Lymphocytic infiltration in the islets of Langerhans is generally recognized as the defining lesion in young patients with recent-onset type 1A diabetes. In a landmark article published in Diabetes in 1965, Gepts (1) described insulitis in 70% of cases with acute diabetes and found that the lesion only affected islets with residual β-cells in pancreatic organs that had otherwise lost most of their β-cell mass. He concluded that the disease was probably caused by a protracted β-cell–specific (auto)immune process, thereby initiating a domain of study that has led to many new insights into the disease process, the identification of preclinical markers, and the definition of new intervention strategies.
Unfortunately, our knowledge of the early disease processes leading to the specific destruction of β-cells is far from complete. Among other things, it is still unknown what event triggers the T-cell–mediated inflammatory process, why the infiltrate of predominantly CD8+T-cells and macrophages is limited to the islets of Langerhans and only β-cells are destroyed, against which antigen the inflammatory infiltrate is directed, and whether this antigen is of an endogenous or exogenous nature (2–5). This lack of knowledge is, to a considerable extent, caused by the scarcity of material regarding those recently diagnosed with type 1 diabetes, of which only several dozen cases have been described in the literature (6). It is especially due to the virtual absence of material regarding pre-diabetic individuals who, although identifiable with increasing confidence by a combination of autoantibody assays and genotyping for HLA-DQ–susceptibility markers (7), cannot easily be investigated due to the difficult accessibility and diffuse location of the endocrine pancreas. Due to this lack of material, researchers have increasingly turned to animal models that resemble the human disease. The spontaneously diabetic NOD mouse has become the object of choice because its diabetes shares several characteristics with human type 1 diabetes, although important differences remain with regard to incidence, sex bias, immune processes, and histopathology (8).
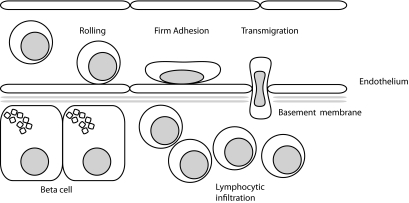
In this issue of Diabetes, an exciting study (9) deals with the question of how circulating immune cells are targeted to the islets of Langerhans. It is generally thought that in an inflammatory response, leukocytes are primed in draining lymph nodes and then home to the tissue where the primary antigen originated. The homing process involves several different phases of interaction with the vasculature of the target tissue; these phases involve rolling, firm adhesion, and transmigration across the endothelial cell layer (Fig. 1). All of these steps are closely regulated by several families of proteins, including the integrins (a family of 24 heterodimeric transmembrane proteins composed of one α-chain and one β-chain), that are involved in intercellular and cell-matrix interactions and are of special importance in T-cell activation and homing (10). The study deals with a subclass of four integrins that are composed of an Itgb2 β-chain and one of four possible α-chains (α-L, αM-, αX-, or αD-chain). The authors used NOD mouse strains knocked out for either Itgb2, thus removing leukocyte expression of all four integrins that contain this subunit, or for the αL-chain, thus removing leukocyte expression of the heterodimer Itgb2/αL (also called LFA-1). Glawe et al. found that both knockouts resulted in the prevention of diabetes and insulitis, indicating that the β2 class of integrins and, especially, LFA-1 are essential for the development of the disease in the NOD mouse. Moreover, using an islet endothelial cell adhesion assay and adoptive transfer experiments, Glawe et al. showed that this prevention was apparently due to two different mechanisms: the Itgb2 knockout interfered with T-cell adhesion, whereas the αL-chain knockout interfered with T-cell activation. These findings are important, as they may open new possibilities for treatment in the early stages of the autoimmune process in autoantibody-positive subjects who are at high risk for developing the disease or in recent-onset subjects who still retain a significant part of their β-cell mass. These findings are also important in view of an existing antibody-based therapy directed against the intregrin αL-chain, thus providing us with a potential new treatment that could reduce T-cell activation and adhesion and could significantly impair the progress of insulitis.
FIG. 1.
Extravasation of leukocytes involves subsequent stages of tethering and rolling over the (peri-)islet endothelium, followed by firm adhesion and transmigration. A plethora of tissue-specific adhesion molecules is involved that trigger, mediate, or modify leukocyte binding and migration.
Clearly, many questions need to be addressed before a clinical intervention can be considered in diabetic subjects. One question is whether the observations in NOD mice also pertain to the human disease. This is not a trivial point, as significant differences have been described between the process of insulitis in the NOD mouse and that in diabetic patients. In the NOD mouse, there is a prolonged phase of relatively benign peri-insulitis followed by massive aggressive intra-insulitis around week 17 (11). This two-step process has not been recognized in patients in whom the insulitis is generally of a much milder nature. There are also a number of anatomical differences between mouse and human islets that may affect leukocytic infiltration. The basement membrane in rodents is single layered, in contrast to human islets where it consists of a double-layered structure, which has been compared with the basement membrane of the blood-brain barrier and may provide a stop signal to lymphocytes (12). On the other hand, there are also significant parallels between NOD and human insulitis; among these is the overexpression of the LFA-1 ligand intracellular adhesion molecule-1 (ICAM1) in inflamed human islets (13,14), suggesting that the LFA-1–ICAM-1 pathway is indeed operative in human diabetes. A second question that remains to be clarified is with regard to previous studies in the NOD mouse model that stressed the importance of other integrins, notably the α4-β1 and α4-β7 heterodimers (15–17). It will be important to investigate whether a knockout of the α4-chain will prevent the disease and whether the β2 integrins indeed dominate over the other classes of molecules. Glawe et al. suggest that in light of their results in the NOD mouse, the clinically available anti–LFA-1 antibody efalizumab could be useful for the treatment of type 1 diabetes in human subjects. Although this is indeed an exciting possibility, there is also need for caution, not only for the reasons stated above, but also because the drug's current use for the treatment of psoriasis is open to debate following reports of serious adverse effects (18,19).
In summary, the study by Glawe et al. provides important new information about the mechanisms of T-cell activation and homing in the NOD mouse. If the data are validated in human subjects, these studies may open the way for new forms of immune intervention that may complement the currently available therapies.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1292.
REFERENCES
- 1.Gepts W: Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965; 14: 619– 633 [DOI] [PubMed] [Google Scholar]
- 2.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PGF, Gamble DR: In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985; 313: 353– 360 [DOI] [PubMed] [Google Scholar]
- 3.Wilcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG: Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2008; 155: 173– 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dotta F, Censini S, van Halteren AGS, Marseli L, Masini M, Dionisi S, Mosca F, Boggi U, Onetti Muda A, Del Prato S, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007; 104: 5115– 5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Alen JS, Tree TIM, Zhao M, Dayan CM, Sewell AK, Unger W, Drijfhout JW, Ossendorp F, Roep BO, Peakman M: CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated prepropinsulin epitope. J Clin Invest 2008; 118: 3390– 3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pipeleers D, Ling Z: Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab Rev 1992; 8: 209– 227 [DOI] [PubMed] [Google Scholar]
- 7.In't Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, Pipeleers D: Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 2007; 56: 2400– 2404 [DOI] [PubMed] [Google Scholar]
- 8.Roep BO, Atkinson M, von Herrath M: Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev 2004; 4: 989– 997 [DOI] [PubMed] [Google Scholar]
- 9.Glawe JD, Patrick DR, Huang M, Sharp CD, Barlow SC, Kevil CG: Genetic deficiency of Itgb2 or ItgaL prevents autoimmune diabetes through distinctly different mechanisms in NOD/LtJ mice. Diabetes 2009; 58: 1292– 1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreiro O, de la Fuente H, Mittelbrunn M, Sanchez-Madrid F: Functional insights on the polarized redistribution of leucocyte integrins and their ligands during leucocyte migration and immune interactions. Immunol Rev 2007; 218: 147– 164 [DOI] [PubMed] [Google Scholar]
- 11.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA: Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 1994; 43: 667– 675 [DOI] [PubMed] [Google Scholar]
- 12.Otonkoski T, Banerjee M, Korsgren O, Thorn LE, Virtanen I: Unique basement membrane structure of human pancreatic islets: implications for beta cell growth and differentiation. Diabetes Obes Metab 2008; 10( Suppl. 4): 119– 127 [DOI] [PubMed] [Google Scholar]
- 13.Hänninen A, Jalkanen S, Samli M, Toikkanen S, Nikolarakos G, Simell O: Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest 1992; 90: 1901– 1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernandez-Figuereas MT, Ariza A, Gomis R, Bragado R, Marti M, Jaraquemada D, Pujol-Borrell R: Pancreas in recent-onset insulin-dependent diabetes mellitus: changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol 1994; 153: 1360– 1377 [PubMed] [Google Scholar]
- 15.Baron JL, Reich EP, Visintin I, Janeway CA: The pathogenesis of adoptive murine autoimmune diabetes requires an interaction between alpha 4-integrins and vascular cell adhesion molecule-1. J Clin Invest 1994; 93: 1700– 1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XD, Michie SA, Tisch R, Karin N, Steinman L, McDevitt HO: A predominant role of integrin alpha4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994; 91: 12604– 12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hänninen A, Nurmela R, Maksimow M, Heino J, Jalkanen S, Kurts C: Islet beta-cell-specific T cells can use different homing mechanisms to infiltrate and destroy pancreatic islets. Am J Pathol 2007; 170: 240– 250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluger N, Girard C, Gonzalez V, Guillot B, Bessis D: Efalizumab-induced aseptic meningitis. Br J Dermatol 2007; 156: 189– 191 [DOI] [PubMed] [Google Scholar]
- 19.Wendt M, Wohlrab J, Zierz S, Deschauer M: Efalizumab-induced isolated cerebral lupus-like syndrome. Neurology 2009; 72: 96– 97 [DOI] [PubMed] [Google Scholar]