diabet Med 2007; 24: 1393– 1399 [DOI] [PubMed] [Google Scholar]
- 18.Christesen HB, Tribble ND, Molven A, Siddiqui J, Sandal T, Brusgaard K, Ellard S, Njølstad PR, Alm J, Jacobson BB, Hussain K, Gloyn AL: Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: prevalence in children and characterisation of a novel GCK mutation. Eur J Endocrinol 2008; 159: 27– 34 [DOI] [PubMed] [Google Scholar]
- 19.Meissner T, Marquard J, Cobo-Vuilleumier N, Maringa M, Rodriguez-Bada P, Garcia-Gimeno MA, Baixeras E, Weber J, Olek K, Sanz P, Mayatepek E, Cuesta-Munoz AL: Diagnostic difficulties in glucokinase hyperinsulinism. Horm Metab Res 2009; 41: 320– 326 [DOI] [PubMed] [Google Scholar]
- 20.Matschinsky FM: Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes 200251( Suppl. 3); S394– S404 [DOI] [PubMed] [Google Scholar]
- 21.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y: Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 2004; 12: 429– 438 [DOI] [PubMed] [Google Scholar]
- 22.Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE: Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003; 301: 370– 373 [DOI] [PubMed] [Google Scholar]
- 23.van Schaftingen E, Viega da Cunha M: Discovery and role of glucokinase regulatory protein. In Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics Matschinsky FM, Magnuson MA. Eds. Basel, Switzerland, Karger, 2004, p. 193– 207 [Google Scholar]
- 24.Gloyn AL, Odili S, Buettger C, Njolstad PR, Shiota C, Magnuson MA, Matschinsky FM: Glucokinase and the regulation of blood sugar. In Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics Matschinsky FM, Magnuson MA. Eds. Basel, Switzerland, Karger, 2004, p. 92– 109 [Google Scholar]
- 25.Ferry RJ, Jr, Kelly A, Grimberg A, Koo-Mccoy S, Shapiro MJ, Fellows KE, Glaser B, Aguilar-Bryan L, Stafford DE, Stanley CA: Calcium-stimulated insulin secretion in diffuse and focal forms of congenital hyperinsulinism. J Pediatr 2000; 137: 239– 246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimberg A, Ferry RJ, Jr, Kelly A, Koo-McCoy S, Polonsky K, Glaser B, Permutt MA, Aguilar-Bryan L, Stafford D, Thornton PS, Baker L, Stanley CA: Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes 2001; 50: 322– 328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoffel M, Froguel P, Takeda J, Zouali H, Vionnet N, Nishi S, Weber IT, Harrison RW, Pilkis SJ, Lesage S: Human glucokinase gene: isolation, characterization, and identification of two missense mutations linked to early-onset non-insulin-dependent (type 2) diabetes mellitus. Proc Natl Acad Sci 1992; 89: 7698– 7702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y, Kesavan P, Wang LQ, Niswender K, Tanizawa Y, Permutt MA, Magnuson MA, Matschinsky FM: Variable effects of maturity-onset-diabetes-of-youth (MODY)-associated glucokinase mutations on substrate interactions and stability of the enzyme. Biochem J 1995; 309: 167– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meglasson MD, Matschinsky FM: Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev 1986; 2: 163– 214 [DOI] [PubMed] [Google Scholar]
- 30.Zelent B, Odili S, Buettger C, Shiota C, Grimsby J, Taub R, Magnuson MA, Vanderkooi JM, Matschinsky FM: Sugar binding to recombinant wild-type and mutant glucokinase monitored by kinetic measurement and tryptophan fluorescence. Biochem J 2008; 413: 269– 280 [DOI] [PubMed] [Google Scholar]
- 31..Guex N, Peitsch MC: SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 1997; 18: 2714– 2723 [DOI] [PubMed] [Google Scholar]
- 32.Thornton PS, Alter CA, Katz LE, Baker L, Stanley CA: Short- and long-term use of octreotide in the treatment of congenital hyperinsulinism [see comments]. J Pediatr 1993; 123: 637– 643 [DOI] [PubMed] [Google Scholar]
- 33.Heredia VV, Thomson J, Nettleton D, Sun S: Glucose-induced conformational changes in glucokinase mediate allosteric regulation: transient kinetic analysis. Biochemistry 2006; 45: 7553– 7562 [DOI] [PubMed] [Google Scholar]
- 34.Heredia VV, Carlson TJ, Garcia E, Sun S: Biochemical basis of glucokinase activation and the regulation by glucokinase regulatory protein in naturally occurring mutations. J Biol Chem 2006; 281: 40201– 40207 [DOI] [PubMed] [Google Scholar]
- 35.Pal P, Miller BG: Activating mutations in the human glucokinase gene revealed by genetic selection. Biochemistry 2009; 48: 814– 816 [DOI] [PubMed] [Google Scholar]
- 36.Gloyn AL, Odili S, Zelent D, Buettger C, Castleden HA, Steele AM, Stride A, Shiota C, Magnuson MA, Lorini R, d'Annunzio G, Stanley CA, Kwagh J, van Schaftingen E, Veiga-da-Cunha M, Barbetti F, Dunten P, Han Y, Grimsby J, Taub R, Ellard S, Hattersley A, Matschinsky MA: Insights into the structure and regulation of glucokinase from a novel mutation (V62M), which causes maturity-onset diabetes of the young. J Biol Chem 2005; 280: 14105– 14113 [DOI] [PubMed] [Google Scholar]