Abstract
OBJECTIVE
The aim of this study was to elucidate whether age plays a role in the expansion or regeneration of β-cell mass.
RESEARCH DESIGN AND METHODS
We analyzed the capacity of β-cell expansion in 1.5- and 8-month-old mice in response to a high-fat diet, after short-term treatment with the glucagon-like peptide 1 (GLP-1) analog exendin-4, or after streptozotocin (STZ) administration.
RESULTS
Young mice responded to high-fat diet by increasing β-cell mass and β-cell proliferation and maintaining normoglycemia. Old mice, by contrast, did not display any increases in β-cell mass or β-cell proliferation in response to high-fat diet and became diabetic. To further assess the plasticity of β-cell mass with respect to age, young and old mice were injected with a single dose of STZ, and β-cell proliferation was analyzed to assess the regeneration of β-cells. We observed a fourfold increase in β-cell proliferation in young mice after STZ administration, whereas no changes in β-cell proliferation were observed in older mice. The capacity to expand β-cell mass in response to short-term treatment with the GLP-1 analog exendin-4 also declined with age. The ability of β-cell mass to expand was correlated with higher levels of Bmi1, a polycomb group protein that is known to regulate the Ink4a locus, and decreased levels of p16Ink4aexpression in the β-cells. Young Bmi1−/− mice that prematurely upregulate p16Ink4afailed to expand β-cell mass in response to exendin-4, indicating that p16Ink4alevels are a critical determinant of β-cell mass expansion.
CONCLUSIONS
β-Cell proliferation and the capacity of β-cells to regenerate declines with age and is regulated by the Bmi1/p16Ink4apathway.
Hyperglycemia in type 1 and 2 diabetes is, by definition, caused by insufficient insulin secretion to meet insulin demand. Defective insulin secretion in both forms of diabetes is caused in part by loss of β-cell mass (1–4). Diabetes can be reversed in type 1 and 2 diabetes by replacement of β-cell mass, as demonstrated by pancreas and islet transplantation (5,6). However, given the shortage of organ donors and the need for chronic immunosuppression, pancreas transplantation has limited applicability in the treatment of diabetes.
Regeneration of β-cell mass is one promising approach to replace the deficit in β-cell mass in diabetic patients. Regeneration occurs in rodents after injury or genetic ablation of β-cells (7). Lineage tracing experiments show that new β-cells can arise from proliferation of preexisting β-cells (8). However, both the capacity of regeneration and the mechanism involved can differ significantly depending on the experimental model. An alternative source of β-cells has recently been proposed showing that facultative progenitors can be found in regenerating pancreatic ducts (9). Several studies have shown that the endocrine pancreas has endogenous renewal capacity in response to metabolic demands such as pregnancy and insulin resistance (10). Changes in insulin demand caused by physiological states such as insulin resistance have been shown to lead to adaptive changes in β-cell mass. The cell source and mechanism leading to the endogenous renewal is not clear, although proliferation of β-cells appears to play an important role (11). Elucidating mechanisms of regeneration and endogenous renewal in response to metabolic demands may provide novel insights into approaches to restore functional β-cell mass in diabetes.
Most of the studies exploring the capacity of endogenous renewal have been carried out on rodents at relatively young ages, and several studies suggest that the capacity to expand or regenerate β-cell mass may decline with age. For example, a threefold increase of insulin content was measured in the residual pancreas after 90% pancreatectomy in 1-month-old rats; however, a comparable increase was not observed in rats that were 5 or 15 months old (12). Moreover, consistent with the adaptive increase in pancreatic insulin content in the 1-month-old but not older animals after a 90% pancreatectomy, blood glucose values in the 1-month-old rats declined 2 weeks after surgery, whereas no such decline was observed in 5- and 15-month-old rats (12). The rate of β-cell proliferation gradually declines with aging in rats to a steady state by 7 months of age (13). Furthermore, long-term bromodeoxyuridine labeling in 1-year-old mice also suggests that β-cell replication rates decline with age (14). The decline in β-cell proliferation with age correlates with increased expression of the cell cycle regulator p16Ink4a in islet cells (15). p16Ink4a inhibits the cyclin-dependent kinase 4 (CDK4)-cyclin D2 complex and can inhibit cell cycle progression and regeneration of islet cells. Transgenic mice that overexpressed p16Ink4a showed reduced islet cell proliferation and a reduction in the regenerative capacity of islets after toxin-mediated destruction. However, the mechanisms that regulate the increase in p16Ink4a with aging are not known.
Establishing the basis of aging in affecting the capacity of adaptive changes in β-cell mass in adult humans versus young rodents has important clinical implications. If it is a species difference, then caution will need to be exercised extrapolating findings in rodents to the potential for β-cell regeneration in humans. For example, partial pancreatectomy in young mice is followed by extensive regeneration of β-cells through β-cell replication. In contrast, partial pancreatecomy in adult humans does not lead to β-cell regeneration (16). To date, it is not clear whether this different outcome is a species difference or a consequence of partial pancreatectomy at different ages. Also of interest, genetically obese mice have a several-fold increase in β-cell mass, whereas obese adult humans have only a much more modest 0.5-fold increase in β-cell mass (1). Again, it is not known whether this is a species difference or the consequence of a different response to obesity-induced insulin resistance during aging.
Studies to date that have reported an increase in β-cell mass with glucagon-like peptide 1 (GLP-1)-1 based therapies were undertaken in young rodents (17–21). On the strength of those observations, it has been proposed that these therapies might serve to foster β-cell regeneration in humans with either type 1 or type 2 diabetes (22–25). However, it is plausible that GLP-1–induced expansion of β-cell mass may only be achievable in young subjects. Similarly, the rapid regeneration of β-cell mass in young mice after a single dose of the β-cell toxin streptozotocin (STZ) has been widely used as a model for β-cell regeneration in type 1 diabetes, but it is not yet known whether there is comparable recovery of β-cell mass in mice during the adult phase of β-cell turnover, a circumstance more clinically relevant to most humans.
In the current studies, the capacity of endogenous renewal of β-cells in response to either a high-fat diet or the GLP-1 analog exendin-4 as well as the capacity to regenerate after toxin administration was examined in young and old mice. β-Cell mass and metabolic measurements were measured after high-fat diet or exendin-4 treatment. β-Cell proliferation was measured to probe the mechanism by which age could affect the capacity for β-cell renewal. Furthermore, the levels of p16ink4a were linked to the capacity of β-cell proliferation. Because several studies have established a role for the polycomb group protein Bmi1 in the regulation of p16Ink4a (26–28), we analyzed the levels of Bmi1. We further showed that loss of Bmi1 results in premature increase in the expression of p16ink4a. Because aging can lead to many changes in the β-cell other than p16Ink4a upregulation, we used Bmi1 knockout mice as a model to explore the endogenous capacity to renew in young mice in which p16ink4a is prematurely upregulated. This result indicates that levels of p16ink4a are the primary determinant of the capacity to expand β-cell mass. Our data suggest that the older mice, unlike younger mice, have a limited capacity to expand β-cell mass because of age-related accumulation of p16Ink4a.
RESEARCH DESIGN AND METHODS
We obtained 6-week-old (young) and 7- to 8-month-old (old) male C57BL/6 mice from The Jackson Laboratory. For the high-fat diet experiment, 12 young or 12 old mice were fed with either normal diet (4.4% of total calories derived from fat, 3.9 kcal/g; Harlan Teklad) or high-fat diet (55% of total calories derived from fat, 4.8 kcal/g; Harlan Teklad) for 8 weeks. For exendin-4 experiments, six young and six old mice were injected intraperitoneally with either exendin-4 (10 nmol/kg; Sigma) or PBS for 7 days. For the STZ (Sigma-Aldrich) experiment, six young and six old mice were injected with a single dose of 90 mg/kg freshly prepared STZ in citrate buffer (pH 4.5) and killed at 7 days. Bmi1−/− mice were obtained from Maarten van Lohuizen of the Netherlands Cancer Institute. For Bmi1−/− mice, targeted disruption of the Bmi1 allele has been described before (29). The animals were maintained by mating Bmi1+/− males and females on a C57BL/6J background. Mice were fed ad libitum on standard diet and keep under a 12-h light/dark circle. All animal protocols were approved by the chancellor's animal research committee at the University of California, Los Angeles.
Metabolic analysis.
Fasting blood glucose was measured after overnight fasting. An insulin tolerance test was performed after a 6-h fast. Blood glucose was measured before intraperitoneal injection of insulin (0.75 mU/g body wt) and then 20, 40, and 60 min after injection. No anesthesia was used during the experiment. Glucose tolerance testing was performed after overnight fasting. Blood glucose levels (mg/dl) were measured before intraperitoneal injection of glucose (2 mg dextrose/g body wt) and then 15, 30, 60, and 120 min after injection.
Immunohistochemistry.
Pancreatic tissue was processed as previously described (11). In brief, the pancreas was dissected and fixed in 4% formaldehyde before being embedded in paraffin. Then, 5-μm sections were deparaffinized and rehydrated, followed by antigen retrieval using antigen unmasking buffer (Vector Labs), and then permeabilized in 0.4% Triton X-100/Tris-buffered saline. Slides were blocked with 3% IgG-free BSA (Jackson ImmunoResearch Laboratories) and treated with anti-mouse insulin (Dako), anti-ki67 (BD Pharmingen), anti–proliferating cell nuclear antigen (PCNA; Lab Vision), anti-Bmi1 (Millipore), anti-p16Ink4a (Santa Cruz), anti–cyclin D2 (Santa Cruz), or anti-p27 (Santa Cruz) antibody followed by fluorescein isothiocyanate– or Cy3-conjugated secondary antibodies (The Jackson Laboratory). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was detected using an in situ cell death detection kit (Roche) according to manufacturer's instructions. Slides were mounted with Vectashield with 4′6-diamidino-2-phenylindole (Vector Labs), and images were obtained with a Leica DM6000 microscope using Openlab software (Improvision). The immunofluorescence data presented are representatives of at least five animals per group in each case.
β-Cell mass.
β-Cell mass was measured as previously described (11). In brief, five to eight sections from each pancreas were stained with anti-mouse insulin antibody (Dako) and scanned by a Leica DM6000 microscope. Montage images were made by ImageJ software. The cross-sectional areas of pancreas and β-cells were determined by ImagePro software. β-Cell mass per pancreas was estimated as the product of the relative cross-sectional area of β-cells per total tissue and the weight of the pancreas and was calculated by examining pancreata from at least three animals for each genotype.
Islet isolation and immunoblotting.
Liberase propidium iodide–purified enzyme blend for rodent islet isolation (Roche) was infused at 3.5 mg/ml into the pancreas via the bile duct. Inflated pancreata were then removed and incubated in Liberase propidium iodide for 18 min at 37°C. Islets were dissociated from the exocrine tissues by shaking vigorously several times, followed by Histopaque (Sigma-Aldrich) gradient. Islets were handpicked under a dissecting microscope and lysed by tissue extraction buffer (Invitrogen). Lysates with equal amounts of protein were resolved by SDS-PAGE, followed by transferring to polyvinylidene fluoride membrane for immunoblotting. The membranes were probed with specific antibodies against p16Ink4a (Santa Cruz), Bmi1 (Millipore), and β-tubulin (Sigma-Aldrich). The data presented are representative of at least three experiments.
Chromatin immunoprecipitation.
We performed chromatin immunoprecipitation (ChIP) analysis using a Millipore ChIP kit (no. 17-295) according to the manufacturer's instructions, with minor modifications. The islets (150–200 islets per group) were treated with 2% paraformaldehyde at room temperature for 20 min to cross-link the DNA with bound proteins. After washing, the islets were resuspended in SDS lysis buffer with protease inhibitors and sonicated to shear the chromatin. The chromatin was then precleared and incubated with 2–5 mg of anti–acetyl histone H3 lysine 9 (H3K9; no. 07-532; Millipore), or normal mouse IgG as a control, overnight at 4°C with agitation. After immunoprecipitation, the chromatin was harvested, the cross-links were reversed, and the DNA was purified and precipitated. The resulting DNA was quantified and served as a template for the real-time PCR, performed using a LightCycler FastStartPLUS DNA SYBR Master kit (Roche) and Light Cycler PCR equipment (Roche). The DNA enrichment after ChIP was estimated as the percentage bound-to-input ratio, determined by real-time PCR. The primers used to amplify the Ink4a/Arf locus are as follows: primer set 1: forward 5′GAGTACAGCAGCGGGAGCAT-3′, reverse 5′-GAACTTCACCAAGAAAACCCTCTCT-3′; primer set 2: forward 5′-GTCCGATCCTTTAGCGCTGTT-3′, reverse 5′-AGCCCGGACTACAGAAGAGATG-3′; primer set 3: 5′CCGGAGCCACCCATTAAACTA-3′, reverse 5′-CAAGACTTCTCAAAAATAAGACACTGAAA-3′; primer set 4: forward 5′-CCCAACACCCACTTGAGGAA-3′, reverse 5′-CAGAGGTCACAGGCATCGAA-3′; and primer set 5: (negative control, HoxC13 exon 2) forward 5′-CATTTTTCACTGATTTCCTAAGCA-3′, reverse 5′-CAATGATGTCACCCCTCCTC-3′.
Cell culture and transfection.
Min6 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions with 1 mg of Bmi1 construct (in pcDNA3 myc-HisA, resulting in expression of NH2-terminal myc-tagged Bmi1) and the control enhanced green fluorescent protein construct (in pcDNA3), allowing expression from the cytomegalovirus promoter in either case.
Statistical analyses.
All data were expressed as the means ± SE. Mean and SE values were calculated from at least triplicates of a representative experiment. The statistical significance of differences was measured by unpaired Student's t test and confirmed by one-way ANOVA for repeat measures. P < 0.05 indicated statistical significance. The P values indicated in the graphs are from Student's t test.
RESULTS
Adaptive expansion of β-cell mass associated with insulin resistance is age dependent.
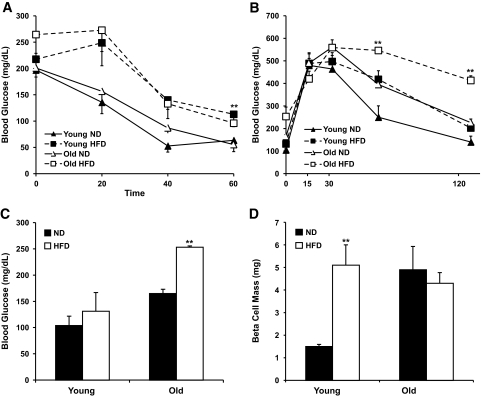
To ascertain whether age plays a role in the adaptive β-cell mass expansion associated with insulin resistance, young (6-week-old) and old (7- to 8-month-old) mice were fed a high-fat diet. Insulin tolerance testing performed after 8 weeks of high-fat diet showed that both young and old mice displayed decreased insulin sensitivity compared with mice on a normal diet (Fig. 1A). No statistically significant difference in body weight between groups was noted at the beginning of the study. The body weight of both young and old mice showed a 50% increase after 8 weeks of high-fat diet (data not shown). Glucose tolerance tests showed that blood glucose levels of old mice after 8 weeks of high-fat diet remained >400 mg/dl by the end of the 120-min testing period. By contrast, young mice after 8 weeks of high-fat diet reached baseline by the end of the 120-min testing period (Fig. 1B). Measurement of fasting blood glucose revealed that older mice displayed fasting blood glucose levels consistently >200 mg/dl after 8 weeks of high-fat diet, indicating that these mice were unable to maintain glucose homeostasis (Fig. 1C). In contrast, young mice fed a high-fat diet did not show fasting blood glucose levels that were significantly different from young mice fed a normal diet.
FIG. 1.
Old mice failed to compensate diet-induced insulin resistance and developed diabetes after an 8-week high-fat diet. Young (6-week-old) and old (7-month-old) mice were fed normal diet (ND; 12.2% calories from fat) or high-fat diet (HFD; 55% calories from fat) for 8 weeks. A: Insulin tolerance test in young and old mice after 8 weeks of high-fat diet or normal diet (n = 6 in each group). Mice were fasted 6 h before testing. High-fat diet–fed mice were compared with normal diet–fed mice. **P < 0.005 (60 min). B: Glucose tolerance test in young and old mice after 8 weeks of high-fat diet or normal diet feeding. Mice were fasted overnight, and the blood glucose level was measured before and after glucose challenge. **P < 0.005. C: Blood glucose levels after overnight fasting. High-fat diet–fed mice were compared with normal diet–fed mice. **P < 0.005. D: Analysis of β-cell mass of young and old mice after 8 weeks of normal diet or high-fat diet feeding. Values are representative of 5–6 slides spanning the whole pancreas of each mouse and three mice per group. **P < 0.005.
The inability of old mice, unlike young mice, after 8 weeks of high-fat diet to maintain glucose homeostasis led us to analyze whether the adaptive expansion of β-cell mass associated with insulin resistance differed in the different age-groups. Young mice after 8 weeks of high-fat diet showed a fivefold increase in β-cell mass compared with the normal-diet group. In contrast, old mice fed high-fat diet did not show any changes in β-cell mass compared with control old mice (Fig. 1D). Moreover, pancreas weight did not show any significant increase after 8 weeks of high-fat diet in both young and old mice (data not shown). Thus, young mice, unlike old mice, have the capacity to expand β-cell mass to adapt to insulin resistance.
Expansion of β-cell mass is correlated with p16Ink4alevels in the β-cells.
To analyze whether increased proliferation could account for the expansion of β-cell mass in young mice fed a high-fat diet, the number of Ki67- and PCNA-positive β-cells was quantified in mice fed high-fat diet and those fed normal diet. A 7.5-fold increase of Ki67-positive β-cells and a 2.3-fold increase of PCNA-positive β-cells was observed in the young mice fed high-fat diet compared with the control group. In contrast, the number of Ki67- and PCNA-positive β-cells did not change in old mice after a high-fat diet compared with those fed normal diet (Fig. 2A and B). No TUNEL staining (as a measure of apoptosis) was observed in all groups (data not shown). Because decreased proliferative capacity has been correlated with increased expression of p16Ink4a with age (15), we determined the levels of p16Ink4a in isolated islets from mice of increasing age. As shown inFig. 2C, p16Ink4a levels increase with age in isolated islets. Immunohistochemistry revealed that β-cells of 1-month-old mice showed little expression of p16Ink4a, in contrast to high levels of p16Ink4a in 6-month-old mice (Fig. 2D). To determine whether p16 levels played a role in the adaptive expansion of β-cell mass in response to high-fat diet, we isolated islets from young and old mice fed normal diet or high-fat diet and measured p16ink4a levels by Western blot. In islets isolated from young mice, low levels of p16ink4a were apparent, which decreased after 8 weeks of high-fat diet feeding. In contrast, high levels of p16Ink4a in islets from old mice did not change with high-fat diet (Fig. 2E). We next examined whether levels of Bmi1, a polycomb group protein that is known to regulate the Ink4a locus, were also affected. High Bmi1 levels were apparent in islets isolated from young mice, and increased levels of Bmi1 were apparent after high-fat diet. In contrast, islets isolated from old mice did not show appreciable levels of Bmi1.
FIG. 2.
Increased β-cell proliferation in high-fat diet young mice correlates with increased Bmi1 levels. A: Percentage of Ki67-positive β-cells of young and old mice after 8 weeks of normal diet or high-fat diet feeding. Values are averaged from three slides for each mouse and three mice for each group. ***P < 0.005. B: Percentage of PCNA-positive β-cells of young and old mice after 8 weeks of normal diet or high-fat diet feeding. Values are averaged from three slides for each mouse and three mice in each group. **P < 0.05 for young mice fed normal diet vs. young mice fed high-fat diet. C: Protein levels of p16 in isolated islets from 1-, 4-, 6-, and 11-month-old mice. Data were representative of pooled islets from 3–5 mice per group. D: Representative picture of pancreatic sections from 1- and 6-month-old wild-type mice stained with antibodies to p16 (red) and insulin (green). E: Levels of p16 and Bmi1 protein expression in the pooled islet cells isolated from 3–4 normal diet or high-fat diet young and old pancreata. Data were representative of pooled islets from 3–4 mice per group. Graph demonstrates densitometric analysis of immunoblot of p16 and Bmi1 normalized to β-tubulin. For all panels, data are representative of three experiments. **P < 0.05 for high-fat diet young mice vs. high-fat diet old mice. HFD, high-fat diet; ND, normal diet; Tub, β-tubulin. (A high-quality digital representation of this figure is available in the online issue).
Aging affects the glucoincretin exendin-4–induced β-cell mass expansion.
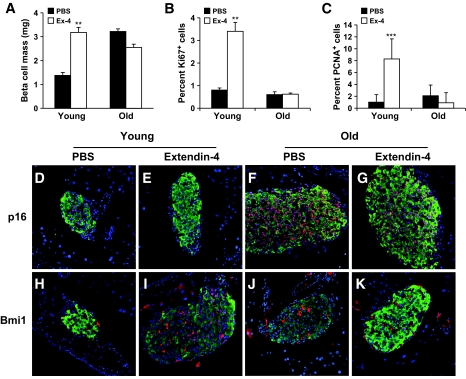
Glucoincretin hormones GLP-1 and its long-acting peptide analogue exendin-4 have been suggested to induce β-cell mass expansion by increasing β-cell proliferation (30). A 1-week daily injection of exendin-4 resulted in a twofold increase in β-cell mass (30). To further investigate the effect of aging on β-cell mass expansion and β-cell proliferation, young and old mice were injected intraperitoneally with exendin-4 daily for 7 days, and β-cell mass was analyzed. After exendin-4 treatment, β-cell mass in young mice showed a twofold increment compared with control group injected with PBS. In contrast, old mice treated with exendin-4 did not show any increases in their β-cell mass (Fig. 3A). These data suggest that as adaptive expansion associated with insulin resistance, the capacity of β-cell mass to expand in response to glucoincretin hormones challenge is severely diminished with aging.
FIG. 3.
Increased Bmi1 and decreased p16 levels are coincident with adaptive β-cell mass expansion on exendin-4 treatment in young mice. A: Analysis of β-cell mass of young and old mice after 7 days' constitutive injection of exendin-4. Values are representative of five slides spanning the whole pancreas of each mouse and three mice per group. B: Percentage of Ki67-positive β-cells of young and old mice after 7 days of PBS or exendin-4 treatment. Values are averaged from slides for each mouse and three mice in each group. **P < 0.05. C: Percentage of PCNA-positive β-cells of young and old mice after 7 days of PBS or exendin-4 treatment. Values are averaged from slides for each mouse and three mice in each group. ***P < 0.005 comparing PBS– vs. exendin-4–treated groups. D– K: Pancreatic sections from young (D, E, H, and I) and old (F, G, J, and K) mice treated with either exendin-4 or PBS were immunostained with antibodies to insulin (green) and p16 (red) (D, E, F, and G), or to insulin (green) and Bmi1 (red) (H, I, J, and K). Ex-4, exendin-4. (A high-quality digital representation of this figure is available in the online issue).
To analyze whether increased proliferation could account for the expansion of β-cell mass in young mice treated with exendin-4, the number of Ki67- and PCNA-positive β-cells was quantified. A 4-fold increase of Ki67-positive β-cells and a 5.5-fold increase of PCNA-positive β-cells was observed in the young mice treated with exendin-4 compared with the control group. In contrast, the number of Ki67- and PCNA-positive β-cells did not change in old mice after treatment with exendin-4 compared with untreated old mice (Fig. 3B and C). We next examined whether the failure of old mice to expand their β-cell mass on exendin-4 treatment is dependent on levels of p16Ink4a. A marked decline of p16Ink4a expression levels was observed in islets of young mice (Fig. 3D and E), which is concomitant with increased Bmi1 levels (Fig. 3H and I). In contrast, exendin-4 treatment did not lead to a decrease of p16Ink4a levels in the β-cells in the old mice (Fig. 3F and G). Furthermore, levels of Bmi1 (Fig. 3J and K) did not show much difference between the exendin-4–treated mice and the PBS control group.
We first assessed whether p16Ink4a was a specific cell cycle target of Bmi1 by analyzing expression levels of p16Ink4a along with other cell cycle regulators involved in β-cell proliferation. Immunohistochemistry analysis of pancreatic sections from 14-day-old Bmi1−/− mice and wild-type littermates showed p16Ink4a was upregulated in islets of Bmi1−/− mice (Fig. 4). Other cell cycle targets cyclin D2 and p27 were unaffected by the absence of Bmi1 (Fig. 4). To directly test whether Bmi1 regulates p16Ink4a, we measured the levels of p16Ink4a in islets isolated from 4-week-old wild-type and Bmi1−/− mice. Immunoblots showed increased levels of p16Ink4a in islets isolated from Bmi1−/− mice (Fig. 5A). To further confirm whether Bmi1 represses p16Ink4a, we generated an expression vector that allowed expression of Bmi1 fused with an NH2-terminal myc tag. The expression of myc-tagged Bmi1 in the Min6 cells resulted in reduced levels of p16Ink4a protein, thus confirming the repressive effect of Bmi1 on p16Ink4a expression (Fig. 5B). We next assessed whether Bmi1 regulated p16Ink4a expression by affecting the chromatin structure of the Ink4a/Arf locus that encodes p16Ink4a. We determined whether histone acetylation at the Ink4a/Arf locus, which results in the loosening of chromatin and lends itself to transcription, was regulated by Bmi1. We examined the levels of H3K9 acetylation associated with the Ink4a/Arf locus in islets isolated from 6-week-old Bmi1−/− mice and their wild-type littermates. In the absence of Bmi1, the levels of H3K9 acetylation were increased, suggesting that Bmi1 mediated repression of p16Ink4a via modulation of chromatin structure (Fig. 5C).
FIG. 4.
Expression patterns of p16, p27, and cyclin D2 in wild-type and Bmi1−/− mice. Pancreatic sections from 2-week-old wild-type and Bmi1−/− mice were immunostained with antibodies to insulin (green) and p16, p27, or cyclin D2 (red) as indicated in the figure. KO, knockout; WT, wild type. (A high-quality digital representation of this figure is available in the online issue).
FIG. 5.
β-Cell mass failed to expand in response to exendin-4 treatment in Bmi1−/− mice. A: Protein levels of Bmi1 and p16 in isolated islets from 6-week-old wild-type and Bmi1−/− mice. Data were representative of pooled islets from three experiments with 3–5 mice per group. B: Protein levels of Bmi1 and p16 in Min6 cells transfected with Bmi1 fused with an NH2-terminal myc tag. C: Schematic representation of the Ink4a/Arf locus, with blue regions, marked 1–4, indicating the amplified regions in the ChIP studies (top panel) and ChIP analysis for the indicated antibodies at the Ink4a/Arf locus in islets isolated from wild-type (WT) and Bmi1−/− mice (4 weeks old) (bottom panel). On the x-axis, “5” indicates binding for the negative control corresponding to exon 2 in the Hox C13 locus. P values were determined by unpaired Student's t test. D and E: Analysis of β-cell mass (D) and β-cell proliferation (E) of wild-type and Bmi1−/− mice after seven constitutive days' injection of exendin-4. Values are representative of 5–6 slides spanning the whole pancreas of each mouse and three mice per group. Error bars indicate ± SE. *P < 0.05; **P < 0.005. Tub, β-tubulin.
The absence of Bmi1 in young mice, which results in premature increase in p16Ink4aexpression in the β-cells, allowed us to test whether upregulation of p16Ink4a in young Bmi1−/− mice would affect β-cell expansion. We treated 6-week-old Bmi1−/− mice and their wild-type littermates daily with exendin-4 for 7 days and quantified β-cell proliferation and β-cell mass. After exendin-4 treatment, a twofold increase of β-cell mass was observed in the wild-type mice compared with the PBS-injected control group (Fig. 5D). In contrast, no difference in β-cell mass was found between exendin-4– and PBS-treated Bmi1−/− mice. To ascertain whether β-cell proliferation was affected in Bmi1−/− mice treated with exendin-4, we quantified β-cell proliferation in wild-type and Bmi1−/− mice after exendin-4 treatment. We observed a threefold increase in β-cell proliferation after exendin-4 treatment in wild-type mice, whereas no increase was observed in Bmi1−/− mice (Fig. 5E). These data further underscore the importance of the age-dependent rise of p16Ink4a levels in regulating age-dependent decline in β-cell proliferation and β-cell mass expansion.
Regenerative capacity of β-cell decreases with aging.
To assess the effect of aging on β-cell regeneration capacity, we adapted a standard regeneration model that uses a single injection of STZ to lead to a loss of ∼50% of the β-cells (31). We used this model for two reasons:1) an increase in β-cell proliferation is observed 7 days after STZ injection that correlates with regeneration of β-cell mass, and 2) the residual β-cell mass is sufficient to maintain blood glucose levels, thus potentially minimizing the role of hyperglycemia in β-cell proliferation and apoptosis. Young and old mice were subject to a single dose of 90 mg/kg STZ to induce partial loss of β-cells, and Ki67- and PCNA-positive β-cells were evaluated 7 days later. Both young and old mice that had received single-dose STZ administration displayed hyperglycemia (Fig. 6A). A number of proliferating β-cells were observed in young mice after STZ administration. In contrast, old mice injected with STZ had limited numbers of Ki67- or PCNA-positive β-cells (Fig. 6B, C, and E). Quantification of Ki67-positive β-cells in young mice after STZ administration revealed a fourfold increase in proliferating β-cells, whereas no difference was observed in older mice after STZ administration. Quantification of PCNA-positive β-cells showed a fivefold increase in proliferating β-cells in young mice after STZ administration, whereas no difference was observed in older mice. These data indicate that young mice, unlike old mice, have the capacity to proliferate and regenerate β-cell mass after β-cell destruction.
FIG. 6.
β-Cell regeneration capacity is severely diminished in old mice. A: Blood glucose of young and old mice after STZ or sham injection of vehicle alone. B: Percentage Ki67-positive cells of young and old mice after STZ or sham injection. Values are averaged from four slides for each mouse and three mice in each group. C: Percentage PCNA-positive cells of young and old mice after STZ or sham injection. Values are averaged from two slides for each mouse and three mice in each group. D– G: Pancreatic sections from control (D and F) or STZ-administered (E and G) young (D and E) and old (F and G) mice were immunostained with antibodies to PCNA (red) and to insulin (green). 4′6-diamidino-2-phenylindole–stained nuclei appear blue. * P < 0.05; ** P < 0.01. (A high-quality digital representation of this figure is available in the online issue).
DISCUSSION
The experiments presented here were designed to evaluate whether age plays a role in the proliferative and regenerative capacity of the pancreatic β-cell. We used three distinct models of endogenous β-cell renewal and regeneration: adaptive expansion associated with insulin resistance, exendin-4–induced β-cell expansion, and the regeneration of β-cells after toxin-mediated ablation. In all three models, young mice had the capacity to expand or regenerate β-cells, whereas the β-cell mass of old mice did not show similar plasticity. We also show that the age-dependent decline in the capacity for β-cell mass expansion coincides with reduced β-cell proliferation. Our data suggests that age-dependent decline in β-cell proliferation curtails the ability of the endocrine pancreas to expand or regenerate β-cell mass to respond to changes in metabolic demands, growth factors, or injury.
Our data raise an important issue in assessing therapeutic strategies in diabetes research that are largely carried out in young mice, when the β-cell proliferative and regeneration capabilities are still retained. It could be argued that older mice with more limited regeneration capacity would serve as a better model for assessing therapies for humans. For instance, the age of NOD mice in most of the studies could contribute to the success in the reversal of diabetes in NOD mice but failures in humans (32,33). It has been suggested that incretin hormone therapy might serve to foster β-cell regeneration in humans with either type 1 or type 2 diabetes, but it is likely that GLP-1–induced increased β-cell mass may not be achievable in typical middle-aged patients with the capacity for β-cell expansion so predictably low in these human subjects (34).
The plasticity of β-cell mass was correlated with β-cell proliferation and the regulation of the Ink4/Arf locus. The product of the Ink4a/Arf (Cdkn2a) locus, p16Ink4a (a negative regulator of CDK4 D-type cyclins), is thought to be involved in aging, and in genome-wide studies it has linked with type 2 diabetes (35,36). Moreover, the age-induced increase in p16Ink4a has been shown to limit the regenerative capacity of β-cells with aging (15). Several studies suggest a role for polycomb group proteins in the regulation of expression from the Ink4a/Arf locus, and Bmi1, a key component of the polycomb complex, has been widely implicated in this process (37,38). We have correlated β-cell proliferation with changes in expression of Bmi1 and p16Ink4a in β-cells that serve as both biological markers and effectors of aging. Our data suggests that age-related changes within the β-cell may be a significant contributing factor to reduced tissue homeostasis and regeneration in older individuals. Whereas the simplest model is that the Bmi1/p16Ink4a pathway operates in the β-cell to regulate β-cell proliferation via CDK4 cyclin D2, it is possible that this pathway acts in putative stem cells that lead to regulation of β-cell mass. Activation of the Bmi1 in distinct cell types within the pancreas will shed light on the compartment that regulates β-cell mass.
Our data indicate that age-related changes that lead to β-cell senescence may be a significant contributing factor to reduced tissue homeostasis and regeneration in older mice. In fact, these senescence-inducing mechanisms presumably act to prevent the development of malignancy in the aging organism, but this protection comes at the cost of lost proliferative potential (39). However, we need to be cautious in interpreting experimental data from short-lived rodents to long-lived humans, where cellular senescence might be regulated by alternative mechanisms. We suggest diminished proliferative capacity of β-cells is the primary cause of why β-cells in old mice expand less successfully. It is also worth pointing out that the β-cell population is heterogeneous, and aging may change the composition of the β-cell population (34). Other distinct processes that may operate in humans to account for diminished regenerative capacity with aging include altered stem/progenitor cell function, an increased tendency for apoptosis, alterations in growth factor profiles, and important changes in immune responses. It will be crucial in the future to better characterize these underlying mechanisms to develop new therapeutic strategies for diabetes because the progression of diabetes is likely to alter how the pancreas copes normally with tissue homeostasis. Understanding the mechanisms that regulate declining capacity for β-cell expansion with aging is not only important from the perspective of regeneration, but it also should be taken into account when selecting donor islets to be used in cellular therapies.
In summary, we have shown here that the ability to expand β-cell mass in mice declines with age and is correlated with reduced β-cell replication. Furthermore, we have correlated the age-dependent decline in the regenerative capacity of β-cells with the Bmi1/Ink4/Arf pathway. The latter pathway might therefore represent a molecular target in efforts to foster β-cell regeneration in the treatment of diabetes.
Acknowledgments
This study was supported by National Institutes of Health Grant R01 DK-068763 and the Larry Hillblom and Juvenile Diabetes Research Foundations (to A.B.).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Peter Butler for advice and helpful discussion. We thank Maarten van Lohuizen, Netherlands Cancer Institute, for the Bmi1−/− mice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying original article, p. 1365.
REFERENCES
- 1.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 2.Gepts W: Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965; 14: 619– 633 [DOI] [PubMed] [Google Scholar]
- 3.Gepts W, De Mey J: Islet cell survival determined by morphology: an immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes 1978; 27( Suppl. 1): 251– 261 [DOI] [PubMed] [Google Scholar]
- 4.Junker K, Egeberg J, Kromann H, Nerup J: An autopsy study of the islets of Langerhans in acute-onset juvenile diabetes mellitus. Acta Pathol Microbiol Scand [A] 1977; 85: 699– 706 [DOI] [PubMed] [Google Scholar]
- 5.Dean PG, Kudva YC, Stegall MD:. Long-term benefits of pancreas transplantation. Curr Opin Organ Transplant 2008; 13: 85– 90 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Sharma A: Are there pancreatic progenitor cells from which new islets form after birth? Nat Clin Pract Endocrinol Metab 2006; 2: 240– 241 [DOI] [PubMed] [Google Scholar]
- 8.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41– 46 [DOI] [PubMed] [Google Scholar]
- 9.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008; 132: 197– 207 [DOI] [PubMed] [Google Scholar]
- 10.Bouwens L, Rooman I: Regulation of pancreatic beta-cell mass. Physiol Rev 2005; 85: 1255– 1270 [DOI] [PubMed] [Google Scholar]
- 11.Zhong L, Georgia S, Tschen SI, Nakayama K, Nakayama K, Bhushan A: Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 2007; 117: 2869– 2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanigawa K, Nakamura S, Kawaguchi M, Xu G, Kin S, Tamura K: Effect of aging on B-cell function and replication in rat pancreas after 90% pancreatectomy. Pancreas 1997; 15: 53– 59 [DOI] [PubMed] [Google Scholar]
- 13.Montanya E, Nacher V, Biarnes M, Soler J: Linear correlation between β-cell mass and body weight throughout the lifespan in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes 2000; 49: 1341– 1346 [DOI] [PubMed] [Google Scholar]
- 14.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA: Very slow turnover of β-cells in aged adult mice. Diabetes 2005; 54: 2557– 2567 [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE: p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 2006; 443: 453– 457 [DOI] [PubMed] [Google Scholar]
- 16.Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ: Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 2008; 57: 142– 149 [DOI] [PubMed] [Google Scholar]
- 17.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R: Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002; 143: 4397– 4408 [DOI] [PubMed] [Google Scholar]
- 18.Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ: Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology 2008; 149: 1338– 1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ: Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003; 278: 471– 478 [DOI] [PubMed] [Google Scholar]
- 20.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC: Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007; 148: 5136– 5144 [DOI] [PubMed] [Google Scholar]
- 21.Xu G, Stoffers DA, Habener JF, Bonner-Weir S: Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999; 48: 2270– 2276 [DOI] [PubMed] [Google Scholar]
- 22.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA: Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 1996; 19: 580– 586 [DOI] [PubMed] [Google Scholar]
- 23.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S: Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326: 1316– 1322 [DOI] [PubMed] [Google Scholar]
- 24.Kjems LL, Holst JJ, Volund A, Madsbad S: The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003; 52: 380– 386 [DOI] [PubMed] [Google Scholar]
- 25.Meneilly GS, McIntosh CH, Pederson RA, Habener JF, Ehlers MR, Egan JM, Elahi D: Effect of glucagon-like peptide 1 (7–36 amide) on insulin-mediated glucose uptake in patients with type 1 diabetes. Diabetes Care 2003; 26: 837– 842 [DOI] [PubMed] [Google Scholar]
- 26.Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, van Lohuizen M: Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev 2005; 19: 1438– 1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M: The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999; 397: 164– 168 [DOI] [PubMed] [Google Scholar]
- 28.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ:. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006; 443: 448– 452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF: Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003; 423: 302– 305 [DOI] [PubMed] [Google Scholar]
- 30.Buteau J, Spatz ML, Accili D: Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes 2006; 55: 1190– 1196 [DOI] [PubMed] [Google Scholar]
- 31.Bonner-Weir S, Trent DF, Honey RN, Weir GC: Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes 1981; 30: 64– 69 [DOI] [PubMed] [Google Scholar]
- 32.Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL: Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 2003; 302: 1223– 1227 [DOI] [PubMed] [Google Scholar]
- 33.Roep BO, Atkinson M: Animal models have little to teach us about type 1 diabetes. 1. In support of this proposal. Diabetologia 2004; 47: 1650– 1656 [DOI] [PubMed] [Google Scholar]
- 34.Schuit FC, In't Veld PA, Pipeleers DG: Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A 1988; 85: 3865– 3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341– 1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park IK, Morrison SJ, Clarke MF: Bmi1, stem cells, and senescence regulation. J Clin Invest 2004; 113: 175– 179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M: Stem cells and cancer; the polycomb connection. Cell 2004; 118: 409– 418 [DOI] [PubMed] [Google Scholar]
- 39.Kim WY, Sharpless NE: The regulation of INK4/ARF in cancer and aging. Cell 2006; 127: 265– 275 [DOI] [PubMed] [Google Scholar]